Children and adolescents often require sedation and analgesia in emergency situations. With the emergence of new therapeutic options, the obsolescence of others, and recent discoveries regarding already known drugs, it became necessary to review the literature in this area.
Data sourcesNon-systematic review in the PubMed database of studies published up to December 2016, including original articles, review articles, systematic reviews, and meta-analyses. References from textbooks, publications from regulatory agencies, and articles cited in reviews and meta-analyses through active search were also included.
Data synthesisBased on current literature, the concepts of sedation and analgesia, the necessary care with the patient before, during, and after sedoanalgesia, and indications related to the appropriate choice of drugs according to the procedure to be performed and their safety profiles are presented.
ConclusionsThe use of sedoanalgesia protocols in procedures in the pediatric emergency room should guide the professional in the choice of medication, the appropriate material, and in the evaluation of discharge criteria, thus assuring quality in care.
Crianças e adolescentes necessitam frequentemente de sedação e analgesia em situações de emergência. Com o surgimento de novas opções terapêuticas, a obsolescência de outras e descobertas recentes das drogas já conhecidas, fez-se necessário uma nova revisão da literatura nesta área.
Fontes dos dadosRevisão não sistemática na base de dados PubMed de estudos publicados até dezembro de 2016, incluindo artigos originais, artigos de revisão, revisões sistemáticas e meta-análises. Também foram incluídos referências de livros-texto, publicações de agências reguladoras além de artigos citados nas revisões e meta-análises através de busca ativa.
Síntese dos dadosCom base na literatura atual, são apresentados os conceitos de sedação e analgesia, os cuidados necessários com o paciente antes, durante e após a sedoanalgesia, além de indicações quanto à escolha apropriada dos fármacos de acordo com o procedimento a ser realizado e o perfil de segurança destes.
ConclusõesO emprego de protocolos de sedoanalgesia em procedimentos no Pronto-Socorro pediátrico deve orientar o profissional na escolha da medicação, do material adequado e na avaliação dos critérios de alta, garantindo assim qualidade na assistência.
Children and adolescents often require sedation and analgesia when treated in an emergency situation. Invasive and noninvasive procedures are part of diagnostic and therapeutic techniques in pediatrics, and are often uncomfortable for the child, the parents, and health professionals.1 Although necessary, sedation and analgesia may have adverse effects, requiring management in an adequate environment and performed by trained professionals.2
Sedation for procedures has advanced and expanded in the last decades; no longer the exclusive scope of anesthesiology, now it is being routinely used by the most varied of medical specialties, such as gastroenterology, cardiology, neurology, radiology, emergency medicine, and pediatric intensive care.3 In a qualitative study with physicians from Ireland and the United Kingdom, McCoy et al. identified the lack of training and education in this area as a significant barrier. The standardization of sedation practices and standardization of guidelines and recommendations remains a challenge.4 In Brazil, few review articles have been published on this topic in recent years5,6; protocols are suggested in two of them.6,7
Sedation reduces the state of consciousness, while analgesia reduces or eliminates the perception of pain. Many analgesics have some sedative effect, but few sedatives have the property of analgesia. The aim of sedation in pediatrics differs from that in adult patients, as it is administered to control behavior and allow safe completion of the procedure.2 For cooperative children, non-pharmacological modalities should be considered, such as parental presence, hypnosis, distraction, and topical anesthetics, as they may reduce the need for or depth of pharmacological sedation.8,9
Levels of sedationSedation is described as a continuum, represented by progressive stages, from mild to general anesthesia. Ketamine, as an exception, is a dissociative agent that has the particularity of producing a sedation that does not follow this pattern; the effect is present or absent, with maintenance of spontaneous breathing, protective reflexes, and cardiovascular stability.10
In 2002, the American Society of Anesthesiologists (ASA) defined the four levels of sedation11:
- •
Minimal sedation (anxiolysis): a state induced by medication in which patients respond normally to verbal commands. Although cognitive function and coordination may be impaired, ventilatory and cardiovascular functions are not affected.
- •
Moderate sedation: also called “conscious sedation,” it is a state of drug-induced decrease in consciousness, in which the patient responds purposefully to verbal commands, isolated or accompanied by light tactile stimuli. No intervention is necessary to maintain airway patency and spontaneous ventilation is adequate. In general, cardiovascular function is usually maintained.
