To guide the diagnostic and therapeutic management of severe forms of food allergy.
Data sourcesSearch in the Medline database using the terms “severe food allergy,” “anaphylaxis and food allergy,” “generalized urticaria and food allergy,” and “food protein-induced enterocolitis syndrome” in the last ten years, searching in the title, abstract, or keyword fields.
Summary of dataFood allergy can be serious and life-threatening. Milk, eggs, peanuts, nuts, walnuts, wheat, sesame seeds, shrimp, fish, and fruit can precipitate allergic emergencies. The severity of reactions will depend on associated cofactors such as age, drug use at the onset of the reaction, history and persistence of asthma and/or severe allergic rhinitis, history of previous anaphylaxis, exercise, and associated diseases. For generalized urticaria and anaphylaxis, intramuscular epinephrine is the first and fundamental treatment line. For the treatment in acute phase of food-induced enterocolitis syndrome in the emergency setting, prompt hydroelectrolytic replacement, administration of methylprednisolone and ondansetron IV are necessary. It is important to recommend to the patient with food allergy to maintain the exclusion diet, seek specialized follow-up and, in those who have anaphylaxis, to emphasize the need to carry epinephrine.
ConclusionSevere food allergy may occur in the form of anaphylaxis and food-protein-induced enterocolitis syndrome, which are increasingly observed in the pediatric emergency room; hence, pediatricians must be alert so they can provide the immediate diagnosis and treatment.
Abordar o manejo diagnóstico e terapêutico das formas graves de alergia alimentar.
Fontes dos dadosBusca ativa na base de dados Medline dos te rmos “severe food allergies”, “anaphylaxis and food allergy” e “food protein-induced enterocolitis” nos últimos dez anos e com busca nos campos título, resumo ou palavra-chave.
Síntese dos dadosA alergia alimentar pode ser grave e ameaçadora à vida. Leite, ovo, amendoim, castanha, noz, trigo, gergelim, crustáceo, peixe e frutas podem precipitar emergências alérgicas. A gravidade das reações vai depender de fatores associados tais como idade, uso de medicamentos no início da reação, persistência de asma e/ou rinite alérgica grave, história de prévia anafilaxia, exercício e doenças intercorrentes. Para anafilaxia, a adrenalina intramuscular é uma indicação bem estabelecida. Para o tratamento da síndrome da enterocolite induzida pela proteína alimentar na fase aguda no setor de emergência, faz-se necessária a pronta reposição hidroeletrolítica, a administração de metilprednisolona e odansetrona IV. Importante recomendar ao paciente com o diagnóstico de alergia alimentar grave que mantenha a dieta de exclusão, procure acompanhamento especializado e, naqueles que apresentaram anafilaxia, enfatizar a necessidade de portar adrenalina.
ConclusãoAlergia alimentar grave pode se manifestar como anafilaxia ou síndrome da enterocolite induzida por proteína alimentar em fase aguda as quais, por serem condições cada vez mais presentes e reconhecidas no setor de emergência pediátrica, demandam diagnóstico e tratamento imediatos.
Severe food allergy refers to the abnormal immune response to a certain food in a susceptible host, causing life-threatening clinical syndromes to the latter.1 These reactions are reproducible each time the food is ingested and, most of the time, are dose-independent.1 They comprise food-induced anaphylaxis, which is mediated by IgE and the acute form of the food-protein-induced enterocolitis syndrome (FPIES), thought to be mediated by cells.2
Anaphylactic reactions to eggs and fish have been described since the 16th and 17th centuries. Decades ago, it was a rare event, but a progressive increase in its prevalence has been observed; currently, severe food allergy is the main cause of emergency care due to anaphylaxis.3 Milk, eggs, peanuts, nuts, walnuts, wheat, sesame seeds, crustaceans, fish, and fruit are some of the foods that can precipitate allergic emergencies.
In Brazil, a survey aimed at allergists indicated food allergy as the second cause of anaphylaxis. The main culprits were cow's milk and egg whites in infants and preschoolers, and crustaceans in older children, adolescents, and adults.4 In a meta-analysis of the literature, the estimated incidence of fatality in high-income countries due to food anaphylaxis in children under 19 years of age was 3.25 per million persons/year.5 The early establishment of the correct and immediate diagnosis and treatment by the emergency pediatrician can prevent lethality and effectively save lives.
