To describe the role of intravenous magnesium sulfate (MgSO4) as therapy for acute severe asthma in the pediatric emergency department (ED).
SourcePublications were searched in the PubMed and Cochrane databases using the following keywords: magnesium AND asthma AND children AND clinical trial. A total of 53 publications were retrieved using this criteria. References of relevant articles were also screened. The authors included the summary of relevant publications where intravenous magnesium sulfate was studied in children (age <18 years) with acute asthma. The NAEPP and Global Initiative for Asthma expert panel guidelines were also reviewed.
Summary of the dataThere is a large variability in the ED practices on the intravenous administration of MgSO4 for severe asthma. The pharmacokinetics of MgSO4 is often not taken into account with a consequent impact in its pharmacodynamics properties. The cumulative evidence points to the effectiveness of intravenous MgSO4 in preventing hospitalization, if utilized in a timely manner and at an appropriate dosage (50–75mg/kg). For every five children treated in the ED, one hospital admission could be prevented. Another administration modality is a high-dose continuous magnesium sulfate infusion (HDMI) as 50mg/kg/h/4h (200mg/kg/4h). The early utilization of HDMI for non-infectious mediated asthma may be superior to a MgSO4 bolus in avoiding admissions and expediting discharges from the ED. HDMI appears to be cost-effective if applied early to a selected population. Intravenous MgSO4 has a similar safety profile than other asthma therapies.
ConclusionsTreatment with intravenous MgSO4 reduces the odds of hospital admissions. The use of intravenous MgSO4 in the emergency room was not associated with significant side effects or harm. The authors emphasize the role of MgSO4 as an adjunctive therapy, while corticosteroids and beta agonist remain the primary acute therapeutic agents.
Descrever o papel do sulfato de magnésio intravenoso (MgSO4) como terapia para asma grave aguda no serviço de emergência pediátrica (SE).
FonteAs publicações foram pesquisadas no banco de dados PubMed e Cochrane utilizando as seguintes palavras-chave: magnésio E asma E crianças E ensaio clínico. Foi encontrado um total de 53 publicações utilizando esses critérios. As referências de artigos relevantes também foram examinadas. Incluímos o resumo de publicações relevantes quando o sulfato de magnésio intravenoso foi estudado em crianças (idade<18 anos) com asma aguda. Revisamos também as diretrizes do Programa Nacional para a Educação e Prevenção da Asma (NAEPP) e do painel de especialistas da Iniciativa Global para Asma.
Resumo dos dadosHá uma grande variabilidade nas práticas do SE na administração intravenosa do MgSO4 para asma grave. A farmacocinética do MgSO4 normalmente não leva em conta um impacto posterior em suas propriedades farmacodinâmicas. A comprovação cumulativa aponta para a eficácia do MgSO4 intravenoso na prevenção da internação, se utilizado quando necessário e em uma dosagem adequada (50-75mg/kg). Uma internação hospitalar pode ser evitada para cada cinco crianças tratadas no SE. Outra modalidade de administração é a infusão prolongada de alta dose de sulfato de magnésio (HDMI) a 50mg/kg/hora/4 horas (200mg/kg/4 horas). O uso precoce da HDMI, para asma não infecciosa mediada, pode ser superior a um MgSO4 em bolus para evitar internações e antecipar as altas do SE. A HDMI parece ter bom custo-benefício se aplicada precocemente em uma população selecionada. O MgSO4 intravenoso possui um perfil de segurança semelhante a outras terapias de asma.
ConclusõesO tratamento com MgSO4 intravenoso reduz as chances de internações hospitalares. O uso de MgSO4 intravenoso no pronto socorro não é associado a efeitos colaterais ou danos significativos. Enfatizamos o papel do MgSO4 como uma terapia adjuvante, ao passo que os corticosteroides e as beta-agonistas continuam os agentes terapêuticos agudos primários.
Asthma is a reversible, diffuse lower airway obstruction caused by airway inflammation and edema, bronchial smooth-muscle spasm, and mucous plugging. The composite effect leads to expiratory airflow obstruction.1 Asthma could be life-threatening and must be promptly treated. Severe asthma is often defined as failure to improve after 2h of conventional emergency department (ED) treatment, and commonly present with moderate hypoxemia. The presence of hypoxemia should be assessed non-invasively with a pulse oximeter. Blood gas, serological or radiological studies are not necessary to define or determine its severity.
