To explore the effect of erythromycin on hyperoxia-induced lung injury.
MethodsOne-day-old preterm offspring Sprague-Dawley (SD) rats were randomly divided into four groups: group 1, air + sodium chloride; group 2, air + erythromycin;group 3, hyperoxia + sodium chloride; and group 4, hyperoxia + erythromycin. At one, seven, and 14 days of exposure, glutathione (GSH) and interleukin-1 beta (IL-1 beta) were detected by double-antibody sandwich enzyme-linked immunosorbent assay (ELISA), and bicinchoninic acid (BCA) was used to detect GSH protein. γ-glutamine-cysteine synthetase (γ-GCS) mRNA was detected by reverse transcription-polymerase chain reaction (RT-PCR).
ResultsCompared with group 1, expressions of GSH and γ-GCS mRNA in group 3 were significantly increased at one and seven days of exposure (p < 0.05), but expression of γ-GCS mRNA was significantly reduced at 14 days; expression of IL-1 beta in group 3 was significantly increased at seven days of exposure (p < 0.05), and was significantly reduced at 14 days. Compared with group 3, expressions of GSH and γ-GCS mRNA in group 4 were significantly increased at one, seven, and 14 days of exposure (p < 0.05), but expressions of GSH showed a downward trend at 14 days; expression of IL-1 beta in group 4 was significantly reduced at one and seven days of exposure (p < 0.05).
ConclusionsChanges in oxidant-mediated IL-1 beta and GSH are involved in the development of hyperoxia-induced lung injury. Erythromycin may up-regulate the activity of γ-GCS, increasing the expression of GSH, inhibiting the levels of oxidant-mediated IL-1 beta and alleviating hyperoxia-induced lung injury via an antioxidant effect.
Explorar o efeito da eritromicina sobre lesões pulmonares induzidas por hiperóxia.
MétodosUma prole de ratos Sprague-Dawley (SD) prematuros com um dia de vida foi dividida aleatoriamente em quatro grupos: grupo 1 ar + cloreto de sódio, grupo 2 ar + eritromicina, grupo 3 hiperóxia + cloreto de sódio e grupo 4 hiperóxia + eritromicina. Com um, sete e 14 dias de exposição, foram detectadas Glutationa (GSH) e Interleucina-1 beta (IL-1 beta) pelo ensaio imunossorvente ligado à enzima (ELISA), e o ácido bicinconinico (BCA) foi utilizado para detectar a proteína GSH. O mRNA da γ-glutamil-cisteina-sintetase (γ-GCS) foi detectado por reação em cadeia da polimerase via transcriptase reversa (RT-PCR).
ResultadosComparadas ao grupo 1, as expressões do mRNA da GSH e da γ-GCS no grupo 3 aumentaram significativamente com um e sete dias de exposição (p < 0,05), porém a expressão de mRNA da γ-GCS diminuiu significativamente aos 14 dias; a expressão de IL-1 beta no grupo 3 aumentou significativamente aos 7 dias de exposição (p < 0,05) e diminuiu significativamente aos 14 dias. Comparadas ao grupo 3, as expressões do mRNA da GSH e da γ-GCS no grupo 4 aumentaram significativamente com um, sete e 14 dias de exposição (p < 0,05), porém as expressões de GSH mostraram uma tendência de queda aos 14 dias; a expressão de IL-1 beta no grupo 4 foi reduzida significativamente com um e sete dias de exposição (p < 0,05).
ConclusõesAs variações de IL-1 beta e GSH mediadas por oxidantes estão envolvidas no desenvolvimento de lesão pulmonar induzida por hiperóxia. A eritromicina poderá regular positivamente a atividade da γ-GCS, aumentando a expressão de GSH, inibindo os níveis de interleucina-1beta mediada por oxidante e aliviando a lesão pulmonar induzida por hiperóxia por meio de um efeito antioxidante.