The objective of this article is to guide the physician in the diagnostic and therapeutic management of severe forms of food allergy, based on an active search in the Medline database using the terms “severe food allergies,” “anaphylaxis and food allergy,” and “food protein induced enterocolitis” within the last ten years; the search comprised the title, abstract, and keyword fields. The review and recommendation articles that were useful, according to the authors’ evaluation, were selected for reading in full to support the article scope.
The two food allergy situations that lead the patient to emergency care are food anaphylaxis and FPIES, which are clinical entities with different presentations and management and will be approached sequentially in this article. However, in both approaches, the emphasis will be on the pathophysiology and associated aspects in the diagnosis, emergency treatment, and patient guidance.
Anaphylaxis due to food allergyPhysiopathology and associated aspectsFood anaphylaxis is the severe IgE-mediated reaction to food, in which generalized and life-threatening vasodilation occurs. The release of vasoactive mediators into the bloodstream can lead to vascular collapse, anaphylaxis, and shock. Vasodilation is accompanied by hypotension and hypoperfusion, which can compromise vital organs such as the brain and heart, resulting in ischemia and death. When cardiovascular symptoms, such as hypotension and shock, and neurological symptoms, such as mental confusion, loss of consciousness, and sphincter relaxation are present, the risk of death is high. Epinephrine administration and lower limb elevation, restoring vascular tonus and venous return, are life-saving measures.4
Anaphylaxis occurs with the progressive increase in vascular permeability, in which relatively minor symptoms appear earlier and foretell a potentially fatal condition. It all starts with the exposure to a food allergen, which forms a bivalent binding with the specific IgE that is fixated in the high-affinity receptors of mast cells. Through the ion channels, this binding leads to reticular activation and to the release of mediators such as histamine (the main mediator of erythema and pruritus) and several other potent neo- and pre-formed vasodilators, which cause edema in the superficial and deep dermis and subcutaneous cell tissue, resulting in urticaria and angioedema. In the digestive system, this process causes nausea, vomiting, and diarrhea and, in the respiratory tract, intense coryza, sneezing, coughing, bronchospasm, laryngeal edema, and even apnea.4,6,7
The presence of generalized urticaria and angioedema are often the initial symptoms, indicating that vasodilation and bronchoconstriction are imminent and the patient must be identified and treated urgently to restore the vascular tonus.8,9 The association with asthma increases reaction severity, and response to treatment is much more difficult. It has been observed that up to 75% of the patients with fatal anaphylaxis had concomitant asthma.6,7,9
Sometimes, severe food allergy develops during exercise, in association with a specific food (up to 4h after ingestion), characterizing a food-dependent exercise-induced anaphylaxis. Exercise may promote increased absorption of the inadequately processed allergen and/or promote the degranulation of sensitized basophils and mast cells, or further promote an over-synthesis of arachidonic acid metabolites.10 In this case, anaphylaxis results from the association of food and exercise, while food or exercise, alone, are well tolerated. Wheat is the most common allergen, but other grains, nuts, and other foods have also been implicated.4,10
Food-dependent exercise-induced anaphylaxis may be precipitated by associated factors, such as the consumption of anti-inflammatories, especially aspirin, and the use of alcohol, which is common among adolescents.10,11
