The objective of this meta-analysis is to evaluate the diagnostic value of serum Cystatin C in acute kidney injury (AKI) in neonates.
SourcesPubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), and WanFang Database were searched to retrieve the literature related to the diagnostic value of Cystatin C for neonatal AKI from inception to May 10, 2021. Subsequently, the quality of included studies was determined using the QUADAS-2 tool. Stata 15.0 statistical software was used to calculate the combined sensitivity (SEN), specificity (SPE), positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR). Additionally, meta-regression analysis and subgroup analysis contributed to explore the sources of heterogeneity.
Summary of the findingsTwelve articles were included. The pooled sensitivity was 0.84 (95%CI: 0.74-0.91), the pooled specificity was 0.81 (95%CI: 0.75-0.86), the pooled PLR was 4.39 (95%CI: 3.23-5.97), the pooled NLR was 0.19 (95%CI: 0.11-0.34), and the DOR was 22.58 (95%CI: 10.44-48.83). The area under the receiver operating characteristic curve (AUC) was 0.88 (95%CI: 0.85-0.90). No significant publication bias was identified (p > 0.05).
ConclusionsSerum Cystatin C has a good performance in predicting neonatal AKI; therefore, it can be used as a candidate biomarker after the optimal level is determined by large prospective studies.
Acute kidney injury (AKI) is an independent risk factor for neonatal death.1 Its incidence is reported to be about 8%-24% in neonates hospitalized in a neonatal intensive care unit (NICU), and its mortality is approximately 10 %-61 %.2,3 Early renal injury is a potentially reversible syndrome characterized by a sharp decline in the glomerular filtration rate. Since neonatal early renal injury usually has no specific clinical symptoms, many AKI cases are often missed the best time of early intervention if there is no relevant examination.4 A survey of 91 hospitals showed that the number of children with chronic kidney disease (CKD) in China increased by 4.3 times between 1990 and 2002,5 and many adult CKD is the continuation of childhood. Therefore, how to accurately identify early renal injury and reduce neonatal mortality and complications in childhood and adulthood has been a difficult problem for researchers to solve.
Serum creatinine (SCr) and blood urea nitrogen (BUN) are classic indicators for clinical detection of kidney injury. Relevant studies have confirmed that SCr of newborns reflects SCr level of the mother during the first two days of life; SCr level of full-term healthy newborns gradually decreases with the days after birth and stabilizes to a normal level two weeks after birth.6 By contrast, the decline of SCr in preterm infants has an unstable trend. It has been reported that in the case of preterm birth with gestational age less than 26 weeks, SCr level transiently increases, peaks on the third day after birth, and then gradually decreases.7,8 In addition, SCr and BUN can only reflect the consequences of renal injury, but are not sensitive to the diagnosis of the injury in the early stage. The reason is that it is difficult to detect significant changes in SCr and BUN levels in the early stage of renal dysfunction.9 Besides, SCr level is also affected by many non-renal factors, such as age, sex, muscle mass, diet, liver disease, chronic organic disease; affect the level of SCr; BUN increases with urea synthesis and changes with endogenous and exogenous protein, which interferes with the assessment of early renal injury.10,11 Therefore, some biochemical indicators instead of classical detection indexes are being studied and reported.
Cystatin C (Cys-C), compared with the classical detection indexes, is not affected by age, sex, race, infection, liver disease, or inflammation.12 Secondly, unlike SCr, Cys-C is produced at a constant rate and is not secreted through renal tubules, and serum Cys-C concentration is measured by glomerular filtration. Thirdly, Cys-C is distributed in extracellular fluid, while SCr is distributed in the internal environment of the whole body, so when glomerular filtration rate decreases, the increase of serum Cys-C is faster than that of SCr.13 Collectively, serum Cys-C may be used as a reliable biological index to predict AKI, especially in guiding early clinical detection of neonatal AKI. Therefore, by using meta-analysis, this study systematically explores and confirms the predictive value of serum Cys-C in the diagnosis of neonatal AKI.
Materials and methodsRetrieval strategyThe present systematic review and meta-analysis were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.14 Two researchers independently searched four English databases (PubMed, Embase, Cochrane Library, Web of Science) and two Chinese databases (China National Knowledge Infrastructure (CNKI) and WanFang Database) from inception to May 10, 2021, without language restrictions, aiming to retrieve the literature on the diagnostic value of serum Cys-C for neonatal AKI. The search strategy was: ("serum cystatin C" OR "Cys-C"), AND ("acute kidney injury" OR "AKI") AND ("newborn" OR "neonates" OR "neonatal"). The references of the retrieved literature were further manually searched to prevent the omission of relevant literature. The disagreement between the two researchers would be determined by a third researcher.
Inclusion and exclusion criteriaInclusion criteria(1) Literature related to the diagnostic accuracy of serum Cys-C for AKI in neonates; (2) Literature that directly or indirectly provided values of sensitivity (SEN) and specificity (SPE); (3) Serum Cys-C samples that collected from neonatal serum; (4) Diagnosis of AKI was confirmed by the standard of pathological scholarship.
Exclusion criteria(1) Literature reviews, case reports, systematic reviews, conference summaries, letters, animal or laboratory studies; (2) There were duplicate samples.
Data extractionThe data were extracted by two researchers independently. Differences in the inclusion of the data between the two would be solved by a third researcher. The extracted data included the first author, year of publication, country, gestational age, detection time, detection method, number of patients, cut-off value, true positive (TP), false positive (FP), false negative (FN), true negative (TN).
Quality evaluationThe quality of included articles was estimated by using Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2).15 QUADAS-2 consists of four domains: patient selection, index test, reference standard, flow, and timing. Each is assessed in terms of risk of bias and rated as "high", "low" or "unclear". Any controversial questions between the two researchers were resolved by a third researcher through discussion.
Statistical analysisThe Spearman correlation coefficient and the typical "shoulder arm shape" in the summary receiver operating characteristic (SROC) curve were used to determine whether there was a threshold effect in the study. With I2 for estimating heterogeneity across studies, if the heterogeneity was large, meta-regression analysis and subgroup analysis would be carried out. Stata15.0 statistical software, with a bivariate mixed-effects model, was applied to calculate SEN, SPE, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR). The area under the curve (AUC) was obtained, ranging from 0.5 to 1.0; 0.5 indicated worst diagnostic performance, while 1.0 suggested best diagnostic performance. Deeks' funnel plot was adopted for detecting publication bias, and QUADAS-2 for evaluating the quality of the included literature.