With the rapid development of maternal health technology and perinatology, the survival rate of premature infants is increasing, especially in very low birth weight infants (VLBWI).1 However, the lungs of premature infants are often immature and in direct contact with oxygen, and they are one of the most sensitive organs to oxygen toxicity. Moreover, premature infants need to receive various oxygen therapies for a long time after birth. Unfortunately, this undoubtedly aggravates oxidative stress in the immature lungs of premature infants, which may lead to acute and chronic lung injury.2
Hyperoxia-induced lung injury is a major cause of chronic respiratory disease from infancy to adulthood, and has become one of the most difficult problems in the neonatal intensive care unit. However, its etiology and pathogenesis are not fully understood.3 Nowadays, most researchers believe that immature lung tissue directly exposed to the hyperoxic environment results in oxidative stress, which has a crucial role in the development of hyperoxia-induced lung injury.4,5 Oxidative stress can disturb the oxidant/antioxidant balance, and is one of the primary pathogenic factors.6 Glutathione (GSH) is an important intracellular antioxidant and has a key role in maintaining integrity and preventing oxidative damage in alveolar epithelial cells.7 γ-glutamine-cysteine synthetase (γ-GCS) is the rate-limiting enzyme of GSH protein synthesis, and regulates intracellular levels of GSH.8 IL-1 beta is present in the early phase of bronchopulmonary dysplasia (BPD) in premature infants, and may have an important role in the development of BPD. However, the exact pathogenesis of BPD remains unclear, and clinically effective treatments remain limited.
The non-antibacterial effect of erythromycin has gradually attracted the attention of several researchers.9 It exhibits many important physiological functions, including: effective antibacterial activity, non-specific anti-inflammatory effects in asthma, immune regulation, induced chemical adhesion, promoted gastrointestinal motility, and an anti-tumor effect.10 Erythromycin effectively treats many non-bacterial, infective chronic inflammatory diseases, some of which show imbalanced redox reactions.11 However, it remains unclear how the expression levels of GSH, γ-GCS, and IL-1 beta are affected in hyperoxia-exposed lung tissue. In the present study, the authors explored the effect of erythromycin on hyperoxia-induced lung injury in premature rats and examined the expression levels of GSH, γ-GCS, and IL-1 beta in premature rat pulmonary tissues.
Materials and methodsExperimental animal models12 and groupingAdult Sprague-Dawley (SD) rats (weighing 200-250g, and including 100 females and 35 males) were provided by the Experimental Animal Center of the Chinese Minority Ethnic Groups Traditional Medicine Research Center of the Central University for Nationalities, Beijing, China. The first day of pregnancy was recorded when sperm was detected in the vaginal sections of female rats by microscopic examination. On day 21 of gestation (term = 22 days) fetuses were delivered by hysterectomy. The one-day-old preterm SD rats were randomly divided into four groups (eight pups in each group): group 1 received air (21% O2) + sodium chloride; group 2, air + erythromycin; group 3, hyperoxia + sodium chloride; and group 4, hyperoxia + erythromycin. Rats in the air groups were exposed to room air, whereas those in the hyperoxia groups were exposed to O2 concentrations > 85% and CO2 < 0.5%. Temperatures were kept at 25-26°C and humidity at 60-70%, and the oxygen and CO2 levels in the chamber were monitored continuously with gas analyzers.12 The caudal vein of the preterm rats was injected with sodium chloride (0.15mL/kg) in the sodium chloride groups, and erythromycin (50mg/kg) in the erythromycin groups. At one, seven, and 14 days of exposure, eight pups from each group were anesthetized and euthanized. Protein was extracted from the left lung, and the right lung was frozen and stored at –70° C in a refrigerator for RT-PCR.
The study was approved by the experimental animal welfare management and ethics committee of Shanghai Children's Hospital, Shanghai Jiao Tong University, Shanghai, China.
Detection of GSH and IL-1 beta in pulmonary tissue homogenates by ELISALung tissues were collected, and total proteins were extracted using a protein extraction kit. Protein concentration was measured using the Bradford method (Bio-Rad - California, USA). GSH and IL-1 beta in pulmonary tissue homogenates were detected by ELISA kits obtained from Nanjing Jiancheng Biological Technology Co. Ltd., Nanjing, China and Wuhan Huamei Cusabio Biological Technology Co. Ltd., Wuhan, China, respectively.