The severity of food allergic reactions will depend on the amount of ingested allergen, its stability against digestion, and epithelial permeability.10,11 Associated factors, such as age, drug use at the reaction onset, persistence of severe allergic rhinitis, history of previous anaphylaxis, exercise, and concomitant diseases, must be considered.10,11 The dose and type of food allergen that sensitizes and causes severe food allergy may vary between individuals, and may even vary in the same individual on different occasions. When the food allergen is hidden, it can result in delayed identification of the culprit agent and greater risk for patients.11
Adolescents are at increased risk of fatal anaphylaxis because of greater difficulty in following the exclusion diet. Acute infectious diseases facilitate mast cell degranulation and favor the onset of severe food anaphylaxis in the presence of the specific allergen. Similarly, excessively hot showers and use of fever medication may predispose to more severe reactions, as they alter intestinal permeability.11
In infants, cow's milk protein is the most common precipitating agent of food anaphylaxis, but egg, soybean, and other proteins may also be implicated.1,4 In schoolchildren and adolescents, allergies to crustaceans, fish, peanuts, walnuts, and cashews predominate as potentially life-threatening situations.4 Wheat is the most common precipitant of exercise-dependent food-induced anaphylaxis, through a protein fraction found in gluten, 5-omega gliadin.10
Diagnosis of food anaphylaxisThe diagnosis of food anaphylaxis is relatively easy to attain. It is important to emphasize that, because it is a potentially fatal allergic reaction, it should be treated as a medical emergency with the immediate administration of epinephrine; therefore, the diagnosis should be associated with prompt treatment, so that the results are favorable.6,7 Food anaphylaxis occurs suddenly, within a few minutes or a few hours after food intake, with intense pruritus and generalized erythematous plaques that tend to converge. This picture is often accompanied by lip, eye, or even tongue and uvula angioedema, followed by further involvement of at least one of the following organ systems: respiratory (dyspnea, wheezing/bronchospasm, stridor, hypoxemia), cardiovascular (hypotension, hypotonia, shock), gastrointestinal (nausea, vomiting, abdominal pain), and neurological (mental confusion, lipothymia, loss of consciousness). In up to 20% of cases, anaphylaxis can occur with two or more of these affected organ systems, but without cutaneous involvement, which makes the diagnosis much more difficult.4,6 Anaphylaxis should be mainly differentiated from vaso-vagal syndrome (in this case, the skin is cold, pale, and moist), from an acute crisis of severe asthma, and from post-feeding generalized acute urticaria, which, as previously mentioned, can be considered and managed as anaphylaxis.4,6,8,9,11
Although urticaria and angioedema are common signs of food allergic reactions, especially during anaphylaxis, it is important to note that their absence does not exclude the possibility of severe food allergy. Up to 20% of cases of food anaphylaxis may present without cutaneous symptoms, and the absence of these symptoms may result in late identification and treatment delay, as well as increased lethality.8
In addition to classical food anaphylaxis, it is important to recognize food-dependent exercise-induced anaphylaxis. The initial symptoms are fatigue, heat, redness, pruritus, and urticaria, which can sometimes subside when the patient interrupts the physical activity and rests; other times, when the exercise continues, angioedema, gastrointestinal symptoms, laryngeal edema, bronchospasm, hypotension, and shock might occur.10