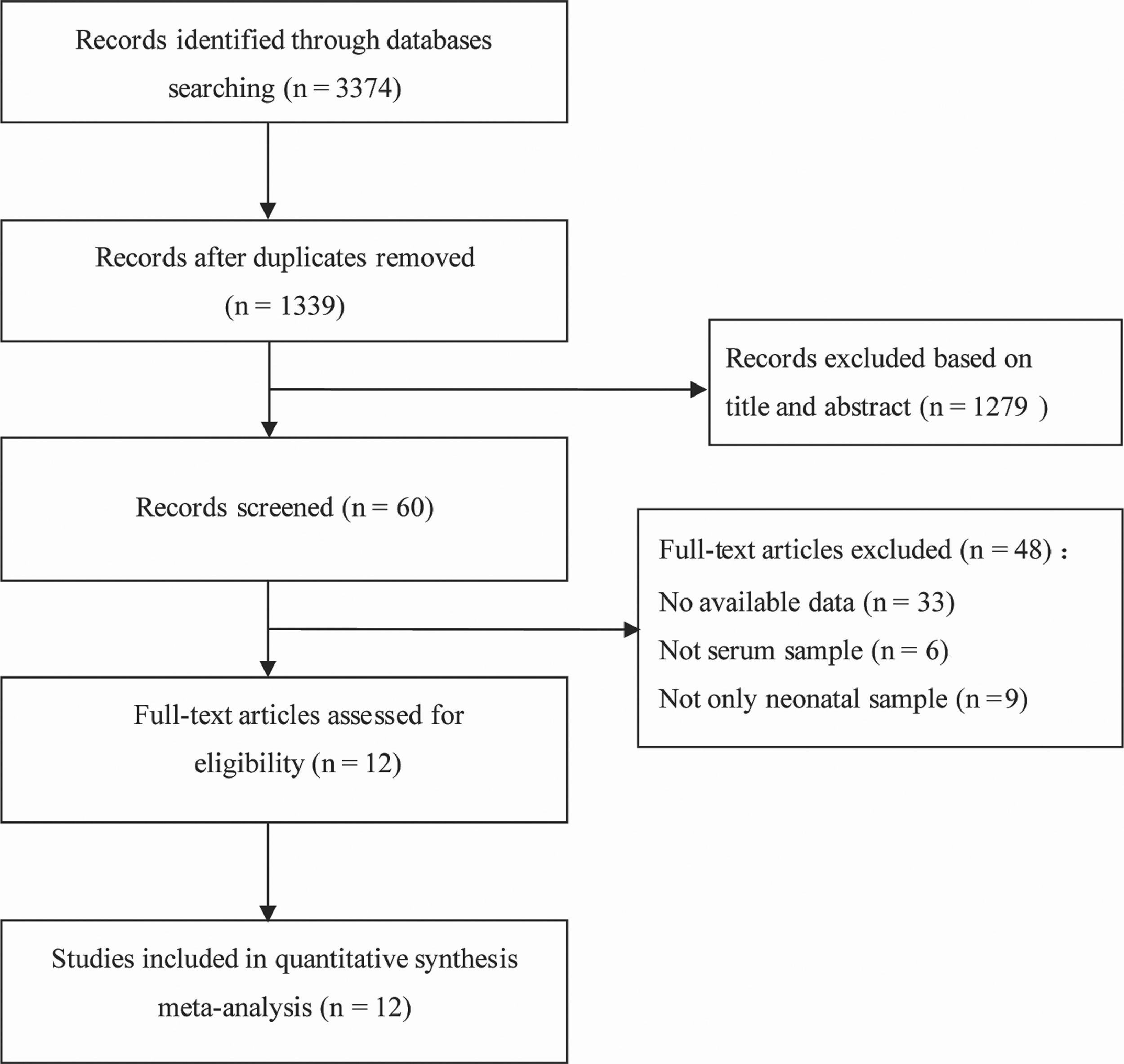
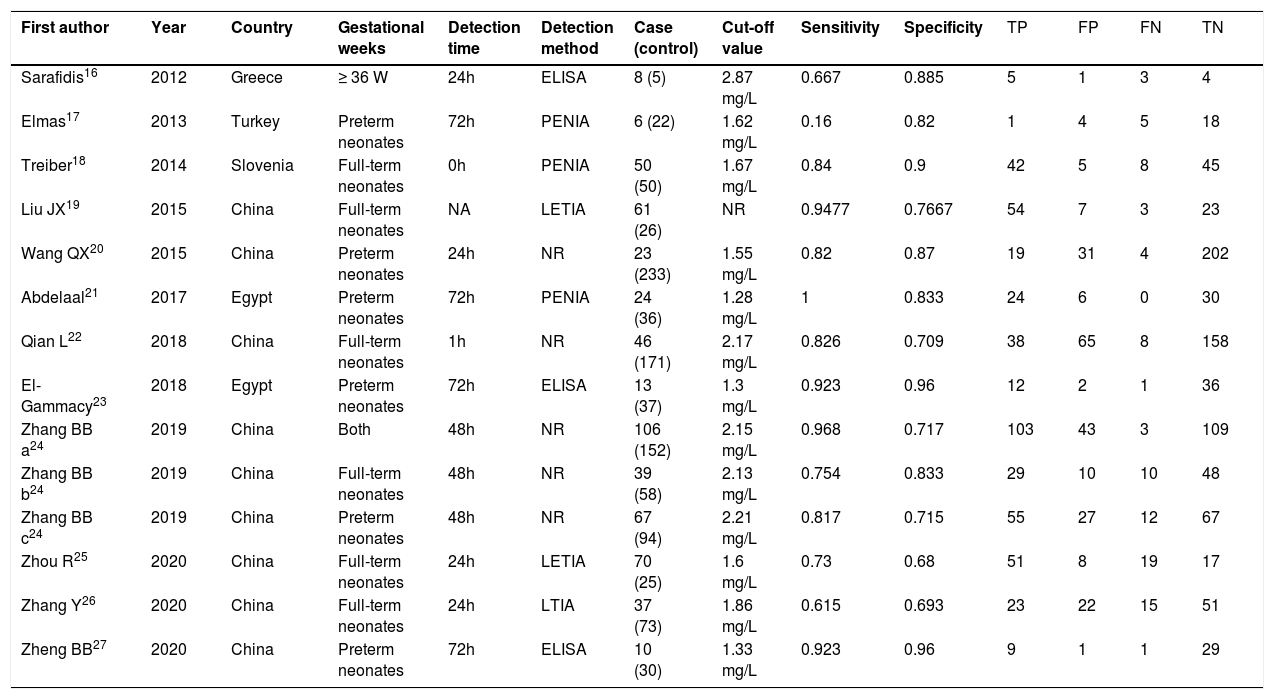
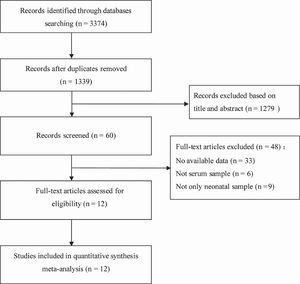
ResultsLiterature retrieval results and basic characteristics of included studiesAmong the 3374 related articles retrieved through the search strategy, 2035 duplicate articles were excluded first. Then 1279 articles were excluded by reading titles and abstracts, and 48 articles by further reading full texts. Finally, a total of 12 articles were included.16-27 The literature screening process and results are shown in Figure 1. Among the 12 included studies, 619,20,22,24,25,27 were in Chinese and 616-18,21,23,26 were in English, including 1572 samples from four countries (556 AKI cases, 1016 controls). The basic characteristics of the included studies are shown in Table 1.