Perspective on a health challengeAsthma is the leading cause of chronic illness in children; 19–24% of Brazilian children have been diagnosed with asthma at some time in their lives.2 It is the third leading cause of hospitalizations among children under the age of 15 years. Severe asthma is one of the most common severe, reversible conditions in EDs.1,2 While asthma-related mortality may be improving, one-third of the deaths occurred before medical attention was provided.3 ED management to revers the progression toward respiratory failure should be structured and aggressive, as invasive mechanical ventilation is fraught with many complications and an elevated mortality.4 Due to the enormous health care burden of asthma, all medical treatments need to be scrutinized regarding their cost-effectiveness.
PathophysiologyAsthma involves a complex inflammatory cascade. There is an antigen-mediated activation of epithelial cells and infiltration of the airways by circulating cells releasing soluble transmitters that intensify the inflammatory cascade. The immediate response is bronchospasm (smooth muscle contraction). The continued release of inflammatory mediators leads to airway edema, mucosal injury, and desquamation of the protective epithelium layer. Airway denudation decreases the production of normal mucus and exposes the terminal nerves to excessive cholinergic stimulation, exacerbating smooth muscle contraction.5
The progression of this physiopathology results in widespread lung heterogeneity with severe bronchoconstriction. Lung areas with mucus plugging and atelectasis alternate with areas of hyperinflation due to air trapping. The combined effects of the aforementioned processes lead to ventilation perfusion mismatch (V/Q mismatch), with the clinical expression of hypoxemia. Air trapping puts the diaphragm in a disadvantageous position, losing its area of apposition and producing an ineffective effort. The respiratory work load increases dramatically, and inspiratory substernal retractions are observed, progressing to a paradoxical thoraco-abdominal breathing pattern. In severe cases, the cardiac output is compromised, with a combination of dehydration, increased pulmonary venous pressure creating a dynamic decreased venous return to the right atrium, and a shift of the intraventricular septum, impinging the left ventricle preload.
Clinical presentationThe majority of severe asthma exacerbations occur after an exposure to allergic triggers or in the setting of a viral upper respiratory infection. Most children present with cough, wheezing, prolonged expiratory phase, and increased work of breathing while under mild hypoxemic conditions and dehydration. The degree of wheezing does not correlate well with severity of the disease. Clinical asthma scoring systems, such as the Woods score, lack granularity, but are helpful in patient follow-up.6 This and other clinical scores express categorical variables (mild/moderate/severe) as a number,1–3 which facilitates the trending of acuity on a single patient; nonetheless, any statistical analysis of these results should be performed as categorical variable. Peak expiratory flow rate (PEFR), in cooperative previously-trained patients, provides a more granular assessment. However, it is an effort-dependent technique and difficult to perform while in respiratory distress, unless the investigators are previously trained to perform spirometry testing.7 The presence of pulsus paradoxus denotes severity, but it is difficult to be repeatedly assessed in a busy ED.
Initial ED managementAn organized and resolute ED initial management is needed, due to the compounded facts that severe asthma is: (a) a condition with a high incidence, (b) has a potential for reversibility, (c) has the risk to progress toward respiratory failure, and (d) the ED needs to judiciously manage hospital admission. The primary goal is to stabilize patients and rapidly identify those in whom the process is not rapidly reversible or who are at a high risk of deterioration.
The initial treatments include oxygen, intravenous fluids, intravenous or oral corticosteroid, repeated or continuous nebulization of a β2 adrenergic (i.e., salbutamol), nebulized muscarinic anticholinergic (i.e., ipratropium), and intravenous MgSO4.
Failure to improve after the aforementioned regimen, assessed as persistence of respiratory distress upon clinical exam, is defined as severe asthma (or status asthmaticus).