When there is doubt regarding the diagnosis of anaphylaxis, the measurement of tryptase levels (collected during or shortly after the event resolution), when available, may be used to subsequently confirm the diagnosis. The measurement of the IgE specific for the food allergen components should be performed later.4
Emergency treatment of food anaphylaxisThe management of anaphylaxis should be performed promptly. The patient should receive oxygen via face mask or catheter and be placed in dorsal decubitus with elevated lower limbs (Trendelenburg position), and epinephrine should be administered intramuscularly in the vastus lateralis. Venous puncture should be performed as soon as possible to maintain blood volume, but always after performing the three fundamental procedures mentioned, without delay. When in doubt, the drug should be promptly administered to prevent fatal anaphylaxis; particularly for food allergies, it is a life-saving drug and reduces the likelihood of hospitalization.7 The action mechanism occurs through the alpha-adrenergic effect that reverses peripheral vasodilation, significantly reduces mucosal edema, upper airway obstruction (laryngeal edema), as well as hypotension and shock, in addition to reducing symptoms of urticaria/angioedema. Its β-adrenergic properties increase myocardial contractility, cardiac output, and coronary flow, and have a potent bronchodilator action.4,6 The intramuscular route is preferable, as it reaches peak concentrations faster than the subcutaneous route, and is ten times safer than the bolus intravenous route; additionally, without any risk of loss of time, the rich vascularization of this muscle allows the medication to be readily absorbed with an immediate effect, even in a state of circulatory insufficiency.4,6,12
Table 1 shows the guidelines for managing food anaphylaxis in the emergency room. Adrenalin should be used at a dose of 0.01mg/kg intramuscularly (IM) up to a maximum dose of 0.3mg/kg in children. If the initial response is insufficient, after 5 to 15min, the dose may be repeated one or more times. It is estimated that up to 20% of treated patients may require a second dose. Late administration may lead to an increased risk of hospitalization, insufficient cardiac perfusion, hypoxic-ischemic encephalopathy, and death. The pharmacological action also includes transient pallor, tremor, anxiety, and palpitations, which, although perceived as an adverse effect, is similar to the physiological reaction of acute stress.7
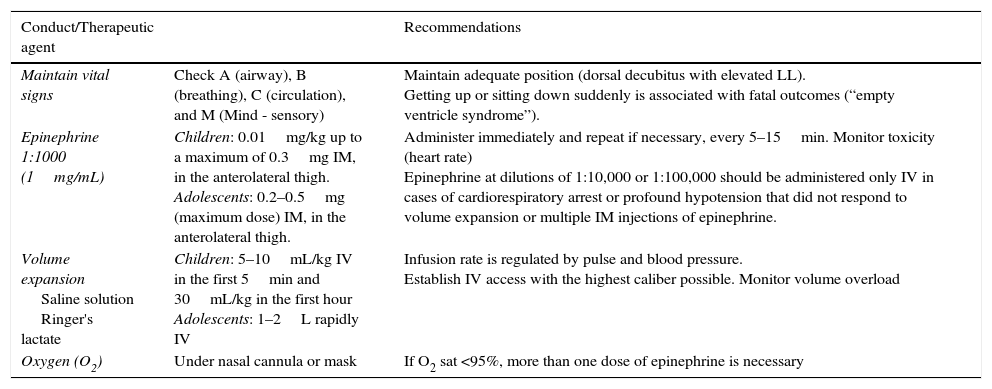
Conduct and main therapeutic agents in anaphylaxis treatment.
| Conduct/Therapeutic agent | Recommendations | |
|---|---|---|
| Maintain vital signs | Check A (airway), B (breathing), C (circulation), and M (Mind - sensory) | Maintain adequate position (dorsal decubitus with elevated LL). Getting up or sitting down suddenly is associated with fatal outcomes (“empty ventricle syndrome”). |
| Epinephrine 1:1000 (1mg/mL) | Children: 0.01mg/kg up to a maximum of 0.3mg IM, in the anterolateral thigh. Adolescents: 0.2–0.5mg (maximum dose) IM, in the anterolateral thigh. | Administer immediately and repeat if necessary, every 5–15min. Monitor toxicity (heart rate) Epinephrine at dilutions of 1:10,000 or 1:100,000 should be administered only IV in cases of cardiorespiratory arrest or profound hypotension that did not respond to volume expansion or multiple IM injections of epinephrine. |
| Volume expansion Saline solution Ringer's lactate | Children: 5–10mL/kg IV in the first 5min and 30mL/kg in the first hour Adolescents: 1–2L rapidly IV | Infusion rate is regulated by pulse and blood pressure. Establish IV access with the highest caliber possible. Monitor volume overload |
| Oxygen (O2) | Under nasal cannula or mask | If O2 sat <95%, more than one dose of epinephrine is necessary |
LL, lower limbs; IV, intravenous; IM, intramuscular.