- •
Deep sedation: a drug-induced decrease in consciousness from which patients cannot be easily awakened, but respond to repeated or painful stimuli. The ability to independently maintain ventilatory function may be impaired. Patients may need assistance to maintain airway patency and spontaneous ventilation may be inadequate. Cardiovascular function is generally maintained.
- •
General anesthesia: a state of drug-induced loss of consciousness from which patients are not aroused, even with painful stimuli. The ability to maintain ventilatory function is impaired and patients generally need assistance to maintain airway patency and positive pressure ventilation. Cardiovascular function may be impaired.
One of the important aspects of pain relief in pediatrics implies the understanding of pain assessment methods and their use. Pain can be assessed in children using physiological parameters, behavioral observation, and self-report. The patient with pain will have tachycardia, pupillary dilatation, sweating, and peripheral vasoconstriction.12,13 No pain assessment should be based solely on these parameters, but rather should be made in combination with validated scales for their adequate measurement. Pain assessment scales have been validated for use in pediatrics, considering the child's developmental phase (verbal or pre-verbal age) and their cognitive ability to report pain.14
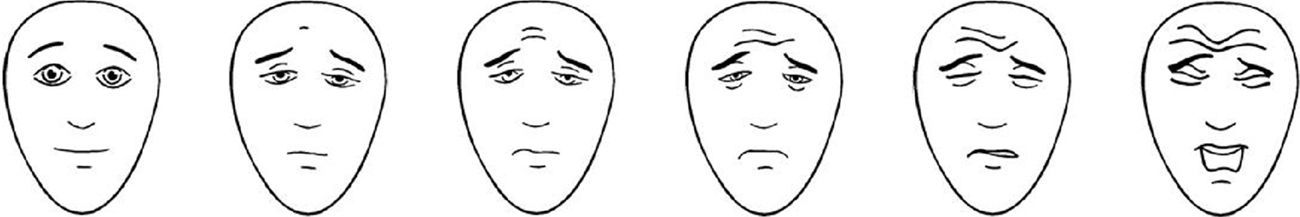
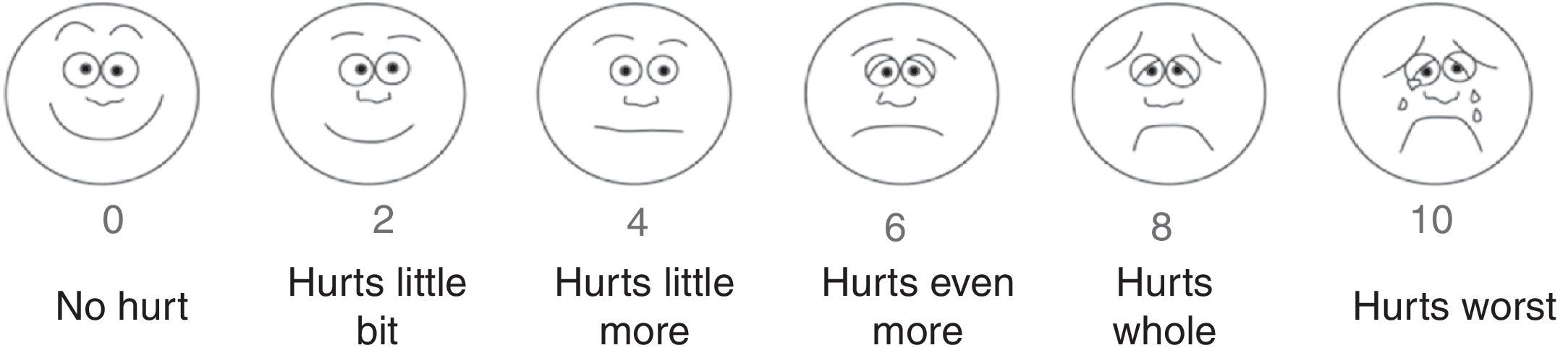
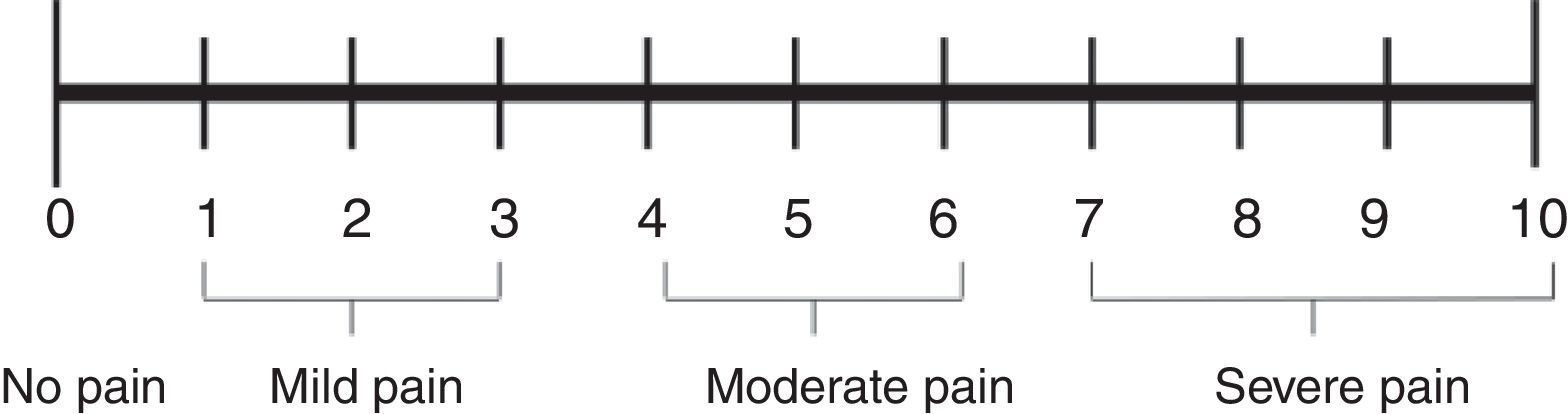
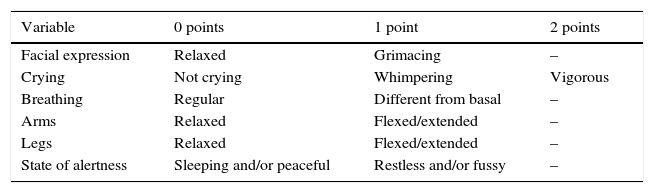
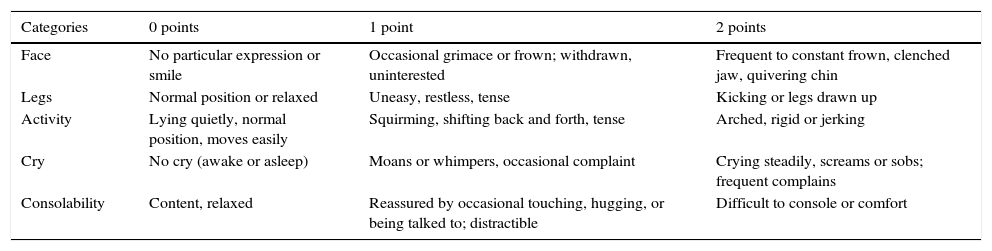
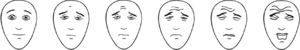
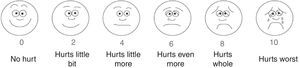
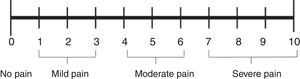
The Neonatal Infant Pain Scale (NIPS) was developed to evaluate the pain response of patients in the neonatal period, assessing six objective parameters (Table 1).15 The Face, Legs, Activity, Cry, and Consolability (FLACC) scale is validated for children between 2 months and 7 years, and scores five reactions to pain on a scale from 0 to 2 (Table 2).16,17 In smaller children, picture-based face are easy to use because they do not require numerical knowledge or certain words (Figs. 1–3).18–20 Children older than 8 years are already cognitively able to use visual analog scales (Fig. 4).21
Neonatal Infant Pain Scale (NIPS).
| Variable | 0 points | 1 point | 2 points |
|---|---|---|---|
| Facial expression | Relaxed | Grimacing | – |
| Crying | Not crying | Whimpering | Vigorous |
| Breathing | Regular | Different from basal | – |
| Arms | Relaxed | Flexed/extended | – |
| Legs | Relaxed | Flexed/extended | – |
| State of alertness | Sleeping and/or peaceful | Restless and/or fussy | – |
Presence of pain: score>3. Adapted from Lawrence et al.15
Face, Legs, Activity, Cry, and Consolability (FLACC) scale.
| Categories | 0 points | 1 point | 2 points |
|---|---|---|---|
| Face | No particular expression or smile | Occasional grimace or frown; withdrawn, uninterested | Frequent to constant frown, clenched jaw, quivering chin |
| Legs | Normal position or relaxed | Uneasy, restless, tense | Kicking or legs drawn up |
| Activity | Lying quietly, normal position, moves easily | Squirming, shifting back and forth, tense | Arched, rigid or jerking |
| Cry | No cry (awake or asleep) | Moans or whimpers, occasional complaint | Crying steadily, screams or sobs; frequent complains |
| Consolability | Content, relaxed | Reassured by occasional touching, hugging, or being talked to; distractible | Difficult to console or comfort |
Adapted from Merkel et al.16
OUCHER scale. Adapted from OUCHER.18 Explain to the child to score that the intensity of the pain increases in the scale from the bottom up and ask her to point to the figure that demonstrates the intensity of pain she is feeling at the moment.
Faces Pain scale – Revised. Adapted from Faces Pain Scale – Revised © 2001, International Association for the Study of Pain (www.iasp-pain.org/FPSR). Used with permission. Explain to the child to score the chosen face as 0, 2, 4, 6, 8, or 10, counting from left to right; 0=no pain and 10=a great deal of pain. Do not use words like “happy” or “sad.” This scale aims to measure how children feel internally and not how they appear to be.
Visual analog scale. Adapted from McCaffery et al.21 Simple numerical scale. Ask the patient to indicate the intensity of the current, the best and the worst level of pain in the last 24h on a scale of 0 (no pain) to 10 (worst pain imaginable).
Sedation and analgesia for procedures should lead to a state of decreased level of consciousness that allows the patient to maintain airway patency independently and continuously. For this purpose, the correct choice of drugs, doses, and forms of administration are important. Younger and severely ill children often require deeper sedation for painful procedures.