Methylxanthines and subcutaneous or intravenous β-agonists are not routinely utilized as a first line therapy in the United States. However, a study from Porto Alegre that assessed the effects of intravenous salbutamol in ED, observed a decrease in the β2 adrenergic nebulization requirements subsequent to the patients’ hospital admission.8 That study only addressed changes in respiratory rate and did not control for alterations in other clinical findings. It also did not state whether vital signs monitoring was performed in a blinded fashion. Heliox may improve the aerosol delivery of β2 adrenergic to the lower airway; nonetheless, it is expensive and does not appear to offer a consistent and significant clinical benefit.9,10 and cost-effective studies are required. BIPAP support in the ED appears to stabilize patients with status asthmaticus before the hospital admission; however, the cumulative data (two publications) is scarce to recommend it as standard therapy.11,12
In turn, the use of intravenous MgSO4 has emerged as a proven strategy to reduce hospital admissions. This study aimed to review the different regimens for MgSO4 administration and its contribution in the management of severe asthma.
MgSO4 mechanism of action and kineticsThe primary mechanism of action of intravenous MgSO4 is thought to be secondary to its spasmolytic properties. Supra-physiologic unbound serum magnesium (Mg), directly related to ionized Mg, produces a transient block of the N-methyl-d-aspartate receptor-gated calcium channels with subsequent muscle relaxation. Blocking the Ca entry into the airway smooth muscle interferes with smooth muscle contraction, inducing bronchodilation.13–15 While other mechanisms modulating the inflammatory reaction, such the attenuation of the neutrophil respiratory burst, have putative beneficial effects, their degree of contribution in the therapeutic management of acute asthma is less clear.16 The Mg2+ ion, due to its effects on Ca, also inhibits the release of acetylcholine from motor nerve terminals, inhibiting histamine release from mast cells and decreasing the production of mucus in the secretory glands.17
Intravenous MgSO4 has a rapid onset of action and, similarly, a rapid renal elimination. This presents a therapeutic challenge and an opportunity. Achieving sustained spasmolytic effects is difficult, as renal tubular reabsorption of Mg is at maximal capacity with normal serum levels and renal clearance rises linearly with higher concentrations.18 Therefore, the maximum serum level during therapy depends more on the rate of infusion rather than on the total dose or duration of the infusion. In children, a retrospective study described that the MgSO4 distribution volume was 0.3L/kg, with a half-life of 2–2.7h.19 Often, the bolus dose of intravenous MgSO4 has been limited to 2000mg, regardless of patient size and renal function. This practice is contradictory with a pharmacokinetic rationale and affects its pharmacodynamic properties.
Experience in the use of intravenous MgSO4 in asthmatic childrenMgSO4 is inexpensive, has minimal adverse effects at the doses indicated, and is widely available. The onset of action of intravenous MgSO4 is rapid (within minutes), a necessity in emergency settings. Since its original description in 1936, the optimal dose of IV MgSO4 as a bolus has not been established, leading to the utilization of a wide dose range, from 25 to >100mg/kg.18–22 Multicenter studies have failed to demonstrate a consistent decrease in hospital admissions or early discharge.23–25
These inconsistent results could be in part due to: (a) failure to take into consideration the MgSO4 pharmacokinetics, (b) failure to conceptualize MgSO4 as a time-sensitive therapy, (c) the inherent challenges of the aforementioned “outcome variables” in asthma, or (d) enrollment of individuals with current infectious process, with ongoing stimuli for bronchoconstriction and damage to the airway.
Some clinical studies indicate the need for higher-dose regimens.22,26 Ciarallo et al. reported positive results in two clinical trials, separated by a period of four years, where the dose was increased from 25mg/kg to 40mg/kg, administered over 20min.22 A retrospective pharmacokinetic study involving 54 children suggested the need for 50–75mg/kg bolus to attain a Mg level near 4mg/dL (1.64mmol/L).19 In a study conducted in India with 47 children, Devi et al. used 100mg/kg over 35min with a co-administration of intravenous aminophylline. The results show an improvement in clinical and PEFR scores; the graphic display implied beneficial effects in oxygenation starting in the first few hours and continuing for 10–12h.27 Nevertheless, the co-administration of aminophylline in this study leads to doubts about whether MgSO4 alone caused this effect.