Adapted and modified by Lockey et al.12
The use of secondary drugs is described in Table 2. Antihistamines are known to prevent pruritus and urticaria, but they do not alleviate respiratory symptoms, hypotension, or shock. Thus, similarly to corticosteroids, they are adjuvant drugs that are not indicated for the initial treatment. Corticosteroids may help prevent the secondary phase of anaphylaxis, which might recur within 12–24h after the initial event, but this biphasic presentation is considerably less common in food allergy anaphylaxis. In cases of asthma crises, the use of inhaled beta-2 agonists should be considered.7
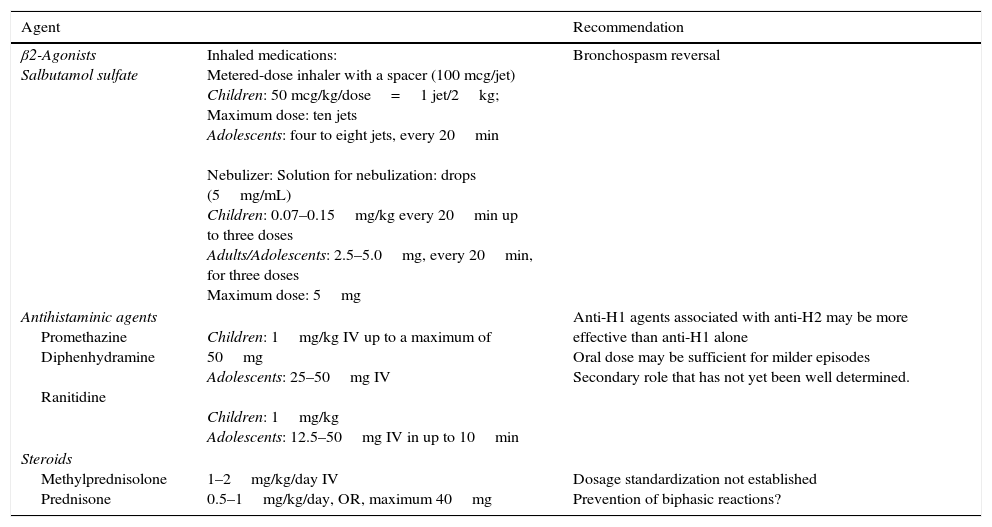
Secondary medications in the treatment of anaphylaxis.
| Agent | Recommendation | |
|---|---|---|
| β2-Agonists Salbutamol sulfate | Inhaled medications: Metered-dose inhaler with a spacer (100 mcg/jet) Children: 50 mcg/kg/dose=1 jet/2kg; Maximum dose: ten jets Adolescents: four to eight jets, every 20min Nebulizer: Solution for nebulization: drops (5mg/mL) Children: 0.07–0.15mg/kg every 20min up to three doses Adults/Adolescents: 2.5–5.0mg, every 20min, for three doses Maximum dose: 5mg | Bronchospasm reversal |
| Antihistaminic agents Promethazine Diphenhydramine Ranitidine | Children: 1mg/kg IV up to a maximum of 50mg Adolescents: 25–50mg IV Children: 1mg/kg Adolescents: 12.5–50mg IV in up to 10min | Anti-H1 agents associated with anti-H2 may be more effective than anti-H1 alone Oral dose may be sufficient for milder episodes Secondary role that has not yet been well determined. |
| Steroids Methylprednisolone Prednisone | 1–2mg/kg/day IV 0.5–1mg/kg/day, OR, maximum 40mg | Dosage standardization not established Prevention of biphasic reactions? |
IV, intravenous; IM, intramuscular; OR, oral route; anti-H1, antihistamine H1; anti-H2, antihistamine H2.