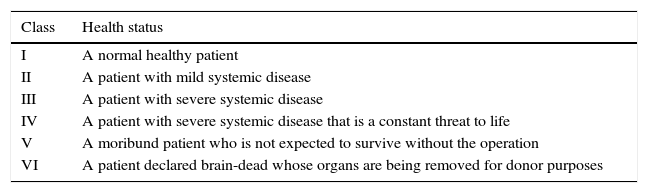
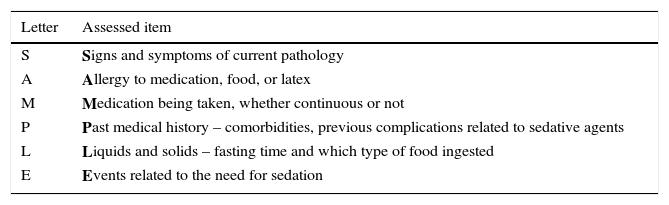
Pre-sedation assessmentThe American Society of Anesthesiologists (ASA) recommends the classification of patients into six categories, according to their baseline health (Table 3). Patients classified as ASA I and II are suitable candidates for minimum, moderate, or deep sedation, whereas ASA III and IV patients, those with special needs or anatomic airway abnormalities, require additional considerations, implying moderate or deep sedation.11 Through the mnemonic acronym “SAMPLE” it is possible to recall the essential components of the patient's medical history that should be considered for sedation assessment (Table 4).
Classification of the patient's baseline health status, according to the American Society of Anesthesiologists.
| Class | Health status |
|---|---|
| I | A normal healthy patient |
| II | A patient with mild systemic disease |
| III | A patient with severe systemic disease |
| IV | A patient with severe systemic disease that is a constant threat to life |
| V | A moribund patient who is not expected to survive without the operation |
| VI | A patient declared brain-dead whose organs are being removed for donor purposes |
Brief and systematic evaluation of the patient submitted to sedation (“SAMPLE”).
| Letter | Assessed item |
|---|---|
| S | Signs and symptoms of current pathology |
| A | Allergy to medication, food, or latex |
| M | Medication being taken, whether continuous or not |
| P | Past medical history – comorbidities, previous complications related to sedative agents |
| L | Liquids and solids – fasting time and which type of food ingested |
| E | Events related to the need for sedation |
Adverse events of the airway, cardiovascular system, and respiratory system are the main causes of morbidity and mortality associated with sedoanalgesia in the pediatric population.22 A meta-analysis (2016) including 41 studies with 13,883 procedures under sedation in children identified vomiting, restlessness, hypoxia, and apnea as the most frequent complications. In that study, the incidence of severe respiratory adverse events (laryngospasm and need for intubation) was less than 0.5% and respiratory depression occurred in 1.5% of the sedations, with no reports of bronchoaspiration; 97% of laryngospasm cases were associated with the use of ketamine.23 Cravero et al. published a multicenter study reporting even lower rates of adverse events, with an incidence of laryngospasm and aspiration of 0.3 and 4.3 per 10,000 cases, respectively.24 A meta-analysis of 9652 adults undergoing sedation for procedures also found an incidence of adverse reactions similar to that observed in the pediatric population.25
Sedative agents have the potential to impair airway protective reflexes, particularly during deep sedation, with the risk of pulmonary aspiration representing one of the reasons to proceed with caution and evaluate the time of fasting before performing a procedure (Table 5). The 2011 ASA recommendations are based on the extrapolation of patients submitted to general anesthesia at the surgical center, not consistent with the reality of sedoanalgesia at the emergency room.26 As of 2014, the American College of Emergency Physicians (ACEP) started to recommend that sedation for procedures should not be delayed according to the time of fasting, because there is no evidence that it is related to a reduction in the risk of aspiration or vomiting.27–29 Clark et al. demonstrated that patients with shorter fasting times submitted to deep sedation for elective procedures outside the surgical room experienced similar complication rates when compared to those with longer fasting duration.30 Despite ACEP's current recommendations, the authors of this article believe that the risk of sedation and the possibility of aspiration should be weighed against the potential benefits of the procedure, since the number of studies in pediatric emergency units is very small.
Adequate fasting time for sedation, according to the American Society of Anesthesiologists.
| Type of food | Fasting time |
|---|---|
| Clear liquidsa | 2h |
| Breast milk | 4h |
| Infant formula and nonhuman milk | 6h |
| Light meal (toast and liquids) | 6h |
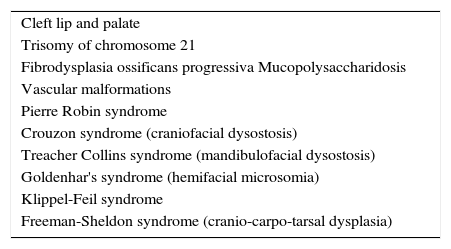
When performing the physical examination, special attention should be given to cardiac, pulmonary, renal, hepatic, and genetic abnormalities that may alter the child's expected response to analgesic and sedative medications.31–34 Some authors have shown an increased risk of adverse events associated with sedation of patients with comorbidities (ASA>1).32–34 Airway examination should be thorough, with an active search for characteristics that increase the risk of airway obstruction during the procedure, such as micrognathia, macroglossia, significant tonsil hypertrophy, limited airway opening, extreme obesity, short neck, excessive secretion, or decreased airway protective reflexes.11 Some genetic and congenital diseases that occur with craniofacial malformations require a more cautious approach due to the difficult airway35 (Table 6).
Genetic and congenital diseases associated with difficult airway.
| Cleft lip and palate |
| Trisomy of chromosome 21 |
| Fibrodysplasia ossificans progressiva Mucopolysaccharidosis |
| Vascular malformations |
| Pierre Robin syndrome |
| Crouzon syndrome (craniofacial dysostosis) |
| Treacher Collins syndrome (mandibulofacial dysostosis) |
| Goldenhar's syndrome (hemifacial microsomia) |
| Klippel-Feil syndrome |
| Freeman-Sheldon syndrome (cranio-carpo-tarsal dysplasia) |
Source: Butler et al.31
In a retrospective study with 11,219 children undergoing general anesthesia procedures with tracheal intubation, laryngoscopy was considered difficult in 1.35% of the cases, with a higher rate in children younger than 1 year of age when compared with those older than 1 year, with an incidence of 4.7% and 0.7%, respectively. A higher risk of difficult laryngoscopy was also identified in patients classified as ASA III and IV, with low body mass index (BMI), submitted to oral cavity, maxillofacial, and cardiac surgery.36 A similar study analyzed a cohort of 102,305 cases of adult patients submitted to general anesthesia and found a difficult laryngoscopy incidence of 4.9%, three-fold that of the general pediatric population.37
Patient vital signs should be recorded prior to the start of sedation, after each medication dose, at regular intervals during the procedure, at the end, during the recovery phase, and at hospital discharge.11 The American Academy of Pediatrics (AAP) recommends that vital signs should be recorded every ten minutes in patients submitted to moderate sedation and every five minutes for those under deep sedation.38
Excluding the minimal sedation, where the observation of the level of consciousness is sufficient, patient monitoring must be continuous and include a pulse oximeter, cardiac, respiratory, and blood pressure monitor.38 Capnography can be used in association with the pulse oximeter, being capable of detecting apnea before the latter; it is recommended in moderate sedation and is mandatory in deep sedation.11,27,38
A meta-analysis from 2011, based on studies with adult patients submitted to sedation for procedures, identified a 17.6-fold greater probability of detecting respiratory depression when monitoring with capnography.39 Langhan et al. randomized 154 children submitted to sedation in the emergency department and found that patients monitored with capnography had earlier interventions in hypoventilation episodes, leading to a lower number of episodes of decreased oxygen saturation in relation to the control group.40
Special care should be taken when covering the face and trunk of the child, since the observation of mucosal color and rib cage movement becomes impaired. In other situations of impaired observation, such as magnetic resonance imaging (MRI), continuous non-invasive monitoring equipment (cardiac monitor, oximeter, capnography) should be used. In addition to adequate sources and routes to supply oxygen and suction material, it is mandatory to always have an emergency cart at hand with a defibrillator, resuscitation drugs, antidotes, and equipment for difficult airways.11
The presence of a trained professional who knows how to recognize airway impairment and intervene to provide ventilatory support is essential.41 The AAP recommends that one professional be present for patient monitoring and another with training in airway management and suctioning, bag-mask ventilation, vascular access, and cardiopulmonary resuscitation, both with advanced life support training in pediatrics.38 A study of the Pediatric Sedation Research Consortium analyzed data from 131,751 cases of pediatric patient sedation for procedures in hospitals in the United States, observing that there was no statistical difference in rates of severe adverse events when sedation was performed by different specialists.42 The training of professionals involved in this type of practice is important to decrease adverse events and promote better patient comfort and safety, and can be carried out using traditional models or through simulations.43–47
One of the periods of greatest risk related to sedation is the recovery phase, so monitoring during this period is mandatory. Patients should be eligible for discharge if they wake up easily, talk and sit unaided, are able to follow age-appropriate commands, are hydrated, and show stable cardiovascular function and patent airways. For very young children or those with some cognitive disorder, with difficulty in interaction, the return to the level of pre-sedation responsiveness must be sought.38 The time of recovery to the baseline state varies with the drug and dose used, but most patients can be discharged after 1–2h. It is recommended that patients not be submitted to activities that require concentration or motor skills in the first hours after recovery from sedation. Caregivers should be instructed to report any adverse events that occur within 24h of discharge.41
Protocols for sedation and analgesia in the emergency room are essential. The implementation of a specific procedure sedation protocol in a Canadian tertiary hospital reduced the median time between sedation administration and discontinuation of patient monitoring from 49 to 19min, releasing important resources in a high-demand emergency department48 (Table 7).