Characteristics of the included studies.
| First author | Year | Country | Gestational weeks | Detection time | Detection method | Case (control) | Cut-off value | Sensitivity | Specificity | TP | FP | FN | TN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sarafidis16 | 2012 | Greece | ≥ 36 W | 24h | ELISA | 8 (5) | 2.87 mg/L | 0.667 | 0.885 | 5 | 1 | 3 | 4 |
| Elmas17 | 2013 | Turkey | Preterm neonates | 72h | PENIA | 6 (22) | 1.62 mg/L | 0.16 | 0.82 | 1 | 4 | 5 | 18 |
| Treiber18 | 2014 | Slovenia | Full-term neonates | 0h | PENIA | 50 (50) | 1.67 mg/L | 0.84 | 0.9 | 42 | 5 | 8 | 45 |
| Liu JX19 | 2015 | China | Full-term neonates | NA | LETIA | 61 (26) | NR | 0.9477 | 0.7667 | 54 | 7 | 3 | 23 |
| Wang QX20 | 2015 | China | Preterm neonates | 24h | NR | 23 (233) | 1.55 mg/L | 0.82 | 0.87 | 19 | 31 | 4 | 202 |
| Abdelaal21 | 2017 | Egypt | Preterm neonates | 72h | PENIA | 24 (36) | 1.28 mg/L | 1 | 0.833 | 24 | 6 | 0 | 30 |
| Qian L22 | 2018 | China | Full-term neonates | 1h | NR | 46 (171) | 2.17 mg/L | 0.826 | 0.709 | 38 | 65 | 8 | 158 |
| El-Gammacy23 | 2018 | Egypt | Preterm neonates | 72h | ELISA | 13 (37) | 1.3 mg/L | 0.923 | 0.96 | 12 | 2 | 1 | 36 |
| Zhang BB a24 | 2019 | China | Both | 48h | NR | 106 (152) | 2.15 mg/L | 0.968 | 0.717 | 103 | 43 | 3 | 109 |
| Zhang BB b24 | 2019 | China | Full-term neonates | 48h | NR | 39 (58) | 2.13 mg/L | 0.754 | 0.833 | 29 | 10 | 10 | 48 |
| Zhang BB c24 | 2019 | China | Preterm neonates | 48h | NR | 67 (94) | 2.21 mg/L | 0.817 | 0.715 | 55 | 27 | 12 | 67 |
| Zhou R25 | 2020 | China | Full-term neonates | 24h | LETIA | 70 (25) | 1.6 mg/L | 0.73 | 0.68 | 51 | 8 | 19 | 17 |
| Zhang Y26 | 2020 | China | Full-term neonates | 24h | LTIA | 37 (73) | 1.86 mg/L | 0.615 | 0.693 | 23 | 22 | 15 | 51 |
| Zheng BB27 | 2020 | China | Preterm neonates | 72h | ELISA | 10 (30) | 1.33 mg/L | 0.923 | 0.96 | 9 | 1 | 1 | 29 |
TP, true positive; FP, false positive; TN, true negative; FN, false-negative; ELISA, enzyme-Linked Immunosorbent assay; PENIA, Particle Enhanced Nephelometry Immunoassay; LTIA, latex-enhanced immunoturbidimetry assay; LETIA, latex particle-enhanced turbidimetric immunoassay; NR, no report.