All reagents were allowed to reach room temperature. The required number of strips were arranged and labeled. 100-μL of reagents were added to wells of polystyrene ELISA plates, and the wells were thoroughly washed with phosphate buffered saline (PBS) containing 0.1% Tween-20 (PBS-Tween) (Bio-Rad Laboratories, CA, EUA) after each incubation step. All reagents were prepared, including working standards and samples. 100 uL of standards, controls, or samples were added to the wells and were incubated for two hours at 37°C. After the wells were washed, 100 uL of goat anti-mouse GSH (or IL-1 beta) polyclonal antibody was added to each well (incubation, 37°C, 30min). After extensive wash, 100 uL of rabbit anti-goat immunoglobulin G (IgG) was added to each well for one hour at 37° C. After substrate solution and stop solution incubation, the optical density of each well was read within 30minutes, using a microplate reader set to 450nm.
Detecting of GSH protein concentrations in pulmonary tissue homogenates through bicinchoninic acid (BCA)Following the standard protocol for the Micro BCA Protein Assay Kit (Beijing Baitaike Biological Technology Co., Beijing, China), the working solution consisted of 1 volume reagent C mixed with 25 volumes of reagent B; then, 26 volumes of reagent A were added to the C/B mixture. The pH value of the working solution was 11.16 ± 0.06, measured with an Orion 310 (Thermo Scientific, MA, EUA) pH meter. Completely dissolved protein standard (5mg/ml), 10μL diluted to 100μL, so that the final concentration was 0.5mg/ml, would be diluted standards according to 0,1,2,4,8,12,16,20μL respectively to 96-well plate, and ultra pure water would all standard up to 20μL, and 10μL samples to 96-well plate, plus ultra pure water release liquid to 20μL, the hole added with 200μL BCA the working solution, gently tap the plate to ensure thorough mixing with a sample adding gun, cooling the samples to room temperature from 37°C for 30-60min. Each measurement was performed in duplicate. All the absorbances were corrected by the corresponding blank replicate. The absorbance of the blank solution was 0.048 ± 0.006. Absorbance at 562nm was measured by spectrophotometer using glass cuvettes with optical path length of 0.1cm.
Expression of γ-GCS mRNA detected by RT-PCRTotal RNA was extracted using the RNAgent Total RNA Isolation System (Promega Corporation, WI, EUA) according to the manufacturer's instructions. The purity and yield of total RNA were determined spectrophotometrically by measuring the absorbance of an aliquot at 260nm and 280nm. RNA (4μg) was reverse-transcribed into 50μL of complementary DNA (cDNA) using the M-MLV Reverse Transcriptase system (Jingmei Biotech Ltd, Shenzhen, China). The primer sequences were designed by Shanghai Biology Engineering Co., China, in accordance with the literature: γ-GCS, forward: 5′-TTGGCAGCCTT CCTGATTTC-3′, reverse: 5′-AACTTCTCCACAACCCTCTG-3′, product size 78bp; β-actin, forward: 5′-AAC GCAGCTCAGTAACAGTC-3′, reverse: 5′-ATCCGT AAA AGCCTCTATGC -3′, product size 280bp. γ-GCS and β-actin PCR reaction mixtures were subjected to incubation for 5min at 94°C, followed by 35 cycles of 94° C for 45 s, 50° C for one min, and 72° C for 30 s. A final extension was carried out at 72° C for ten min. PCR products were separated by electrophoresis on 2% agarose gels, stained with ethidium bromide (0.5μg/mL), and observed using a UV transilluminator and evaluated using a GDS-8000 gel image system (UVP Co., Cambridge, United Kingdom) by comparing the intensity of target product bands with that of β-actin used as the internal standard.
Statistical AnalysisData were analyzed using the statistical software package SPSS, version 16.0 (IBM, Armonk, NY, USA). All data were presented as means ± standard deviation. Statistical differences between the groups were tested by ANOVA, and data between two groups were analyzed using the q-test. A p-value less than 0.05 was considered to be statistically significant.