Steps for sedation and analgesia.
| Anamnesis | Physical examination | Monitoring | Discharge criteria |
|---|---|---|---|
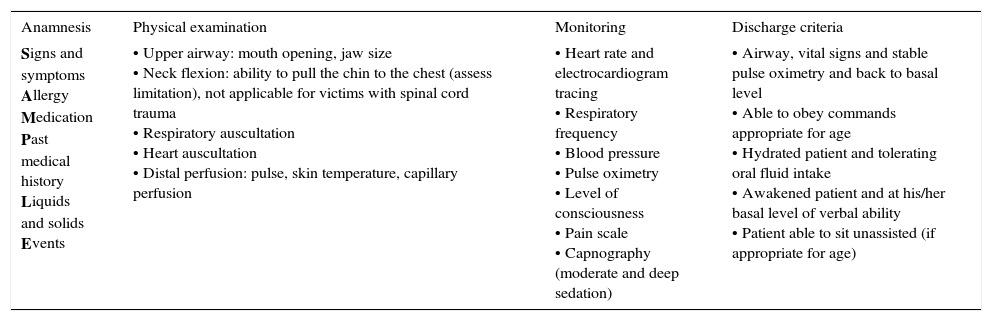
| Signs and symptoms Allergy Medication Past medical history Liquids and solids Events | • Upper airway: mouth opening, jaw size • Neck flexion: ability to pull the chin to the chest (assess limitation), not applicable for victims with spinal cord trauma • Respiratory auscultation • Heart auscultation • Distal perfusion: pulse, skin temperature, capillary perfusion | • Heart rate and electrocardiogram tracing • Respiratory frequency • Blood pressure • Pulse oximetry • Level of consciousness • Pain scale • Capnography (moderate and deep sedation) | • Airway, vital signs and stable pulse oximetry and back to basal level • Able to obey commands appropriate for age • Hydrated patient and tolerating oral fluid intake • Awakened patient and at his/her basal level of verbal ability • Patient able to sit unassisted (if appropriate for age) |
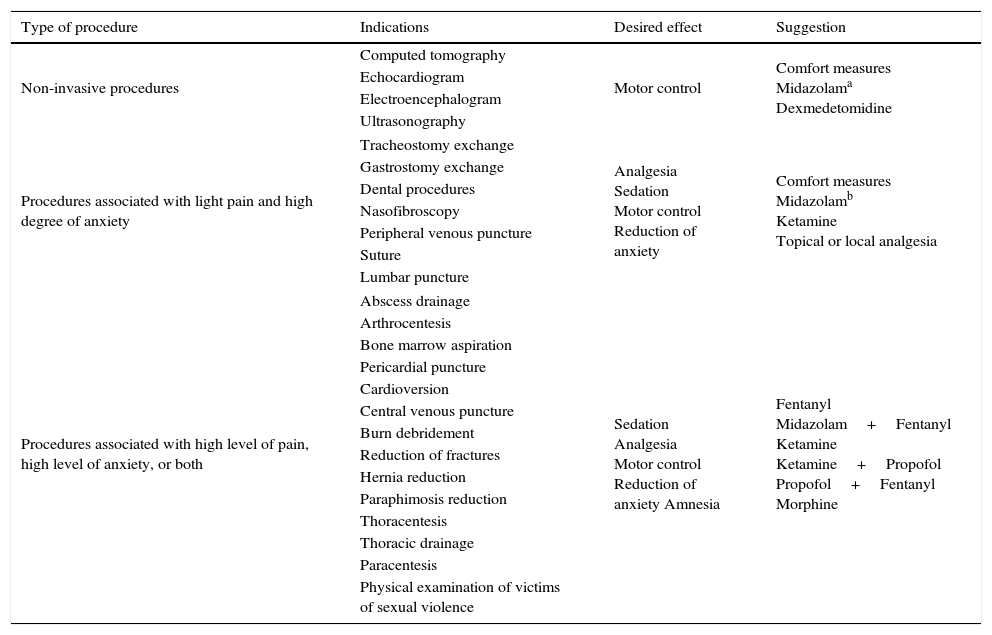
The choice of medication used during the sedation and analgesia process should consider the criteria related to the procedure to which the patient will be submitted, as well as the criteria related to the baseline state and comorbidities. Table 8 shows the suggested medications for different types of procedure. The main drugs used in the pediatric emergency unit will be described below. Table 9, at the end of the chapter, summarizes the different characteristics of each drug.
Sedoanalgesia for emergency procedures.