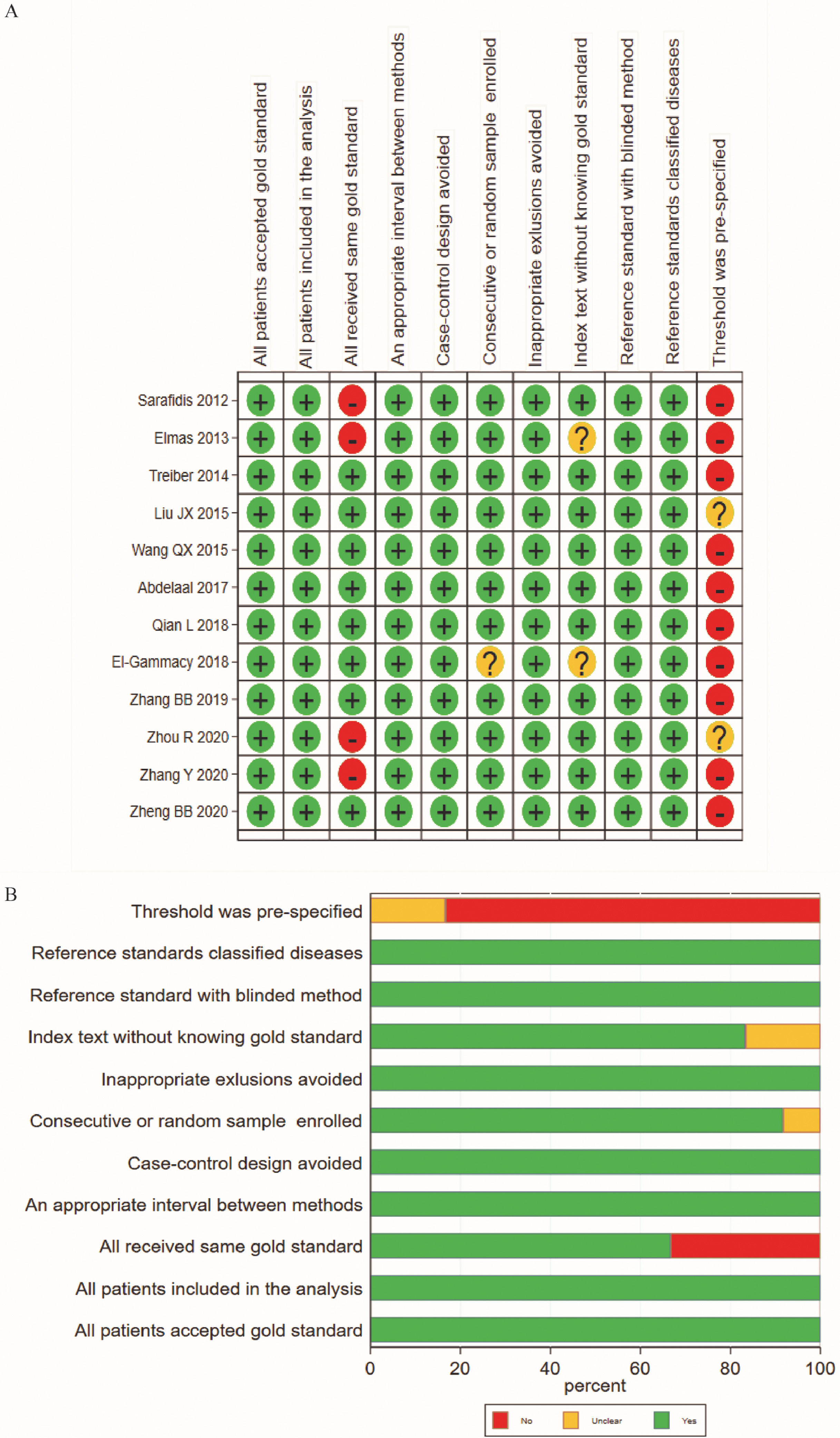
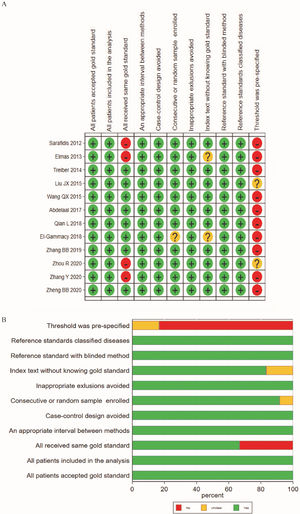
According to the results of QUADAS-2 (Figure 2A-B), all the 12 included studies showed high-level quality. However, there were biases in the index test and reference standard. One reason was that none of the studies adopted a preset threshold. In addition, the specific methods of the reference standard testing in the included studies were inconsistent.
Summary of risk and applicability by QUADAS-2. A, Summary diagram of quality evaluation results of the included studies by using the QUADAS-2 tool; B, Regulation chart of quality evaluation results of diagnostic test literature.
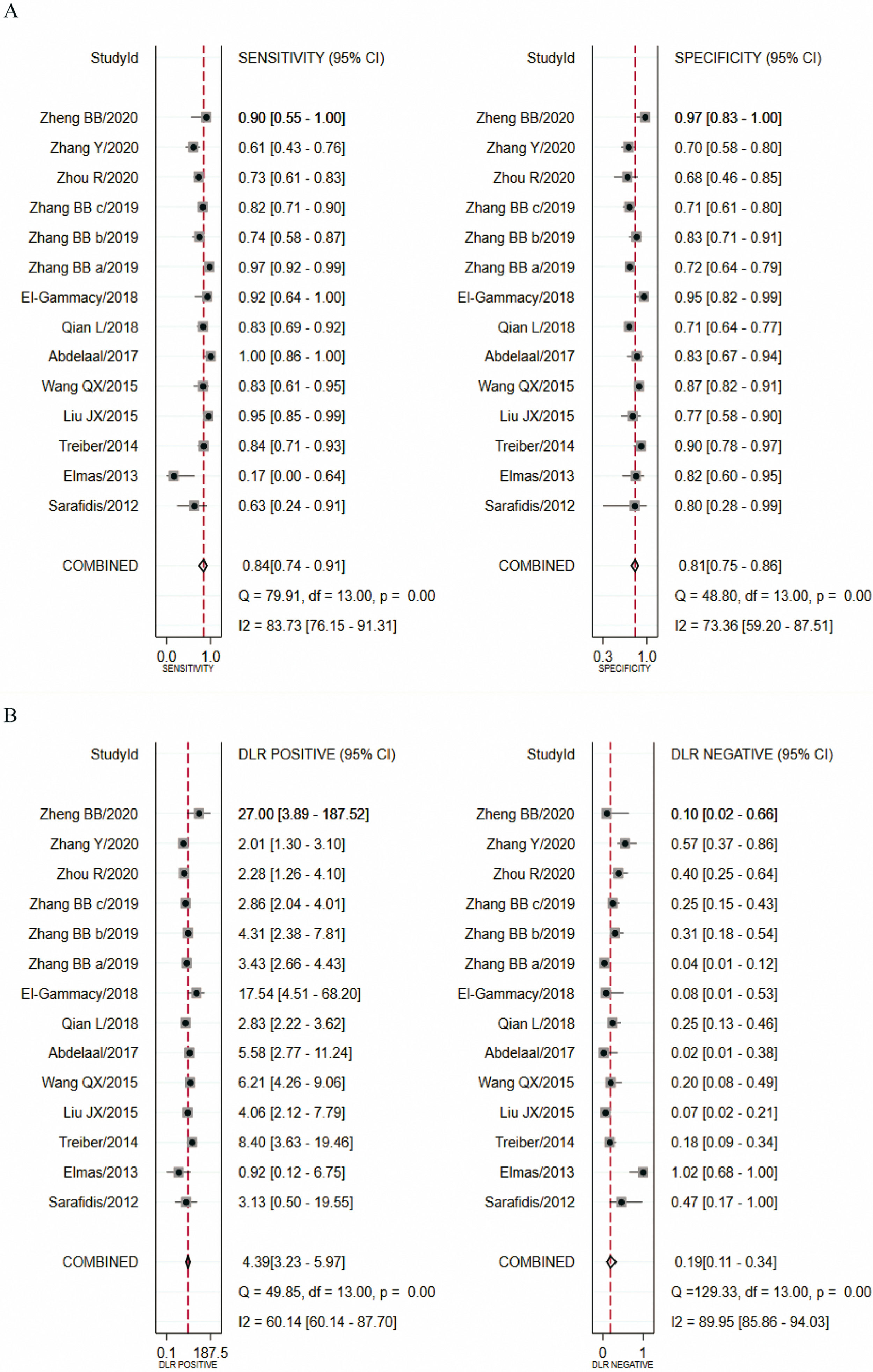
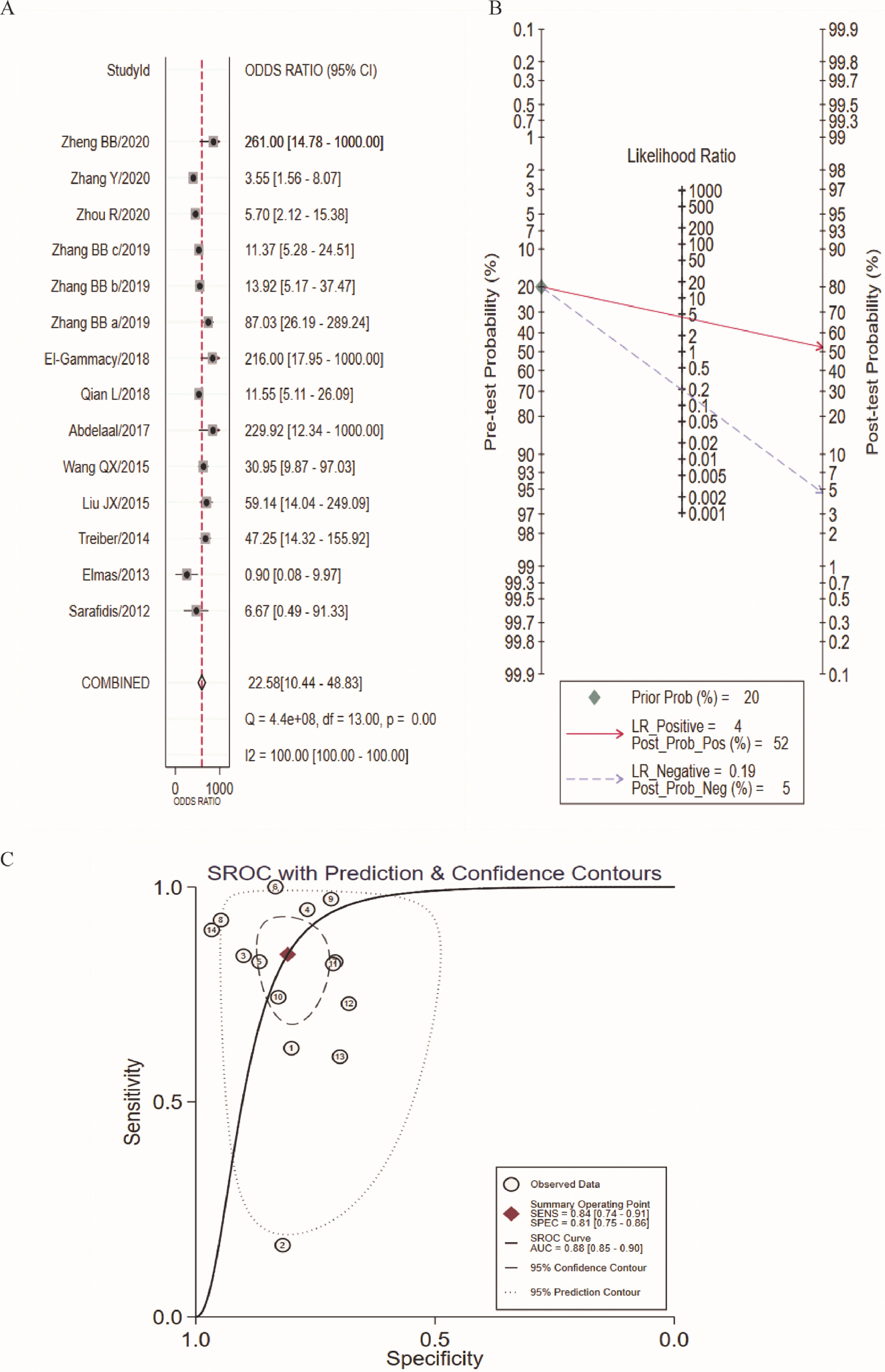
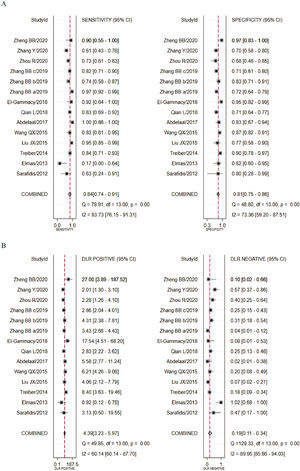