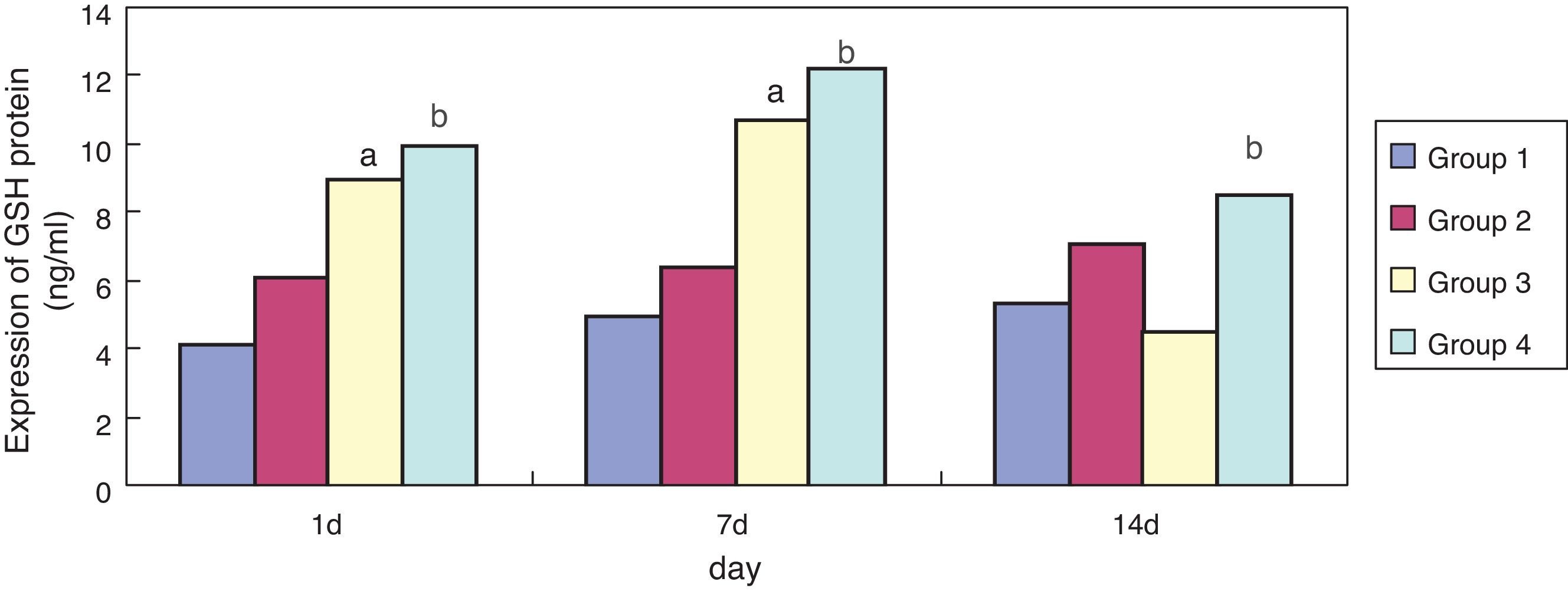
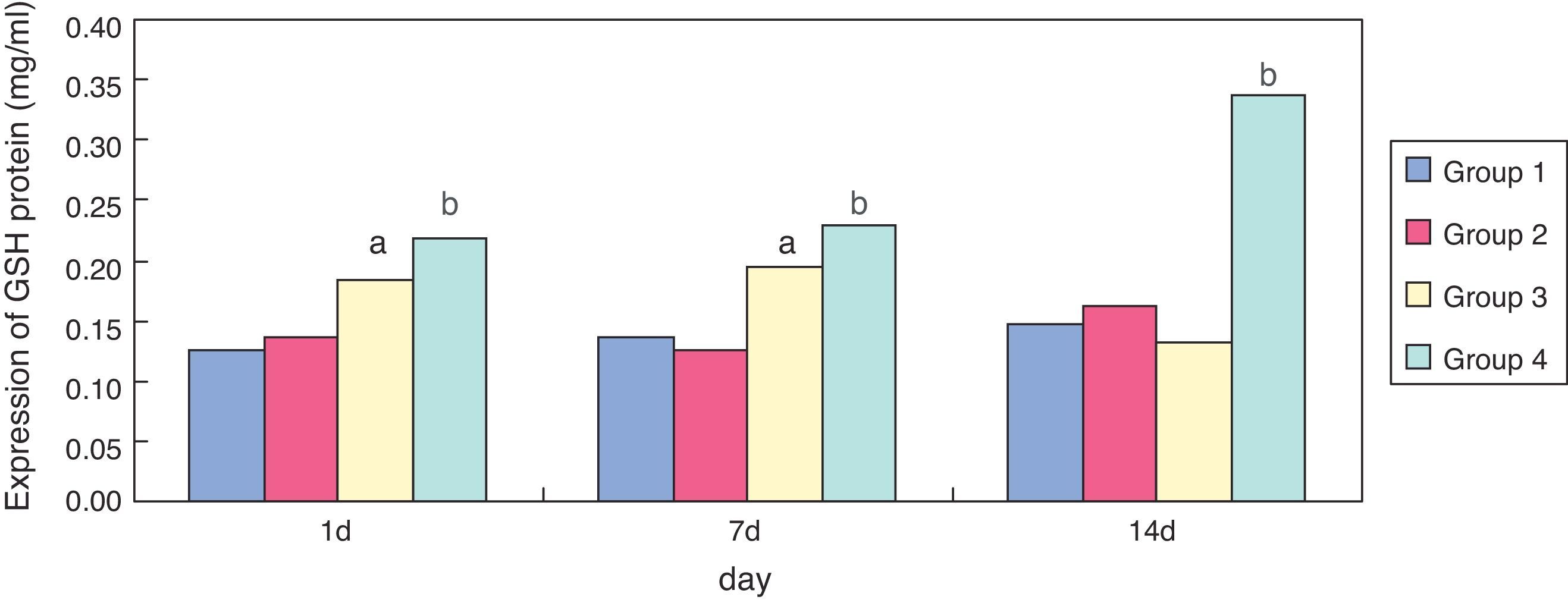
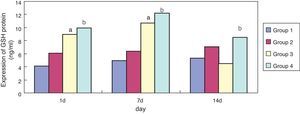
ResultsEffect of erythromycin on GSH in hyperoxia-exposed lung tissueCompared with group 1, expression of GSH in group 3 was significantly increased (p < 0.05) at one and seven days of exposure, but showed no significant reduction (p > 0.05) at 14 days. Compared with group 3, expression of GSH in group 4 was significantly increased at one, seven, and 14 days of exposure (p < 0.05); the general tendency decreased after 14 days (Figs. 1 and 2).
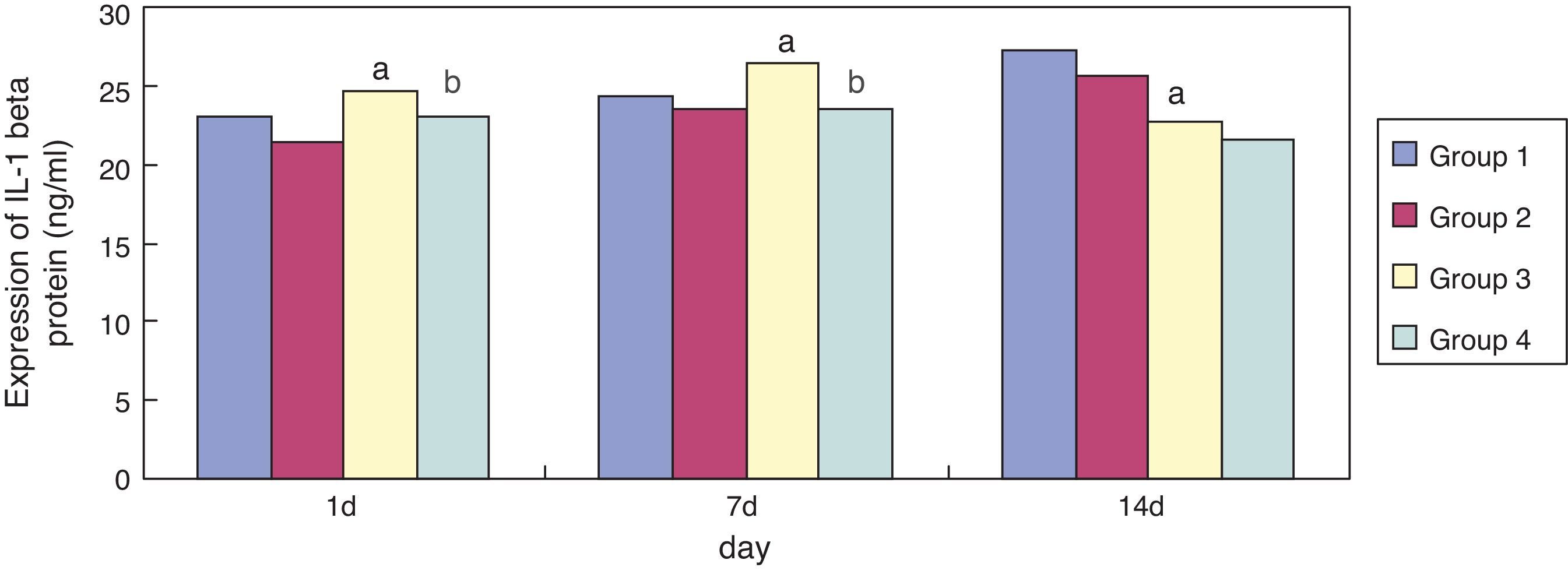
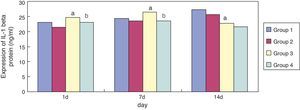
Compared with group 1, expression of IL-1 beta in group 3 was significantly increased (p < 0.05) at seven days of exposure; its expression was significantly reduced (p < 0.05) at 14 days of exposure. Compared with group 3, expression of IL-1 beta in group 4 became significantly reduced at one and seven days of exposure (p < 0.05) (Fig. 3).
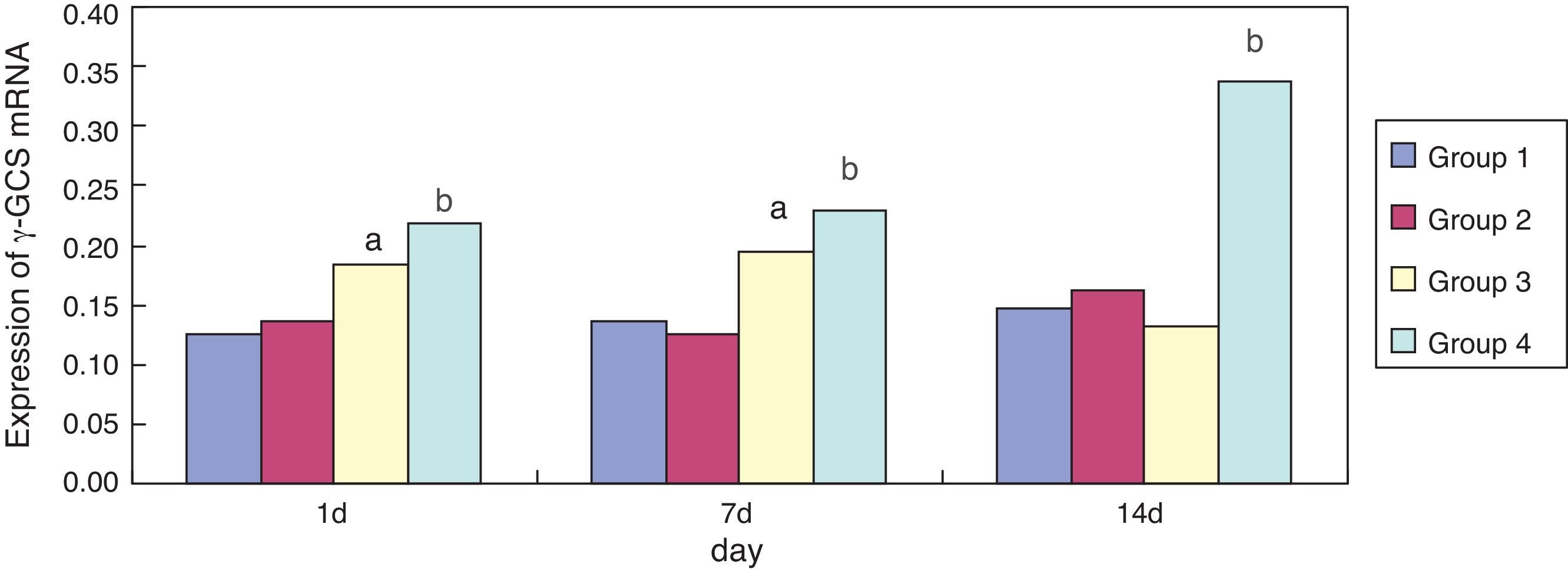
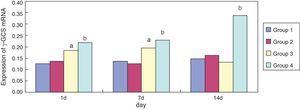
Effect of erythromycin on γ-GCS in hyperoxia-exposed lung tissueCompared with group 1, expression of γ-GCS mRNA in group 3 was significantly increased (p < 0.05) at one and seven days of exposure; its expression was significantly reduced (p < 0.05) at 14 days of exposure. Compared with group 3, expression of γ-GCS mRNA in group 4 was significantly increased at one, seven, and 14 days of exposure (p < 0.05) (Fig. 4). Erythromycin intervention up-regulated the activity of γ-GCS mRNA particularly in the hyperoxia-exposed lung tissues.