| Type of procedure | Indications | Desired effect | Suggestion |
|---|---|---|---|
| Non-invasive procedures | Computed tomography | Motor control | Comfort measures Midazolama Dexmedetomidine |
| Echocardiogram | |||
| Electroencephalogram | |||
| Ultrasonography | |||
| Procedures associated with light pain and high degree of anxiety | Tracheostomy exchange | Analgesia Sedation Motor control Reduction of anxiety | Comfort measures Midazolamb Ketamine Topical or local analgesia |
| Gastrostomy exchange | |||
| Dental procedures | |||
| Nasofibroscopy | |||
| Peripheral venous puncture | |||
| Suture | |||
| Lumbar puncture | |||
| Procedures associated with high level of pain, high level of anxiety, or both | Abscess drainage | Sedation Analgesia Motor control Reduction of anxiety Amnesia | Fentanyl Midazolam+Fentanyl Ketamine Ketamine+Propofol Propofol+Fentanyl Morphine |
| Arthrocentesis | |||
| Bone marrow aspiration | |||
| Pericardial puncture | |||
| Cardioversion | |||
| Central venous puncture | |||
| Burn debridement | |||
| Reduction of fractures | |||
| Hernia reduction | |||
| Paraphimosis reduction | |||
| Thoracentesis | |||
| Thoracic drainage | |||
| Paracentesis | |||
| Physical examination of victims of sexual violence | |||
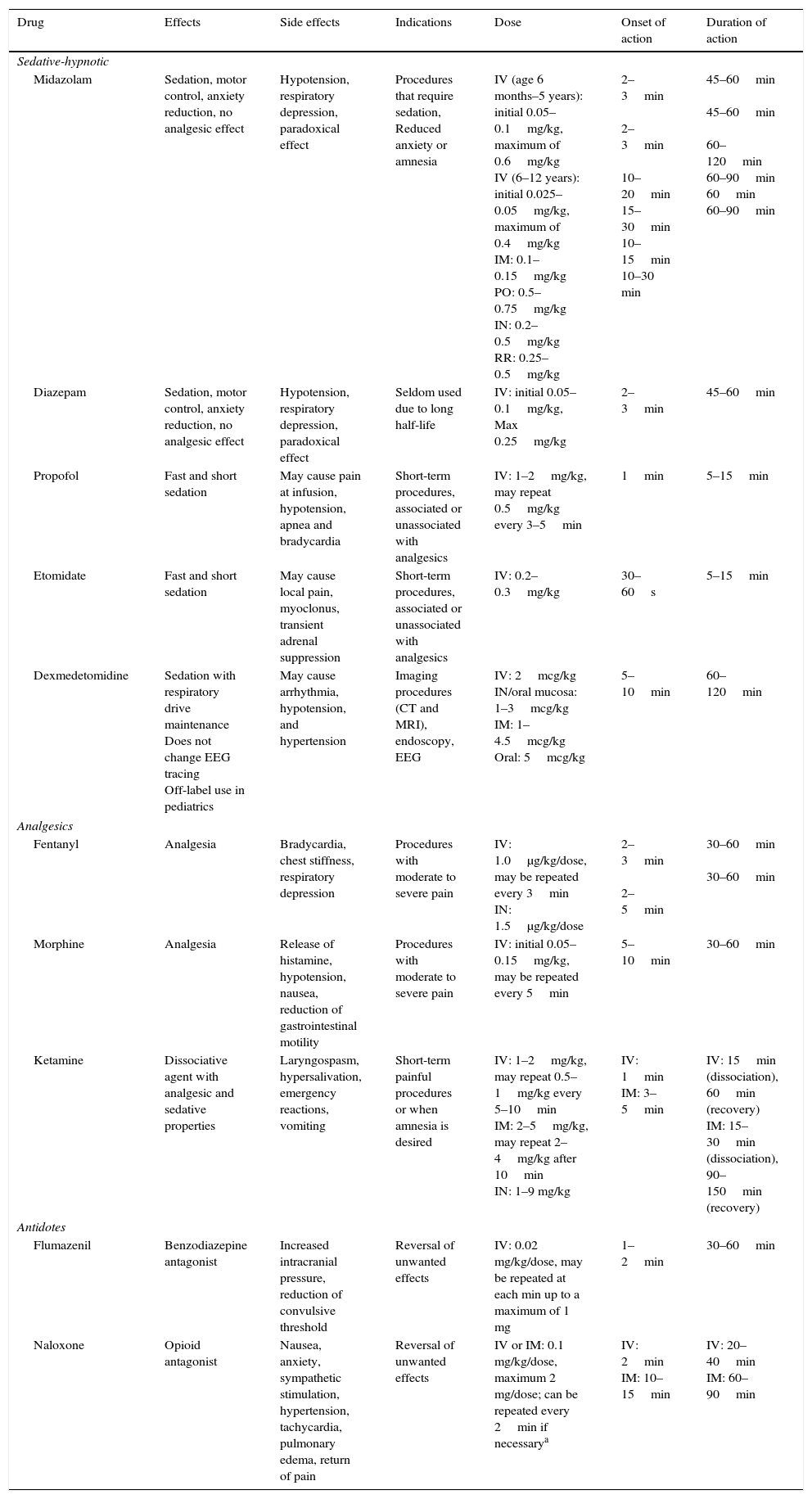
Pharmacological characteristics of drugs used in sedation and analgesia.
| Drug | Effects | Side effects | Indications | Dose | Onset of action | Duration of action |
|---|---|---|---|---|---|---|
| Sedative-hypnotic | ||||||
| Midazolam | Sedation, motor control, anxiety reduction, no analgesic effect | Hypotension, respiratory depression, paradoxical effect | Procedures that require sedation, Reduced anxiety or amnesia | IV (age 6 months–5 years): initial 0.05–0.1mg/kg, maximum of 0.6mg/kg IV (6–12 years): initial 0.025–0.05mg/kg, maximum of 0.4mg/kg IM: 0.1–0.15mg/kg PO: 0.5–0.75mg/kg IN: 0.2–0.5mg/kg RR: 0.25–0.5mg/kg | 2–3min 2–3min 10–20min 15–30min 10–15min 10–30 min | 45–60min 45–60min 60–120min 60–90min 60min 60–90min |
| Diazepam | Sedation, motor control, anxiety reduction, no analgesic effect | Hypotension, respiratory depression, paradoxical effect | Seldom used due to long half-life | IV: initial 0.05–0.1mg/kg, Max 0.25mg/kg | 2–3min | 45–60min |
| Propofol | Fast and short sedation | May cause pain at infusion, hypotension, apnea and bradycardia | Short-term procedures, associated or unassociated with analgesics | IV: 1–2mg/kg, may repeat 0.5mg/kg every 3–5min | 1min | 5–15min |
| Etomidate | Fast and short sedation | May cause local pain, myoclonus, transient adrenal suppression | Short-term procedures, associated or unassociated with analgesics | IV: 0.2–0.3mg/kg | 30–60s | 5–15min |
| Dexmedetomidine | Sedation with respiratory drive maintenance Does not change EEG tracing Off-label use in pediatrics | May cause arrhythmia, hypotension, and hypertension | Imaging procedures (CT and MRI), endoscopy, EEG | IV: 2mcg/kg IN/oral mucosa: 1–3mcg/kg IM: 1–4.5mcg/kg Oral: 5mcg/kg | 5–10min | 60–120min |
| Analgesics | ||||||
| Fentanyl | Analgesia | Bradycardia, chest stiffness, respiratory depression | Procedures with moderate to severe pain | IV: 1.0μg/kg/dose, may be repeated every 3min IN: 1.5μg/kg/dose | 2–3min 2–5min | 30–60min 30–60min |
| Morphine | Analgesia | Release of histamine, hypotension, nausea, reduction of gastrointestinal motility | Procedures with moderate to severe pain | IV: initial 0.05–0.15mg/kg, may be repeated every 5min | 5–10min | 30–60min |
| Ketamine | Dissociative agent with analgesic and sedative properties | Laryngospasm, hypersalivation, emergency reactions, vomiting | Short-term painful procedures or when amnesia is desired | IV: 1–2mg/kg, may repeat 0.5–1mg/kg every 5–10min IM: 2–5mg/kg, may repeat 2–4mg/kg after 10min IN: 1–9 mg/kg | IV: 1min IM: 3–5min | IV: 15min (dissociation), 60min (recovery) IM: 15–30min (dissociation), 90–150min (recovery) |
| Antidotes | ||||||
| Flumazenil | Benzodiazepine antagonist | Increased intracranial pressure, reduction of convulsive threshold | Reversal of unwanted effects | IV: 0.02 mg/kg/dose, may be repeated at each min up to a maximum of 1 mg | 1–2min | 30–60min |
| Naloxone | Opioid antagonist | Nausea, anxiety, sympathetic stimulation, hypertension, tachycardia, pulmonary edema, return of pain | Reversal of unwanted effects | IV or IM: 0.1 mg/kg/dose, maximum 2 mg/dose; can be repeated every 2min if necessarya | IV: 2min IM: 10–15min | IV: 20–40min IM: 60–90min |
IV, intravenous; IM, intramuscular; PO, oral route; IN, intranasal; RR, rectal route.
