To evaluate the association between 3111T/C polymorphism of the CLOCK gene and the presence of obesity and sleep duration in children aged 6‐13 years. In adults, this genetic variant has been associated with duration of sleep, ghrelin levels, weight, and eating habits. Although short sleep duration has been linked to obesity in children, no study has aimed to identify the possible molecular mechanisms of this association to date.
MethodsWeight, height, and circumferences were transformed into Z‐scores for age and gender. Genotyping was performed using TaqMan methodology. A questionnaire regarding hours of sleep was provided to parents. The appropriate statistical tests were performed.
ResultsThis study evaluated 370 children (45% males, 55% females, mean age 8.5 ± 1.5 years). The prevalence of overweight was 18%. The duration of sleep was, on average, 9.7hours, and was inversely related to age (p<0.001). Genotype distribution was: 4% CC, 31% CT, and 65% TT. There was a trend toward higher prevalence of overweight in children who slept less than nine hours (23%) when compared to those who slept more than ten hours (16%, p=0.06). Genotype was not significantly correlated to any of the assessed outcomes.
ConclusionsThe CLOCK 3111T/C polymorphism was not significantly associated with overweight or sleep duration in children in this city.
Avaliar a relação entre o polimorfismo 3111 T/C do gene CLOCK (rs1801260) e a presença de obesidade, bem como a duração do sono, em crianças de 6 a 13 anos. Em adultos, essa variante genética foi associada à duração do sono, níveis de grelina, peso e padrão alimentar. Embora, em crianças, a curta duração do sono tenha sido relacionada à obesidade, até o momento nenhum estudo foi direcionado no sentido de identificar possíveis mecanismos moleculares dessa associação.
MétodosPeso, altura e circunferências foram transformados em escores‐Z para idade e sexo. A genotipagem foi realizada pela metodologia Taqman. Um questionário sobre horas de sono foi entregue aos pais. Testes estatísticos apropriados foram realizados.
ResultadosForam avaliadas 370 crianças (45% meninos, 55% meninas, idade média 8,5±1,5 anos). A prevalência de excesso de peso foi de 18%. A duração do sono foi, em média, 9,7 horas, sendo inversamente relacionada à idade (p<0,001). A distribuição genotípica foi: 4% CC, 31% TC e 65% TT. Houve uma tendência de maior prevalência de excesso de peso em crianças que dormiam menos de 9h (23%), quando comparadas às que dormiam mais de 10h (16%, p=0,06). O genótipo não se correlacionou significativamente a nenhum dos desfechos avaliados.
ConclusõesO polimorfismo CLOCK 3111 T/C não está significativamente associado ao excesso de peso ou à duração do sono em crianças desta localidade.
Childhood obesity is a serious public health problem, and represents a worldwide epidemic. The fact that it is an epidemic is of concern not only because obese children are more likely to become obese adults, but also because of its strong association with high morbidity and mortality events, such as cardiovascular disease, diabetes, and cancer.1 Considering this and the inefficiency of the traditional methods used to fight obesity, new scientific approaches aimed at understanding the mechanisms involved in this epidemic are of paramount importance so that innovative preventive and therapeutic measures can be implemented.