Adapted and modified by Lockey et al.12
In patients with a history of very severe anaphylaxis, it is recommended to start treatment with epinephrine soon after the probable ingestion and onset of the first symptom (even when they are mild symptoms, such as itching of the face/mouth, mild gastric discomfort, or nausea), because the rapid progression to severe anaphylaxis is expected and usual.7 The same conduct is prudent and recommended for children at risk of developing food anaphylaxis with uncontrolled asthma.11
Recommendations to patients after hospital dischargeOnce the patient has been treated, it is essential to identify the possible food culprits. A patient with anaphylaxis should remain on observation for 12–24h, as a secondary delay response may occur; the patient should also be advised to avoid exercises for the next seven days. Prescription of oral corticosteroids (prednisone or prednisolone at a dose of 1–2mg/kg/day, with a maximum dose of 40mg/day) for five to seven days and of second generation antihistamines for at least seven days (e.g., fexofenadine at a dose of 2.5mL twice daily for children under 6 years, 5mL twice daily for those older than 6 years, or 1120mg tablet, twice daily for adolescents). It is also prudent to avoid physical activity in the week following the episode.4
For food-dependent exercise-induced anaphylaxis, which occurs primarily in adolescents, it is also recommended to refer the patient to a specialist. At the outpatient level, it will be assessed whether the IgE specific to the possibly involved food is positive, whether symptoms occur when this food is ingested in the absence of physical activity, and whether symptoms occur during exercise without ingestion of the implicated food.10
The subsequent management of severe IgE-mediated food allergy consists mainly of an exclusion diet for a given period. The proposal of an allergen-specific immunotherapy for food anaphylaxis, especially with baked milk (as cake or cookies) aiming to prevent anaphylaxis, is still an experimental treatment undergoing evaluation.3,13
An important recommendation to the family of a patient who suffered severe anaphylaxis is the need to carry self-injecting epinephrine. These devices are available at fixed doses (0.15mg for children up to 30kg, 0.3mg for older children/adolescents) and are indicated, especially in cases of high risk of antigenic exposure. Unfortunately, the expiration dates for these devices are limited, their cost is high, and they are not available in Brazil and in other countries.4
Where self-injectable epinephrine is not available, doses of epinephrine prepared and assembled by the health care provider according to the patient's weight, in insulin syringes, adequately protected from sunlight and well-conditioned, may be offered to the patient and/or appropriately-trained family members. A scheme that can be simply and safely used intramuscularly is as follows: up to 10kg, 0.1mL IM; 10–20kg, 0.2mL IM; and >20kg, 0.3mL IM. Similarly, caution should be taken not to miss the dose when handling the syringe; patients should be informed that they should be replaced every two to three months, to avoid loss of the drug's effect due to environmental exposure.7,14 It is important that family, teachers, and community leaders increasingly recognize the early signs and know how to handle anaphylaxis with self-injectable epinephrine or even arrange and practice the use of syringe containing the medication, when this presentation is not available (Table 3).14,15
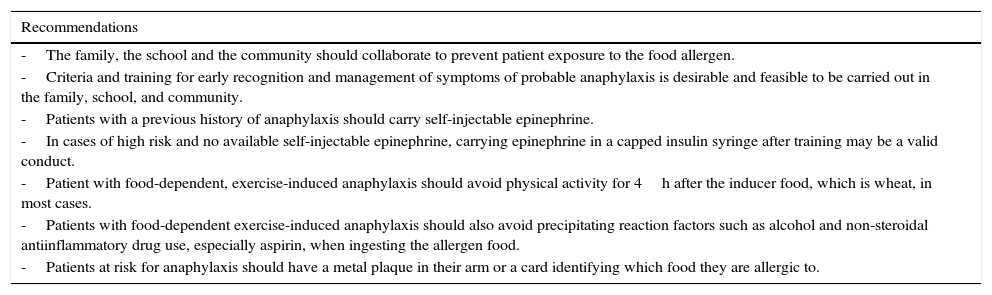
Preventive recommendations for food anaphylaxis for family, teachers, and community leaders.
| Recommendations |
|---|
| -The family, the school and the community should collaborate to prevent patient exposure to the food allergen. |
| -Criteria and training for early recognition and management of symptoms of probable anaphylaxis is desirable and feasible to be carried out in the family, school, and community. |
| -Patients with a previous history of anaphylaxis should carry self-injectable epinephrine. |
| -In cases of high risk and no available self-injectable epinephrine, carrying epinephrine in a capped insulin syringe after training may be a valid conduct. |
| -Patient with food-dependent, exercise-induced anaphylaxis should avoid physical activity for 4h after the inducer food, which is wheat, in most cases. |
| -Patients with food-dependent exercise-induced anaphylaxis should also avoid precipitating reaction factors such as alcohol and non-steroidal antiinflammatory drug use, especially aspirin, when ingesting the allergen food. |
| -Patients at risk for anaphylaxis should have a metal plaque in their arm or a card identifying which food they are allergic to. |
The pathogenesis of non-IgE mediated food allergies has yet to be clarified, because endoscopies and biopsies are not routinely performed. FPIES has been the most assessed allergy; several studies have suggested a key role of T-cells, with secretion of proinflammatory cytokines that may alter intestinal permeability.2 Although neutrophilia and thrombocytosis occur in patients with acute FPIES, the role of these cells in the pathogenic mechanism has not yet been established. The IgEs against allergy-causing foods are not typically detected; however, in a subgroup of children, they may be present in the acute phase or during its evolution.16 These patients tend to develop a longer course and, in some cases, progress to IgE-mediated allergy.2 The neuroendocrine pathway appears to play a role in the pathogenesis of FPIES, based on the efficacy of ondansetron, a serotonin antagonist receptor (5-HT3), in the management of FPIES acute reaction.17,18
Acute FPIES is characterized by uncontrollable vomiting, pallor and/or lethargy within 1–4h after the food ingestion. Diarrhea may occur within 5–10h after ingestion, particularly in young infants with a more severe phenotype (less than 30% are children older than one year). The acute FPIES event may be the first manifestation or it may occur when the food allergen is introduced after a period of exclusion in patients with the chronic form; it would be an episode of acute FPIES occurring in the chronic form of the disease.