DiscussionBased on the histological features, rat fetal lung development can be divided into four periods: the embryonic period (zero to 13 days), gland period (14 to 18 days), canalicular stage (19 to 20 days), and saccular period (21 to 22 days). The saccular period during the development of human lung corresponds to 28 to 34 weeks of gestational age, the age of birth of most preterm neonates. The postnatal rat lung development is divided into three periods: the expansion period (one to four days after birth), alveolar period (four to 13 days after birth), and balanced growth period (14 to 21 days after birth). Thus, the different time points respectively represent the different stages of lung development in preterm infants after birth.
The pathogenesis and prevention of BPD in preterm infants has made significant breakthroughs recently; however, the exact pathogenesis of BPD remains unclear, and effective treatment is still significantly restricted.13 Macrolide antibiotics (MAs) contain the 12-22 carbon chemical structure and belong to the lactone ring carbon antibiotics. Erythromycin A can inhibit the secretion of pro-inflammatory cytokines such as tumor necrosis factor-α and IL-1beta.14 Moreover, it is an extremely broad-spectrum antibiotic, and has antibacterial activity against Gram-positive bacteria and some Gram-negative bacteria, anaerobic bacteria, Legionella, Chlamydia, Mycoplasma, and Rickettsia.15 Long-term clinical practice and in-depth pharmacological studies have showed that MAs not only have antibacterial effects, but also possess non-specific anti-inflammatory, anti-allergic, and immune regulation properties.16 The main role of antibiotics in some chronic pulmonary inflammatory diseases may be related to inhibiting the oxidative burst of neutrophils and the release of inflammatory mediators. In addition, MAs are effective in preventing