Currently, it is well established that most cases of obesity are of multifactorial origin, where multiple genetic variations, with varying frequency in different populations, modulate the magnitude with which behavioral and environmental factors influence the weight of individuals.2 Although the full etiopathological understanding is hindered by gene‐gene and gene‐environment interactions, efforts have been made to unravel this complex web of influences and gradually make it understood.3
Among the environmental factors related to obesity, great importance has been attributed to changes in eating patterns and physical activity that have occurred with modern lifestyle. However, other changes in behavior generated by current lifestyle may be associated with disease, including sleep patterns, identified as an important variable especially in children.4–6 In 2008, a meta‐analysis demonstrated that children with shorter sleep duration have an increase of up to 92% in the risk of obesity. In children younger than 10 years, there was a clear dose‐response association between sleep duration and weight gain, and for each additional hour of sleep, the risk of overweight was reduced on average by 9%.4
It is possible that part of the observed association between sleep and obesity is attributable to interaction effects of environmental factors, such as exposure to light and food intake, as well as the function of the so‐called biological clocks, either central or peripheral, which consist of cells with finely regulated oscillatory gene expression that act as “pacemakers”, dictating the timing of hormones, neurotransmitters, and metabolic, autonomic, and behavioral activities. Experimental studies demonstrate that increasing the duration of exposure to light interferes with the lipogenic activity mediated by lipoprotein lipase, suggesting a central role of the biological clock in determining body weight. Conversely, endogenous changes of the oscillatory rhythm can influence both eating behavior and the pattern of energy expenditure and fat deposition in adipose tissue.7–9
Alterations in genes that regulate the circadian rhythm have been associated with changes in metabolic homeostasis. Among them, the authors highlight the CLOCK (Circadian Locomotor Output Cycles Kaput, OMIM * 601851) gene, the first gene that regulates the biological rhythm identified in mammals.10 Knockout models for this gene exhibit a phenotype of hyperphagia, obesity, hyperlipidemia, hepatic steatosis, and hyperglycemia, which very much resembles the picture of metabolic syndrome observed in humans.11 Furthermore, other studies have demonstrated its action on the regulation of metabolic processes, such as insulin and leptin secretion and action.9,12,13
Recently, CLOCK gene polymorphisms have been associated with the occurrence of obesity in adults, the concentrations of adiponectin produced by fat tissue, the pattern of caloric intake, and sleep‐related cytokines.14–18 An interesting study involving obese adults observed an association between CLOCK 3111T/C (rs 1801260) polymorphism and the capacity to lose weight during obesity treatment, demonstrating that patients with at least one C allele showed greater resistance to weight loss than individuals that were homozygous for the T allele, as well as shorter sleep duration, higher serum ghrelin levels, and changes in eating behavior, including nocturnal feeding.15 Moreover, other studies have shown significant associations between this polymorphism and the occurrence of disorders related to appetite, weight, mood, and attention.18,19
To date, no study of polymorphisms in this gene has been developed for children. Thus, the primary objective of this study was to evaluate the presence of an association between genotype 3111T/C of the CLOCK gene and the presence of overweight, as well as the pattern of fat distribution in schoolchildren of this city. Furthermore, this sample was also evaluated for the presence of an association between this genetic variant and sleep duration, as well as sleep duration and nutritional status.
MethodsSampleThe study, which was approved by the University Research Ethics Committee, was developed with a cross‐sectional design, involving children aged 6‐13 years enrolled in public elementary schools (UMEF) of the city in the year 2012.
Sample size calculation was performed using the GPower® software, release 3.1.6 (Kiel University, Germany), using as parameters a probabilistic error of 0.05, effect size of 0.5, and 80% statistical power, so that the minimum sample was determined as a total of 144 children. Children were recruited from five public schools in the city, randomly selected to represent each of the five political‐geographic regions of the municipality. In each school, student selection occurred in a systematic way, according to their enrollment and consent of the parents or guardians, as several attempts at randomization were abandoned due to the difficulty in obtaining written consent from parents. Aiming to increase the estimated power for the test, the number of patients was increased to 370 children.
Data collectionThe children's parents or caregivers completed questionnaires where data regarding regular physical activity (defined as activity with a frequency of at least one hour twice a week), sleep duration, and mean daily time spent with television, computer, and video games were collected. The children were assessed for height, weight, and waist, hip and neck circumference measurements. All measurements were performed by the main researcher and/or a team of professionals trained in anthropometric measurement technique.
Body weight was measured in an Avanutri® (Avanutri Informática Ltda, Rio de Janeiro, Brazil) digital scale, graded from 0 to 150kg, with a resolution of 0.05kg. Children were weighed without shoes or socks, wearing school uniforms. The scale was placed on a rigid surface and the students were weighed in the standing position, with the limbs stretched along the body, positioned on the center of scale, looking forward.