Chronic FPIES shares clinical characteristics with food-induced enteropathy, such as malabsorption syndromes, anemia, diarrhea, and vomiting in children younger than nine months of age; however, in these patients, diarrhea is a more prominent symptom, but it does not lead to metabolic disturbances and severe dehydration as in acute FPIES. FPIES also occurs in older children and adults, due to exposure to fish or shrimp. In contrast to food protein-induced proctocolitis, FPIES is rare in exclusively breastfed children.19
FPIES caused by solid foods typically occurs later than that caused by cow's milk and soy milk, probably related to the time of their introduction into the child's diet. Most FPIES patients respond to a single food (65–80%), usually cow's milk or soy. However, patients with FPIES caused by cow's milk/soy might react to solids. In the United States, up to 50% of patients with cow's milk/soy allergy react to both foods, and about one-third of patients with cow's milk and/or soy allergy react to solids.19
Most children with FPIES to solids respond to several foods; chiefly those with FPIES caused by rice, oats, or barley have symptoms related to other grains. Patients with FPIES to multiple foods are less common in Japan, Australia, and Italy. These differences may reflect specific dietary habits in each country, and reinforce the hypothesis that early introduction of cow's milk and soybeans is a risk factor for FPIES caused by these proteins and foods at older ages.19
Diagnosis of FPIESThe diagnosis of FPIES is based on clinical history, recognition of clinical symptoms, exclusion of other etiologies, and oral challenge test (OCT) under medical supervision. Although the OCT is the gold standard, most patients do not need to undergo confirmation, especially if they have a history of severe reactions and become asymptomatic after removal of the suspected protein. However, challenge tests are required to determine FPIES resolution or to confirm chronic FPIES.20 The diagnostic criteria for FPIES are shown in Table 4.
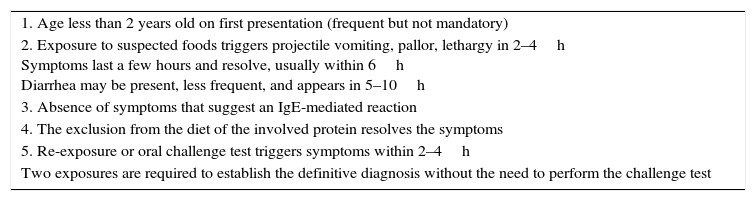
Current criteria used for the diagnosis of FPIES.
| 1. Age less than 2 years old on first presentation (frequent but not mandatory) |
| 2. Exposure to suspected foods triggers projectile vomiting, pallor, lethargy in 2–4h Symptoms last a few hours and resolve, usually within 6h Diarrhea may be present, less frequent, and appears in 5–10h |
| 3. Absence of symptoms that suggest an IgE-mediated reaction |
| 4. The exclusion from the diet of the involved protein resolves the symptoms |
| 5. Re-exposure or oral challenge test triggers symptoms within 2–4h |
| Two exposures are required to establish the definitive diagnosis without the need to perform the challenge test |
FPIES, food protein-induced enterocolitis syndrome.