and treating some respiratory diseases, including asthma, pulmonary fibrosis, diffuse panbronchiolitis, and some non-infectious inflammatory diseases, such as blood diseases, skin diseases, and cancer; these functions have nothing to do with the antibacterial activities.17
Glutathione is a tripeptide-containing sulfonium compound, composed of glycine, glutamic acid, and cystine. γ-GCS is the rate-limiting enzyme of GSH synthesis that regulates intracellular GSH levels.18 GSH is activated by the in vivo oxidation/reduction system, and provides the reductant for cystine, inhibiting the body's production of various substances in the process of oxidation of reactive oxygen species (ROS), inactivating activity of membrane peroxidase and inhibiting ROS, thus reducing ROS. Most researchers have recognized that ROS caused by oxidative stress has an important role in the development of hyperoxia-induced lung injury.19 Several studies with in vivo and in vitro experiments have demonstrated that, as an important antioxidant, GSH played an important role in maintaining the airway epithelial cell integrity and resisting lung injury and inflammation.20
In the present study, compared with the air + sodium chloride group, GSH expression in lung tissues of premature rats was significantly enhanced after erythromycin intervention on day one, seven, and 14 in the erythromycin + sodium chloride group (p < 0.05); its expression was significantly enhanced on day one and seven after exposure to hyperoxia in the hyperoxia + sodium chloride group, and decreased significantly on day 14. GSH expression in the hyperoxia + erythromycin groups was significantly enhanced under the exposure to hyperoxia and erythromycin intervention on day one, seven and 