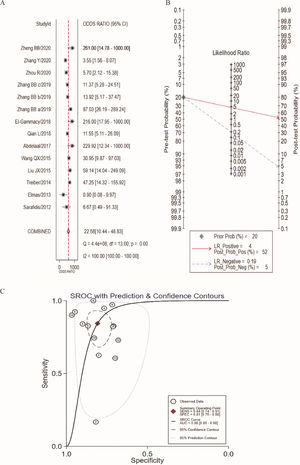
In this study, there was heterogeneity (I2 = 100%) in the combined DOR. In the threshold effect analysis, the Spearman correlation coefficient was 0.4026 (p > 0.05), suggesting that the threshold effect was not significant and the data could be pooled. The pooled SEN, SPE (Figure 3A), PLR, NLR (Figure 3B) and DOR (Figure 4A) were 0.84 (95%CI: 0.74-0.91), 0.81 (95%CI: 0.75-0.86), 4.39 (95%CI: 3.23-5.97), 0.19 (95%CI: 0.11-0.34), 22.58 (95%CI: 10.44-48.83), respectively. The AUC was 0.88 (95%CI: 0.85-0.90). Fagan's Nomogram results showed that, when the pre-test probability ratio was 20%, the post-test probability ratio of PLR was 52%, with an NLR of 5% (Figure 4B). The SROC curve was shown in Figure 4C.