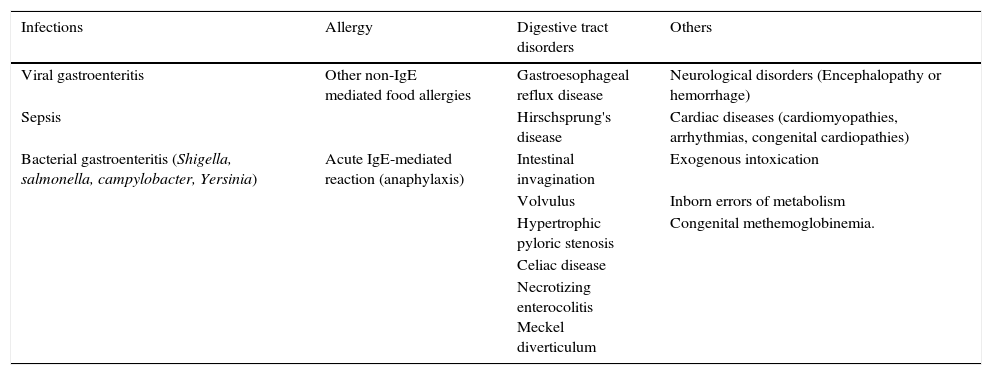
The differential diagnosis of FPIES is extensive and includes infectious diseases, other food allergies, and intestinal obstruction, as well as neurological and metabolic diseases (Table 5). The initial episodes are often diagnosed as acute, viral, or septic gastroenteritis, when profound lethargy and hypotension occurs, and there is a high leukocyte count with a left shift. Many other conditions can also be considered in the differential diagnosis, especially in infants with repeated and prolonged episodes of vomiting. Metabolic disorders are present and lead to dehydration, lethargy, as well as metabolic acidosis.21
Main clinical situations in the differential diagnosis of FPIES.
| Infections | Allergy | Digestive tract disorders | Others |
|---|---|---|---|
| Viral gastroenteritis | Other non-IgE mediated food allergies | Gastroesophageal reflux disease | Neurological disorders (Encephalopathy or hemorrhage) |
| Sepsis | Hirschsprung's disease | Cardiac diseases (cardiomyopathies, arrhythmias, congenital cardiopathies) | |
| Bacterial gastroenteritis (Shigella, salmonella, campylobacter, Yersinia) | Acute IgE-mediated reaction (anaphylaxis) | Intestinal invagination | Exogenous intoxication |
| Volvulus | Inborn errors of metabolism | ||
| Hypertrophic pyloric stenosis | Congenital methemoglobinemia. | ||
| Celiac disease | |||
| Necrotizing enterocolitis Meckel diverticulum |
FPIES, food protein-induced enterocolitis syndrome.
Emergency treatment of acute FPIES is based on three main points18,19:
- 1)
Hydroelectrolytic resuscitation – 10–20mL/kg body weight in bolus;
- 2)
Administration of methylprednisolone – 1mg/kg body weight IV, maximum of 60–80mg;
- 3)
Ondansetron IV or IM – 0.15mg/kg body weight.
After these initial conducts in the emergency unit, the patient should remain hospitalized, maintaining the venoclysis for hydration and loss replacement, monitoring of vital signs (pulse, temperature, capillary filling time, heart rate, and blood pressure). Additional doses of ondansetron may be necessary, as well as corrections of hydroelectrolytic disorders, based on losses. Complementary exams should be requested: whole blood count with platelets, ionogram, and gasometry.
Patient guidance after hospital dischargeThe management of non-IgE food allergy is empirical due to the limited evidence and the divergences in many areas of its pathophysiology. Food protein elimination diet is paramount. In FPIES, exclusive breastfeeding must be preserved. Protein hydrolysate formulas are generally well tolerated, although approximately 20% of patients may require amino acid formulas.20 Follow-up with a specialist is indicated for specific care, especially for nutritional guidance and symptom control during and shortly after hospital admission.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Sarinho ES, Lins MG. Severe forms of food allergy. J Pediatr (Rio J). 2017;93:53–9.