Benzodiazepines are hypnotic sedative agents. Their mechanism of action occurs through their inhibitory effect on the central nervous system (CNS). They bind to postsynaptic gamma-aminobutyric acid (GABA) receptors and increase the permeability to chlorine ions, leading to hyperpolarization and stabilization of the neuronal membrane. They have hepatic metabolism and renal excretion. Their pharmacological effects are sedation, hypnosis, anxiety reduction, amnesia, muscle relaxation, and anticonvulsant effects. This group does not have an analgesic effect and should be associated with other agents, such as opioids, if they are used in painful procedures.49 The two main drugs of the group used for sedation in procedures are diazepam and midazolam.
Diazepam has been widely used for sedation, but may cause prolonged sedation because it has a long and variable half-life. Its use has been replaced by midazolam, which was introduced to the market due to its varied routes of administration and shorter duration.1 Wright et al., in a prospective, multicenter, randomized study, compared diazepam and midazolam for sedation in emergency department procedures, observing that patients receiving midazolam achieved higher levels of early sedation, higher 90-minute score on the alertness scale, less need for a new dose during the procedure, and less pain during infusion.50
MidazolamMidazolam is the most commonly used intravenous sedative in the emergency department for adults and children. Ilkhanipour et al. carried out a survey in 80 emergency residence programs in the United States and found a 82% rate of institutions using midazolam as the drug of choice for sedation of pediatric patients in the emergency department.51 A study conducted in Brazil by Sukys et al. in a pediatric emergency unit found that midazolam was the sedative of choice in 80% of rapid intubation sequences.52 The rationale for this fact is the rapid action onset, short duration, anterograde amnesia, and a wide variety of administration routes.
Midazolam is metabolized by cytochrome p450; therefore, the first passage effect should be considered depending on the route of administration; additionally, its metabolization may be compromised in the presence of inflammatory processes, hypoxemia, or use of other drugs metabolized by the same route. It has high protein affinity (97%), thus, in the presence of other medications with the same characteristic, there is an increase in the free fraction of circulating midazolam. Its elimination is 80% by the renal route.49 Due to these pharmacological characteristics, it should be administered with caution in patients with hepatopathies and nephropathies. The half-life of midazolam may also be increased in neonates, due to the immaturity of kidney and liver function.53
It can be administered through the oral (PO), rectal (RR), intranasal (IN), intramuscular (IM), and intravenous (IV) routes. The IV presentation (5mg/mL) may be used in any route of administration, but it has an irritant effect on the mucosa.54 The oral presentation has a lower concentration (2mg/mL), being more palatable, which results in a better acceptance by the patients according to specialists.55 Smith et al., in a randomized study with 77 pediatric patients, observed less discomfort when atomized IN lidocaine at 4% was used as a premedication.56 Chiaretti et al., in a study of 46 children submitted to sedation for non-painful procedures, found no discomfort or pain related to the use of IN midazolam when in combination with lidocaine spray as a premedication.57
The main side effects are hypotension, respiratory depression, and paradoxical effect. Midazolam reduces peripheral vascular resistance and the action of the sympathetic nervous system, which decreases the cardiac output, with implications mainly in hypovolemic patients or in those with cyanotic heart diseases. Respiratory depression is dose-dependent. In combination with opioids, there is a greater risk of hypotension and respiratory depression; on the other hand, the paradoxical effect is reduced in this association.1,58
FlumazenilFlumazenil is a central-action benzodiazepine antagonist and its administration is exclusively by the intravenous route. Reversal of benzodiazepine effects is contrary to its appearance during sedation, i.e., small doses of midazolam are necessary to generate an anxiolytic effect, while higher doses of flumazenil are necessary to reverse this effect. In turn, high doses of midazolam are required to induce deep sedation, while small doses of flumazenil reverse this effect. Side effects are elevated intracranial pressure and decreased convulsive threshold, so it should be used with caution in patients taking medications that may induce seizures (tricyclic antidepressants, cocaine, lithium, methylxanthines, isoniazid, monoamine oxidase inhibitors, bupropion, theophylline).59
OpioidsOpioids modulate the cortical perception of pain. They act by binding to central and peripheral μ, δ, and κ receptors, causing cellular hyperpolarization, reducing the release of neurotransmitters. Their main indication is for relief of moderate to severe pain.12,13 Morphine and fentanyl are the most commonly used opioids in clinical practice.
MorphineMorphine is an opioid with a delayed action onset (5–10min) and prolonged duration (120–180min). Delay in the clinical effect occurs because of its relatively low lipid solubility. It undergoes significant first passage metabolism and, therefore, oral doses should be six-fold greater than parenteral doses to achieve the same degree of analgesia.60,61 It is indicated in procedures in which the aim is to maintain analgesia for a longer time, e.g., in fracture fixation. Barcelos et al. compared morphine (0.1mg/kg) and ketamine (2mg/kg), both associated with midazolam (0.2mg/kg), for analgesia in fracture reduction in 25 children in an emergency department, and found no statistical difference regarding failure rate, procedure start time, length of hospital stay, pain scales, or rate of satisfaction of parents and orthopedists.62 Another recurrent use in clinical practice is the relief of acute or chronic intense pain, such as in patients with sickle-cell disease.63
The metabolism of morphine is hepatic and extrahepatic, with the metabolites being excreted in the urine. In patients younger than 6 months, the metabolization mechanisms are immature, and there is evidence of decreased clearance in children with cardiovascular instability.61,64 McRorie et al. observed that children receiving IV morphine after cardiac surgery reached adult morphine clearance values at 6 months of age.65 In another study, Lynn et al. identified adult morphine clearance values in infants around 1–3 months of age.66 The variability of morphine duration is high, which makes it difficult to predict the duration of morphine effects.