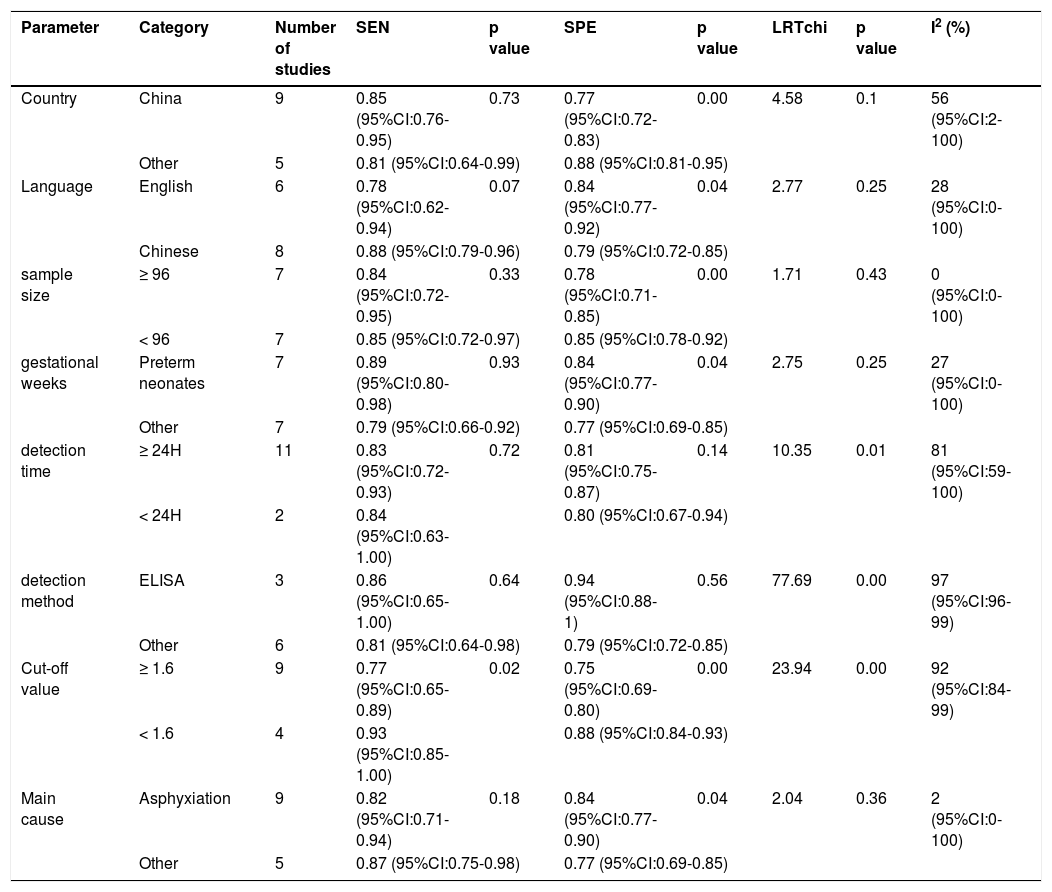
The following indicators were included in the regression model of this study: country of publication (China or other regions), language (Chinese or English), number of samples, neonatal gestational age (full-term or not), serum Cys-C detection time (less than 24 hours after birth or not), detection method (ELISA or not), cut-off value (upper and equal to 1.6 mg/L or below) and cause of AKI (asphyxiation or not). The pooled SEN was only affected by cut-off value; the group with cut-off value more than 1.6mg/L has higher pooled SEN than that of the corresponding group, 0.93 (95%CI:0.85-1) vs. 0.77 (95%CI:0.65-0.89), p < 0.05. Except serum Cys-C detection time and detection method, the pooled SPE was affected by the other indicators. Specifically, higher pooled SPE was found in the group with following characteristics (from other regions, published in English, sample size less than 96, preterm newborns as subjects, cut-off value less than 1.6 mg/L, AKI caused by asphyxiation) when compared with the corresponding group, 0.88 (95%CI: 0.81-0.95) vs. 0.77 (95%CI: 0.72-0.83), p < 0.001; 0.84 (95%CI: 0.77-0.92) vs. 0.79 (95%CI: 0.72-0.85), p < 0.05; 0.85 (95%CI: 0.78-0.92) vs. 0.78 (95%CI: 0.71-0.85), p < 0.001; 0.84 (95%CI: 0.77-0.90) vs. 0.77 (95%CI: 0.69-0.85), p < 0.05; 0.88 (95%CI: 0.84-0.93) vs. 0.75 (95%CI: 0.69-0.80), p < 0.001; 0.84 (95%CI: 0.77-0.90) vs. 0.77 (95%CI: 0.69-0.85), p < 0.05.
Subgroup analysis found significant differences in detection time (χ2 = 10.35, p = 0.01, I2 = 81 (95%CI: 59-100), and detection method (χ2 = 77.69, p = 0.001, I2 = 97 (95%CI: 96-99), cut-off values (χ2 = 23.94, p < 0.001, I2 = 92 (95%CI: 84-99). There were no differences in SEN and SPE between groups of countries (χ2 = 4.58, p = 0.1, I2 = 56 (95%CI: 2-100)), language (χ2 = 2.77, p = 0.25, I2=28 (95%CI: 0-100)), sample size (χ2 = 1.71, p = 0.43, I2 = 0 (95%CI: 0-100)), gestational age (χ2 = 2.75, p = 0.25, I2 = 27 (95%CI: 0-100)), AKI caused (χ2 = 2.04, p = 0.36, I2 = 2 (95%CI: 0-100)). The results can be found in Table 2.
Meta-regression analysis and subgroup analysis.
SEN, sensitivity; SPE, specificity; ELISA, Enzyme-Linked Immunosorbent assay.