14, but showed a significant downward trend on day 14. GSH expression detecting by BCA confirmed the ELISA results. After exposure to hyperoxia on day one and seven, GSH expression was significantly enhanced. The body may have some mechanism for self-protection and can resist hyperoxic injury. As intracellular ROS increases, the sulfur groups of cysteine in GSH have a strong affinity activity, and can be used as electrophilic targets that combine with ROS. They also have a role in eliminating ROS and lipid peroxidation, thus avoiding alveolar cell membrane damage. However, exposure to hyperoxia caused GSH protein in alveolar epithelial cells to be severely damaged by oxidative stress on day 14, and GSH expression showed no significant reduction.
γ-glutamine-cysteine synthetase is the rate-limiting enzyme of GSH protein synthesis, which regulates intracellular levels of GSH.21 The present study demonstrated that the intervention of erythromycin can inhibit up-regulation of GCS protein levels in lung tissues by hyperoxia exposure on day one and seven (p < 0.05); the intervention of erythromycin had no obvious influence on hyperoxia exposure on day 14, but γ-GCS mRNA expression was significantly enhanced on day seven and 14 (p < 0.05), which may be related to relevant regulatory proteins after γ-GCS mRNA transcription because of hyperoxia exposure damage, resulting in erythromycin inhibiting the up-regulation of GCS protein levels by hyperoxia exposure.
Infection and inflammatory reactions are key factors in the pathogenesis of BPD in preterm infants, which has been confirmed by animal and clinical studies.22 It has been reported that IL-1 beta as a proinfammatory cytokine has a central position in the pathogenesis of BPD