Morphine stimulates the release of significant amounts of histamine and inhibits compensatory sympathetic responses. This effect can cause bronchoconstriction and is deleterious in asthmatics. Vasodilation produced by histamine may result in hypotension, especially with rapid infusion administration.67
Important gastrointestinal side effects are also observed with the use of morphine, such as nausea and vomiting that can occur in up to 40% of patients, an effect that tends to decrease with repeated doses of the medication. Another effect, which is common to all opioids, is increased tonus and reduced gastrointestinal motility.67
Discontinuation of morphine infusion is associated with abstinence phenomena. Signs and symptoms include pupillary dilation, tearing, sweating, shivers, hypertension, fever, vomiting, abdominal pain, diarrhea, muscle and joint pain, and behavioral changes.68
FentanylFentanyl is a synthetic opioid with approximately 100 times the analgesic power of morphine. It is highly liposoluble, which explains its rapid action onset. It has a half-life of 2–4h on intermittent administration and 21h after prolonged continuous infusion. This difference occurs due to the saturation of opioid receptors in the lipophilic peripheral tissues.69,70
The metabolism of fentanyl occurs almost exclusively in the liver; it has no active metabolites and a small fraction is excreted unaltered in the urine. Infants and young children have greater clearance than older children and adults, often requiring more frequent doses. Singleton et al. evaluated the plasma concentration of fentanyl in three different age groups and found a higher plasma concentration of fentanyl in adults compared to children, and an even lower concentration in infants.71
Some properties of fentanyl have clinical implications, for instance, changes in blood pH can alter its ionization and distribution between plasma and the CNS, and patients with acidosis may have an increase in the free fraction of fentanyl, putting them at higher risk of toxicity.72
Studies have shown that fentanyl administered by alternative routes is effective in relieving pain. Miner et al., in a randomized clinical trial of 41 children who received 1.5mcg/kg of IV fentanyl or 3mcg/kg of nebulized fentanyl, showed a similar result in the pain improvement score.73 Borland et al., in a study comparing IN fentanyl at a dose of 1.7mg/kg with IV morphine at a dose of 0.1mg/kg, showed similar improvement in pain scores in the two groups of pediatric patients submitted to fracture reduction.74 Similar results were achieved by Young et al. when comparing intranasal fentanyl (1mcg/kg) with IM morphine (0.2mg/kg) in fracture reduction in pediatric patients, and tolerance to administration was better in the IN fentanyl group.75
Fentanyl can cause hemodynamic impairment by bradycardia-induced decreased cardiac output.58 A widely reported adverse effect, although rare, is chest wall stiffness, which is associated with high doses, above 5mcg/kg with bolus administration.60
Respiratory depression is a side effect common to all opioids, and is associated with the administered dose. The incidence of respiratory depression is significantly higher when opioids are used in combination with benzodiazepines. Roback et al. analyzed the occurrence of side effects in 2500 patients who underwent sedation and analgesia in a pediatric emergency department in the United States, and found a rate of respiratory adverse events of 19.3% when there was an association between midazolam and fentanyl, and of 5.8% when midazolam was administered alone.59,76
The potential advantages of fentanyl over morphine for performing the procedure are: faster onset of action, shorter half-life, and avoidance of histamine release, with a low incidence of nausea, vomiting, and generalized pruritus. Additionally, there is no cross-reaction with morphine allergy.77
NaloxoneNaloxone is an opioid receptor antagonist. The most commonly used administration routes are IV and IM, but it can also be administered by the subcutaneous, sublingual, and endotracheal routes. The dose varies according to the desired effect, i.e., partial reduction of the opioid effect (0.01–0.03mg/kg) or complete reversal (0.1–2mg/kg). After its administration, respiration return is observed in one to two minutes, and transient tachypnea may occur. Doses may be repeated every two minutes if the expected effect is not achieved or the reversal has been transient, since the half-life of naloxone is lower than that of opioids.78
Possible side effects are nausea, anxiety, sympathetic stimulation, hypertension, tachycardia, pulmonary edema, and return of pain. At a low dose, naloxone seems to reduce the nausea caused by opioids. A meta-analysis carried out by Barrons and Woods concluded that the use of naloxone reduces the occurrence of postoperative nausea without significantly increasing the need for extra doses of opioids and without increasing pain scores; however, there was no decrease in the occurrence of vomiting.79
KetamineKetamine is a dissociative anesthetic agent that acts on the N-methyl-d-aspartate-glutamate receptor, disconnecting the limbic and thalamocortical systems, dissociating the central nervous system from external stimuli. The cataleptic state allows potent analgesia, sedation, and amnesia, while maintaining airway patency, protective stimuli, and cardiovascular stability.10
It is widely used in painful short-term procedures or in those in which amnesia is desired, such as for the physical examination of patients that are victims of abuse.80 It is not recommended for sedation in computed tomography (CT) of the skull or MRI, because the dissociative state can produce inappropriate movements, resulting in poorer image quality. It has hepatic metabolism and predominantly urinary excretion (91%).10
It is typically administered intravenously or intramuscularly, and may be administered by the intranasal and oral routes. The IV route allows faster recovery and less time until discharge, while the IM route is an independent predictor of emesis.81,82 In a randomized study, the administration of higher doses of ketamine (1.5 and 2mg/kg) compared to a lower dose (1mg/kg) decreased the need for new doses to achieve adequate sedation (2.9% and 5% vs. 16%), with no increase in the risk of adverse effects (14.3% and 10% vs. 10%) or the duration of sedation in minutes (24.5min and 23min vs. 23min).83
Ketamine can cause transient laryngospasm, as well as apnea, hypersalivation, vomiting, and restlessness in recovery. It inhibits the reuptake of catecholamines, resulting in a sympathomimetic effect, which causes an increase in blood pressure, heart rate, and cardiac output. A prospective study of 11 adult patients with ischemic heart disease showed a reduction in left ventricular systolic and diastolic function.84 Reduction of systolic pressure secondary to decreased ejection fraction after ketamine use was demonstrated in a small group of patients in the pediatric age group.85 The catecholaminergic effects mask myocardial depression. Further studies are needed to clarify the hemodynamic effects of this drug.
The post-sedation emergency phenomenon with ketamine, rare in pediatrics, can manifest itself through crying, lucid dreaming, hallucinations, and, more rarely, severe delirium. It is more common in adolescents when the drug is administered intramuscularly or at high doses.86,87 There is no evidence that an infusion of midazolam as premedication to ketamine reduces the incidence of emergency phenomena. Wathen et al. found similar rates of emergence phenomena in pediatric patients receiving ketamine alone (7.1%) and in those receiving it in association with midazolam (6.2%).88 Also, another study published by Sherwin et al. did not demonstrate any additional benefit of midazolam to prevent emergency phenomena.89
Ketamine is contraindicated in patients younger than 3 months due to the risk of airway complications, and in schizophrenic patients, due to the risk of psychotic stimulation.90 Risk factors for adverse airway and breathing events are high intravenous doses, age less than 2 years or greater than 13 years, and co-administration of anticholinergics and benzodiazepines.91 The associated use of atropine may reduce hypersalivation, but does not reduce the frequency of adverse events.92 Two randomized studies have demonstrated the reduction of hypersalivation with the prophylactic use of atropine, with no positive impact on the rate of adverse events.93,94
The use of ketamine should be avoided in patients with heart disease, with active pulmonary pathologies, or with central nervous system abnormalities. Currently, there is no contraindication to its use in traumatic brain injury, as there is no increase in the risk of intracranial hypertension or neurological complications, even in severe cases.95–98 A systematic review showed no significant difference in cerebral perfusion pressure, neurological outcome, length of stay in the intensive care unit (ICU), or mortality when the use of ketamine was compared with other sedatives.99 However, the relative contra-indication still remains in patients with intracranial masses, intracranial anatomical alterations, and hydrocephalus.100
PropofolPropofol is a hypnotic sedative agent with fast and short-acting anesthetic properties, corresponding to 2,6-diisopropylphenol, which exerts its hypnotic action through the activation of GABA, a central inhibitory neurotransmitter.101,102 It can be used in brief procedures, associated or unassociated with analgesic agents, such as fentanyl and ketamine.103,104