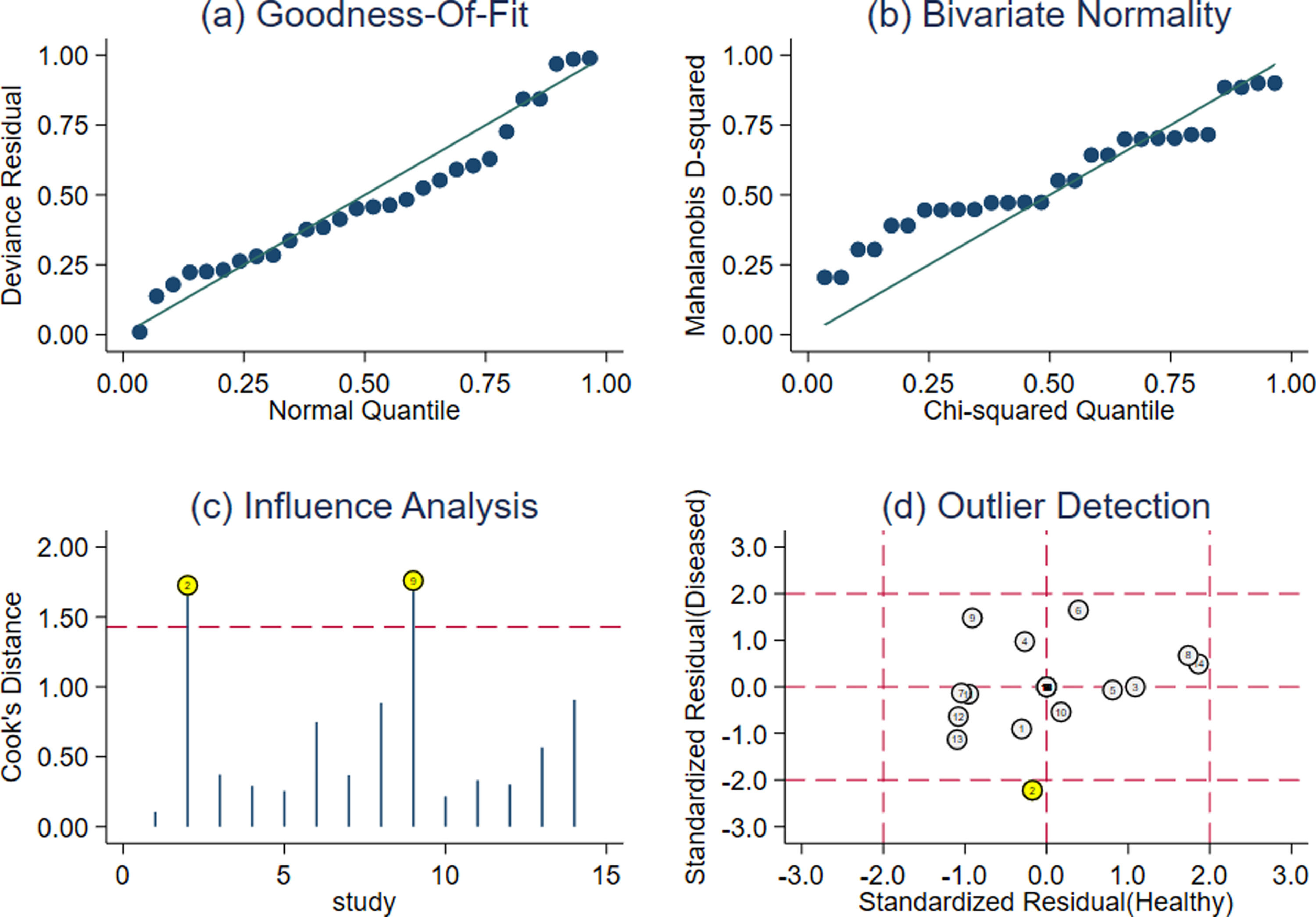
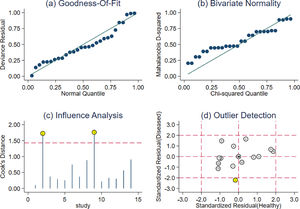
According to the sensitivity analysis results (Figure 5), the goodness of fit and bivariate normality indicated that the random effect bivariate model was suitable for analysis (Figure 5A-B). Impact analysis found two studies were more weight (Figure 5C), and outlier detection identified one abnormal study (Figure 5D). After removing the study, SPE, PLR, DOR, AUC increased a little (Supplementary Table 1), suggesting that the results of this study were stable.
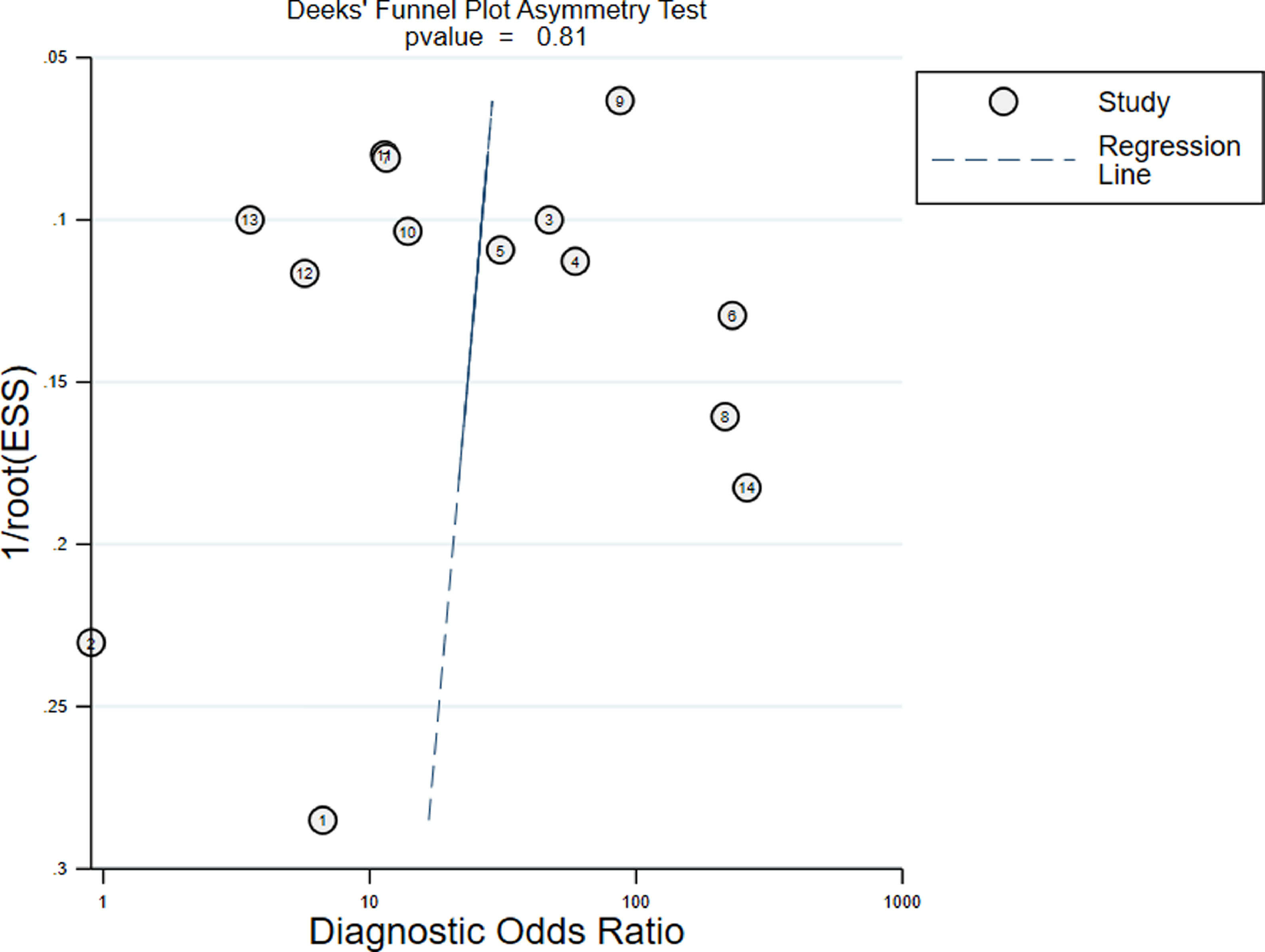
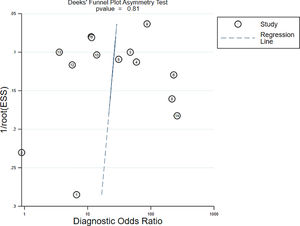
Publication biasAs shown in Deeks' funnel plot (Figure 6), no significant publication bias was found (p > 0.05).
DiscussionIn the clinic, serum SCr level and urine volume are classic indexes used in renal function examination. However, in the clinical diagnosis of newborns, the accuracy of urine 24-hour volume recording is low, and this volume fluctuates widely within a few days after birth.28 Many factors during the neonatal period (such as maternal SCr level, age, height, muscle mass, etc.) may affect the value of SCr.29 Therefore, it is necessary to explore biomarkers that can predict neonatal AKI, but there are a few reports on this field. The purpose of this study is to reveal the diagnostic value of serum Cys-C in neonatal AKI.
This study showed that the pooled SEN and SPN of serum Cys-C in the diagnosis of neonatal AKI was 0.84 and 0.81, suggesting a good diagnostic performance of Cys-C. The pooled PLR was 4.39, indicating that the newborns with AKI were 4.39 times more likely to be diagnosed as positive. The pooled NLR was 0.19, meaning that the risk of newborns with AKI was 19% of being free, which suggested that the diagnostic value of serum Cys-C was high. In this study, the DOR value was 22.58 and the AUC was 0.88, proving that the accuracy of serum Cys-C in the diagnosis of neonatal AKI was general. The heterogeneity of the combined effect in this meta-analysis was not caused by the threshold effect (the Spearman correlation coefficient was 0.4026). Therefore, Meta-regression analysis was used to further explore the source of heterogeneity and the difference of diagnostic value. The results showed that the cut-off value may be the source of heterogeneity, and the difference was statistically significant. Sensitivity analysis was applied to evaluate the stability of this study, which demonstrated that the results of this study were stable. A meta-analysis of AKI after heart surgery in adults showed that Cys-C had a good diagnostic value, with a pooled SEN and SPE of 0.84, 0.82 respectively, and AUC of 0.96.30 Another report showed that serum Cys-C was