Height was measured using an Avanutri® (Avanutri Informática Ltda, Rio de Janeiro, Brazil) stadiometer, graded from 20 to 200cm, with scale accuracy of 0.1cm, and was represented by the mean of three consecutive measures, in order to minimize measurement error. Children were asked to remain in the orthostatic position without shoes, with hips and shoulders perpendicular to the central body axis, heels firmly planted on the floor, knees close and extended, relaxed arms held close to the body, and head in the Frankfurt plane.
Body mass index (BMI) was calculated by the formula weight/height2 and adjusted for the child's age and gender according to the percentile distributions and cutoffs proposed by the Centers for Disease Control (CDC), which considers overweight as a BMI ≥ 85th percentile and<95th percentile, and obesity as a BMI at or above the 95th percentile.
Body fat distribution was assessed through the measurement of circumferences performed in duplicate for control of measurement errors or reading. The measurement of waist circumference was performed according to the standard recommended by the I Brazilian Guideline for Metabolic Syndrome (I Diretriz Brasileira de Síndrome Metabólica ‐ I‐DBSM), with an inelastic tape placed at mid‐distance between the iliac crest and the lower rib cage rim at the end of expiration. Hip measurement was performed in the horizontal plane, at the level of the greatest circumference of the buttocks, with the individual in standing position with feet placed together. Neck circumference was assessed using as reference a horizontal line at the level of half of the thyroid cartilage, with the neck in neutral position.
Molecular studiesThe collection of material for genetic studies was performed with the use of oral mucosal swabs or soft brush cytology, to provide the least possible discomfort to patients. DNA extraction was performed using two methods. Of the 370 samples, 112 had genetic material extracted by means of FTA cards (FTA Elute Microcard, Whatman International Ltd., United Kingdom), a highly practical technology, often used in forensic genetics, wherein the chemical treatment of the card lyses the cells and leaves DNA intact through simple elution in water.20
The remaining 258 DNA samples were obtained through the use of cytological brushes, whose genetic material extraction was performed using the salting‐out method, traditionally described and used in the Endocrinology Genetics Service ‐ Molecular Research Laboratory (LIM)‐25, Faculdade de Medicina, Universidade de São Paulo (FMUSP).21 All genotyping was performed in LIM‐25‐FMUSP, using the TaqMan methodology (Real Time TaqMan® SNP (single nucleotide polymorphisms) Genotyping Assay C‐8746719‐20, Applied Biosystems, Carlsbad, United States), using the equipment for Real Time Applied Biosystems, model StepOnePlus (Applied Biosystems, Carlsbad, United States).
Statistical analysesAll data collected were stored in electronic spreadsheets. Measures of height, weight and BMI were converted into Z‐scores (adjusted for age and gender) according to international reference parameters, using the Growth Analyser software, release 3.5 (Rotterdam, Netherlands). After genotyping, the presence of a distribution compatible with the Hardy‐Weinberg equilibrium was assessed.
Association analyses between variables were performed by comparing groups, correlations, and linear regressions. The groups of children with and without excess weight (overweight or obesity) were compared regarding genetic and nongenetic variables. The genotype groups were compared by both the codominant (CC vs. TC vs. TT) and the dominant model (C* vs. TC). Quantitative variables were analyzed using the Mann‐Whitney test or Student's t‐test as appropriate. Qualitative variables were analyzed using the chi‐squared test. All statistical analyses were performed using SigmaStat® for Windows® (release 3.5 ‐ SPSS Inc., San Rafael, United States) and statistical significance was set at p<0.05.
ResultsA total of 370 children were studied, of whom 167 were boys (45%) and 203 were girls (55%). There was a prevalence of 10% overweight (n=38) and 8% obesity (n=30). There was no statistically significant difference in the prevalence of excess weight between females and males (p=0.6).
The practice of regular physical activity was observed in only 27% of children, with no significant difference regarding gender or age. The number of hours devoted to watching television and playing computer or video games was also verified. There was no significant association between these parameters and the presence or absence of obesity in these children. These data are summarized in Table 1.