and has an important pathogenic role in acute and chronic lung injury in preterm infants.23 In the present study, it was found found that, compared to the air groups, the expression of IL-1 beta in the lung tissue of premature rats of hyperoxia groups was significantly increased on day seven and reduced on day 14. Moreover, compared with the sodium chloride groups, the expression of IL-1 beta was significantly reduced in the erythromycin groups on day one and seven. By contrast, the expression of GSH in the lung tissues was enhanced after the intervention of erythromycin on day one, seven, and 14. These results demonstrated that the primary role of erythromycin may be related to inhibiting the oxidative burst of neutrophils and the release of inflammatory mediators. Thus, one of the main mechanisms of MAs in treating BPD in the preterm infants may be the inhibition of neutrophil oxidative outbreak and the release of inflammatory mediators.
In summary, erythromycin can inhibit the oxidative outbreak of neutral granulocytes in lung tissue, improve the antioxidant role of GSH, inhibit the release of the inflammatory cytokine IL-1 beta, and thus has an important role in reducing oxidative stress in the development of hyperoxia-induced lung injury, which may provide a new theoretical basis for the clinical treatment of hyperoxia-induced lung injury.
FundingThis work was supported by funding from the Shanghai Science and Technology Committee (Project Number: 134119a0500).
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Cai C, Qiu G, Gong X, Chen Y, Zhao H. Effects of erythromycin on γ-glutamyl cysteine synthetase and interleukin-1β in hyperoxia-exposed lung tissue of premature newborn rats. J Pediatr (Rio J). 2014;90:493–9.