For longer procedures, such as in MRI sedation, propofol can be used under continuous infusion.105 Sebe et al. published a prospective pediatric study with a cohort of 200 pediatric patients undergoing imaging examinations, in which propofol was more effective than midazolam in terms of sedation effectiveness and shorter recovery time, with no statistical difference in the rate of adverse events between the drugs.106 Studies comparing propofol, pentobarbital, and dexmedetomidine in MRI sedation were also favorable to the first drug.107–109 More complex and time-consuming imaging studies can be performed effectively with continuous infusion, without increasing recovery time or risk of adverse effects.110
The metabolism of propofol is hepatic, the elimination is biphasic (with the initial phase at 40min and terminal phase at 4–7h), and excretion is mostly urinary (88%). Administration is exclusively IV, usually associated with pain during infusion, an effect that can be reduced with pre-treatment of the vein with lidocaine, i.e., previous administration of small doses of opioid or ketamine or infusion into large-caliber veins.111
Propofol has several cardiovascular effects, with hypotension being the most significant.112 This is due to arterial vasodilation through the reduction of sympathetic tonus, but also because it affects myocardial contractility and cardiac output.113 These effects may be exacerbated in hypovolemic patients, or patients with pre-existing heart disease or the concomitant use of other cardiac depressant drugs. Bolus administration of saline 0.9% at 20mL/kg prior to propofol infusion did not reduce the risk of hypotension in pediatric patients when compared to the control group in a randomized study published by Jager et al.114 The decrease in the systemic vascular resistance induced by the drug may be harmful in patients with congenital heart disease; however, the heart rate does not change significantly. Apnea and airway obstruction is frequent, even at usual doses, since propofol depresses the CNS, which can reduce respiratory rate and pulmonary volume.115 The risk of respiratory depression increases proportionally to the infusion velocity as well as the risk of bradycardia, and hypotension is associated with higher doses.116,117 It is contraindicated in patients with allergy to eggs, soybeans, and their derivatives.118
Despite the adverse effects, propofol is safe and effective when performed with adequate monitoring. Mallory et al., in a retrospective study that analyzed 25,433 episodes of sedation with propofol, showed a severe adverse event rate, such as airway obstruction, apnea, and oxygen saturation drop in 2.2%, with no associated death reports related to this drug.104 Chiaretti et al. reviewed 36,156 procedures and obtained an even lower complication rate of 0.75%.119
Propofol infusion syndrome is defined as refractory acute bradycardia progressing to asystole, metabolic acidosis, rhabdomyolysis, hyperlipidemia, and hepatic steatosis. It is described in severely-ill pediatric patients receiving this medication for a prolonged period of time (>48h) and at high doses (>4mcg/kg/min).120
KetofolThe use of ketamine and propofol in association, known as “ketofol,” has become popular due to the possibility of counterbalancing the side effects of each medication and enhanced sedation, increasing efficacy and safety.121 Ketamine maintains the respiratory drive, prevents hypotension and bradycardia, and provides analgesia, while propofol reduces the incidence of nausea and vomiting, in addition to hypothetically prevents emergency reactions.122
When compared to propofol as a single drug, the use of ketofol in pediatric patients resulted in fewer respiratory adverse events, hypotension, and bradycardia, as well as fewer additional doses of the drug.123 A meta-analysis of 932 patients showed fewer respiratory adverse events with the ketofol group compared to propofol (29% vs. 35.4%), with no significant difference in the proportion of general adverse events (38.8% vs. 42.5%).124 Another meta-analysis by Jalili et al. demonstrated that ketofol significantly reduced respiratory and cardiovascular complications (hypotension and bradycardia).125
There is no consensus regarding which dose of each drug should be used when in combination, with the suggestion of an initial dose of 0.5mg/kg of propofol and 1mg/kg of ketamine. No difference in the quality of sedation or safety profile was observed between mixtures at the proportions of 1:1 and 1:4 of ketamine and propofol in adults.126 Further studies on the use of ketofol in pediatric patients are required to elucidate its advantages and disadvantages as a sedative agent.127
DexmedetomidineDexmedetomidine (DEX) is an alpha-2 adrenergic agonist with an action that is not mediated by GABA, which promotes sedation without decreasing the respiratory drive.128 Despite its off-label use in pediatrics, its use has been increasingly used for short procedures and also for situations requiring prolonged sedation.129 It can be used as a single agent or in combination with midazolam, ketamine, or opioids.130,131 In the emergency department, it is mainly used for imaging studies.132,133 Due to its unique pharmacological characteristics, DEX is used to induce an electroencephalogram (EEG) pattern similar to that of natural sleep.134,135 It is an effective alternative to midazolam in sedation during upper digestive endoscopy, with better sedation potential and fewer adverse effects.136
It has hepatic metabolism and urinary excretion (95%), with IV, IN, oral, and oral gastric mucosa routes, with the latter showing less bioavailability.128 It can cause hypotension, bradycardia, and sinus arrhythmia.130,137 Due to its pharmacological characteristics and its clinical applications, dexmedetomidine appears to be a good alternative to the use of chloral hydrate in pediatric emergency departments after the latter's recent discontinuation of use in Brazil.
Although it has not been approved by the Food and Drug Administration for pediatric use due to lack of data demonstrating its safety profile, studies have already shown that it is a safe sedative, especially in the context of adult and pediatric ICUs.138 Constantin et al. published a meta-analysis of 1994 adult ICU patients, showing a reduction in hospitalization time, mechanical ventilation time, and delirium occurrence in 48h, but an increase in cases of bradycardia and hypotension was observed.139 Berkenbosch et al., in a prospective pediatric study, demonstrated the efficacy and safety of dexmedetomidine for noninvasive procedures.140
EtomidateEtomidate is an imidazole derivative used as an ultra-fast acting sedative-hypnotic agent that binds to GABA receptors in the central nervous system. Commonly used in rapid intubation sequences in children, it may be used in non-painful short-term procedures, such as skull CT, and in painful procedures associated with an analgesic drug. It has few hemodynamic repercussions, and respiratory effects are rare.141,142
It is a highly lipophilic drug, with hepatic metabolism and urinary excretion (75%). It is administered exclusively intravenously, and may be irritating at the infusion site; it is preferable to administer it through larger caliber veins or in combination with lidocaine.141 It can cause myoclonus (without EEG repercussions), nausea, vomiting, apnea, and hypoventilation.142,143 Di Liddo et al. compared etomidate and midazolam in the sedation of pediatric patients submitted to fracture reduction and showed a higher proportion of adequately sedated patients in the etomidate group (92% vs. 36%), with a shorter time of induction and recovery.144
As etomidate inhibits the 11-beta-hydroxylase enzyme, which participates in the production of adrenal hormones, a single dose can suppress the stress-induced cortisol production response and may last 6–8h.145,146 Thus, the use of this drug in septic or critically-ill patients, even in a single dose, remains controversial.145 Nonetheless, its use in healthy patients appears to be well tolerated.147 Two prospective randomized studies in adults did not demonstrate increased morbidity and mortality or hospital length of stay when etomidate was administered by bolus in the rapid intubation sequence.148
ConclusionPainful diagnostic and therapeutic procedures that frequently require patient collaboration are common in the practice of pediatric emergency. Indication of analgesia and sedation for such procedures should occur after a careful patient assessment, considering the purpose, risks, and benefits associated with the procedure and the use of medications. The use of protocols for this purpose should guide the professional in the choice of medication, the appropriate material, and evaluation of the discharge criteria, thus ensuring quality in care. Analgesia and sedation for procedures requires training of health teams, aiming at safety and effectiveness. Studies have shown the best pharmacological choices for certain procedures, considering the age and health status of each patient, making sedoanalgesia an increasingly common and safer practice in the pediatric emergency room.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Ramalho CE, Bretas PM, Schvartsman C, Reis AG. Sedation and analgesia for procedures in the pediatric emergency room. J Pediatr (Rio J). 2017;93:2–18.
Study carried out at Universidade de São Paulo (USP), Faculdade de Medicina, Hospital das Clínicas, Instituto da Criança, São Paulo, SP, Brazil.