Comparison between children with and without overweight in relation to non‐genetic variables.
| With overweight | Without overweight | p | |
|---|---|---|---|
| n | 68 | 302 | – |
| Gender (F:M) | 36:32 | 167:135 | 0.83 |
| Age (years) | 8.4 (7.2–9.4) | 8.4 (7.3–9.5) | 0.77 |
| Regular physical activity (yes:no) | 15:40 | 72:185 | 0.96 |
| Hours spent at TV, computer, or videogames | 3.0 (2.0–5.0) | 3.0 (2.0–4.0) | 0.95 |
| Time of waking up (h)a | 6.6 (6.0–8.5) | 7.0 (6.0–8.5) | 0.72 |
| Time of going to sleep (h)a | 22 (21.2–22.5) | 22 (21.0–22.5) | 0.23 |
| Sleep duration (h)a | 9.0 (8.5–10.5) | 10.0 (9.0–10.5) | 0.12 |
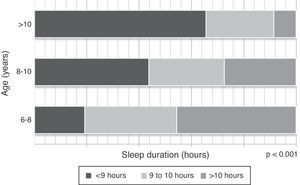
The children slept an average of 9.7hours. An inverse association was observed between sleep duration and the child's age (r=‐0.4, p<0.001). While 47% of children aged between 6 and 8 years slept more than ten hours a day, only 9% of children older than 10 years had this sleep duration profile (p<0.001) (Fig. 1). No significant association was observed between sleep duration and other variables, such as gender or regular physical activity.
Regarding the association between sleep duration and obesity, there was a tendency for a higher prevalence of excess weight among children who slept less than nine hours (23%), when compared with children who slept more than 10hours (16%, p=0.06). Moreover, there was a significant association between sleep duration and the circumferences that represent central fat distribution: neck circumference (r=‐0.1; p=0.01) and waist circumference (r=‐0.2; p<0.001). However, these associations were not independent from the association between sleep duration and age, when analyzed by multiple linear regressions.
The genotype distribution regarding the 3111 T/C polymorphism of the CLOCK gene was 4% of children homozygous for the C allele (n=13), 31% heterozygous (n=106), and 65% homozygous for the T allele (n=221); which is consistent with the Hardy‐Weinberg equilibrium (P=0.98).
Although both the prevalence of excess weight (31%) and the median BMI Z‐score were greater in individuals homozygous for the C allele, this difference did not show statistical significance. Likewise, no significant difference was observed regarding sleep duration in children classified in the different genotype groups (Table 2).
Comparison of genotypic groups of 3111T/C polymorphism of the CLOCK gene in relation to overweight and sleep duration.
| CC | CT | TT | p | |
|---|---|---|---|---|
| n | 13 | 106 | 221 | |
| Gender (F:M) | 6:7 | 53:53 | 130:91 | 0.25 |
| Age (years) | 8.5 (7.4–9.9) | 8.4 (7.3–9.5) | 8.2 (7.3–9.4) | 0.70 |
| Time of going to sleepa | 22 (21.1–22.7) | 22 (21–22) | 22 (21–22.5) | 0.43 |
| Time of waking upa | 6.5 (6.0–8.0) | 7.0 (6.0–8.0) | 6.6 (6.0–9.0) | 0.75 |
| Sleep durationa | 9.0 (8.1–10.0) | 10.0 (9.0–10.5) | 9.9 (8.6–10.5) | 0.21 |
| BMI Z‐scorea | 0.6 (−0.1–1.5) | 0.1 (−0.7–1.0) | 0.2 (−0.6–1.5) | 0.43 |
| Overweight (%) | 31% | 13% | 20% | 0.66 |
Sleep duration has been decreasing in parallel with the increased incidence of obesity. Considering that sleep is a modifiable environmental factor that can be adopted as a preventive measure against obesity, several studies that focused on the impact on health determined by behavioral and/or molecular changes in the sleep‐wake cycle have been performed in the last decade.4,7 In Brazil, this is the first study that attempts to assess the association between sleep duration and obesity in children, correlating it to a genetic variation involved in the biological rhythm control of several hormonal and metabolic variables.22
One of the main genes involved in the modulation of circadian rhythm is the CLOCK gene, a transcription factor expressed in different tissues that has been implicated in the regulation of metabolic processes such as insulin secretion,23 hypothalamic action of leptin,13 nutrient absorption,24 and sensitivity to glucocorticoids.25 The polymorphism 3111T/C, located in the 3′‐untranslated gene region, has been associated with feeding behavior control, hormone secretion, mood, and sleep; therefore, it was selected as a candidate SNP for the study of the molecular association between sleep duration and obesity in children.16,18