also effective in diagnosing critically ill premature infants with AKI, with an SEN and SPE of 0.848, 0.618, and AUC of 0.849, but not better than SCr.31 Compared with SCr, Cys-C seems to be a more specific and sensitive biomarker for urinary neutrophil gelatinase-associated lipocalin (NGIL) positive AKI in infants after cardiopulmonary bypass, with an SEN and SPE of 0.80 and 0.89 respectively and AUC of 0.87.32 Additionally, a meta-analysis that included 24 articles (1948 children) showed the serum and urine levels of Cys-C were significantly higher in children with AKI. The AUC of serum Cys-C and urine Cys-C in the prediction of AKI were 0.83 and 0.85, respectively. The best SEN was 0.85 and SPE was 0.61 for serum Cys-C, and the cut-off value was 0.4-1.0 mg/L. Serum Cys-C rises earlier than SCr in the development of AKI, and serum Cys-C has a higher predictive value for AKI compared to urine Cys-C according to the limited number of studies.33 The pooled SEN of serum Neutrophil Gelatinase Associated Lipocalin (NGAL) for diagnosis of AKI in neonates with perinatal asphyxia was 0.818, the SPE was 0.870, and the AUC was 0.912.34
This study had some limitations. (1) When calculating the pooled effect, there is heterogeneity among the studies due to the limited numbers of studies. And the number of AKI newborns enrolled in this study was relatively small, so the results need to be confirmed by large-scale, prospective clinical studies; (2) This study is only a rough exploration of the potential of serum Cys-C in the diagnosis of neonatal AKI. The methods and procedures for detecting serum Cys-C are not standardized among the including studies. (3) There are various causes of neonatal AKI, such as perinatal hypoxia, asphyxia, hyperbilirubinemia, pre-term delivery, extremely low birth weight, and frequent infection, which can easily lead to AKI.35 But in this meta-analysis, there is no conclusion about the diagnostic performance of serum Cys-C for AKI caused by these causes. The incidence of AKI in newborns after asphyxia is more than 40%,36 and the incidence of severe asphyxia even reaches 61-70%.37 A large number of studies are required to investigate the relationship between serum Cys-C, AKI etiology, and the degree of AKI.
In conclusion, the results of this study prove that serum Cys-C has potential diagnostic value for neonatal AKI. However, a larger sample size and prospective studies are still needed to verify these results. Based on this conclusion, it is recommended that serum Cys-C measurement should be performed in every newborn suspected of AKI to assist the diagnosis of neonatal AKI.
Availability of data and materialThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.