Previous observations attributed a phenotype of overweight and short sleep duration to genotype C*. An interesting study involving 1,290 obese individuals of both genders, aged 20 to 69 years, observed that patients with at least one C allele had shorter sleep duration when compared with individuals homozygous for the T allele, as well as weight loss resistance, higher serum levels of ghrelin, and nocturnal feeding habits.15
In the present sample, a higher prevalence of overweight and shorter duration of sleep was observed in individuals homozygous for the C allele; although consistent with previous results, this difference was not statistically significant. A negative result for this association has been described by other studies.18,26
One possible explanation for the divergent results is the statistical power limitation of this sample, as the study had limitations in terms of cost and logistics for expanding sample size. Another possibility is that the association reported in previous studies is not directly related to a causal effect of this polymorphism. Although the functional role of the polymorphism on the mRNA stability has been demonstrated,27 the possibility of linkage disequilibrium with another truly functional polymorphism cannot be discarded, as the degree of linkage may vary depending on the genetic profile of each population.
Only one study involving this polymorphism in a Brazilian sample was retrieved in the literature, which showed no significant association between genotype and sleep pattern in adults.26 In this study, in which 162 adults of both genders were genotyped for the 3111 T/C polymorphism of the CLOCK gene, the observed genotypic frequencies (7% CC, 40% CT, and 53% TT) were similar to those in the present study, which are also compatible with the classically described frequencies in the dbSNP database (rs 1801260).
Regarding the epidemiological association between sleep duration and excess weight in children, this association was not consistently observed in the present sample, either. Although the method of measuring sleep duration used in the study, performed through questionnaires distributed to the parents, is subject to information bias, this is the method traditionally used in studies that demonstrated these associations,4 and thus it cannot be affirmed that it has adversely affected the present study.
As the association showed borderline significance (p=0.06) in the categorical analyses, statistical power limitation to detect this association cannot be ruled out. Moreover, this was not the first study to show a negative result with regard to this association.28 Despite the many positive studies and the biological plausibility for this association,29 the cross‐sectional nature and methodological limitations of most studies in this area have led some authors to suggest caution when determining the nature of causality and the direction of this association.30
In conclusion, in this sample of children from the city of Vila Velha, ES, Brazil, no significant association was observed between the SNP 3111T/C of the CLOCK gene, the presence of obesity, and sleep duration. However, it is important to perform further studies with larger statistical power to clarify the role of this polymorphism in the population.
Furthermore, it is important to investigate other molecular circadian rhythm regulators that might possibly be involved in the modulation of the obesity susceptibility profile. The identification of risk genotypes related to “clock‐genes” is a new area of research on the etiology and pathogenesis of obesity, which broadens the knowledge on the subject and provides a new method for its control, allowing individualized therapies to help treat this complex and impactful disease in the future.
FundingConcessão de bolsa de mestrado ao primeiro autor pela Fundação de amparo à pesquisa do estado do Espírito Santo (FAPES). Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) 304678/2012‐0.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Giovaninni NP, Fuly JT, Moraes LI, Coutinho TN, Trarbach EB, Jorge AA, et al. Study of the association between 3111T/C polymorphism of the CLOCK gene and the presence of overweight in schoolchildren. J Pediatr (Rio J). 2014;90:500–5.
Study conducted at the Universidade Vila Velha (UVV), Vila Velha, ES, Brazil.