The prompt initiation of therapy may be correlated with its efficacy. A study performed in Argentina indicated that early administration in the ED was associated with fewer patients, later on, requiring mechanical ventilation in a pediatric intensive care unit (PICU).25 Of note, the control group comprised younger subjects and may have included infants with bronchiolitis. A large randomized clinical trial with 100 patients in India, using a modified asthma clinical severity score, demonstrated the superiority of an early intravenous MgSO4 bolus over terbutaline or aminophylline infusions20; of note, many of these patients were very young and infection-mediated asthma was not identified. Another randomized trial in a Brazilian ED demonstrated the superiority of intravenous MgSO4 over placebo, with almost identical effects to intravenous Salbutamol while using surrogate variables of efficacy in patients that were later hospitalized.8 An earlier meta-analysis conducted by Cheuk et al. including five trials that assessed MgSO4versus placebo demonstrated MgSO4 effectiveness in preventing hospitalization.28 A more recent and stricter meta-analysis involving three trials (115 children) concluded that the true estimated reduction in admission was between 86% and 26%, due to the wide confidence interval (odd ratio: 0.32, 95% CI: 0.14–0.74). Nevertheless, an number needed to treat (NNT) of 5 could be ascribed to the use of intravenous MgSO4 in the ED to prevent one hospital admission.29
The need to return to the ED after discharge has not been well documented. One study with 47 children reports a reduced length of stay of 5.3h on patients who were admitted.28 This is an elusive variable as, once the patient is admitted, discharge may not be solely dependent on the patient's condition but rather on the availability of medical personnel, time of the day, and day of the week.
High-dose MgSO4 continuous infusion (HDMI)Much of the magnesium in serum is attached to albumin, while ionized Mg (IoMg; the free form) is the pharmacologically active form in asthma. IoMg makes up 55% of extracellular Mg; however, the relation of Mg/IoMg is adversely altered in asthma and critically ill patients.24,25,27 Animal studies indicate that IoMg concentrations ≥1mmol/L are required to produce smooth muscle relaxation.15
These and other factors lead the authors to study high-dose MgSO4 continuous infusion (HDMI) in children in the setting of severe asthma and status asthmaticus. HDMI has been used in patients with pulmonary hypertension, brain injury, and subarachnoid hemorrhage, as well as extensively in preeclampsia.30–33 In these scenarios, the strategy is to maintain a consistent therapeutic level to compensate for MgSO4's rapid elimination. This approach has been rarely adopted in asthma cases.28,34 The obstetrics and gynecology literature targets Mg infusion to clinical signs of weakening, but not losing, patellar reflexes as reflection of adequate spasmolysis. This usually represents serum Mg of 4.8–8.4mg/dL with IoMg 0.9–1.6mmol/L.15,35,36
A retrospective study by Glover et al. on continuous intravenous MgSO4 use in children attempted to assess safety, but had many confounding variables. Those authors described a heterogeneous group with a large variation in dosage and regimen duration, bolus of 35.3±12.7mg/kg, infusion of 21.6±6mg/kg/h for 93.8h±89.2h, without significant side effects.26
In this practice, the present authors retrospectively analyzed the use of HDMI in the setting of status asthmaticus within the confines of the pediatric intensive care unit (PICU).37 The HDMI regimens consisted of an initial bolus that was weight-dependent: 50mg/kg (>30kg) or 75mg/kg (≤30kg) over a period of 30–45min; followed by a continuous infusion of 50mg/kg/h, for 4h. Serum magnesium levels were 4.4±0.8mg/dL, and IoMg 0.95±0.2mmol/L at the end of the infusion, within the target range. In 12 patients, troponin levels and electrocardiograms were all normal.37
In a following prospective study, the authors determined the HDMI pharmacokinetics VD of 0.4±0.13L/kg, clearance of 1.58±0.24mL/kg/min, and safety, documented levels of IoMg associated with smooth muscle relaxation and the absence of significant side effects.38 This regimen was pharmacologically precise, but complex, and lead to errors in administration. After trying several regimens, a simplified regimen of 50mg/kg/h for 4h was adopted. In a subsequent study, the authors compared the initial and the simplified regimen, demonstrating similar serum levels.39
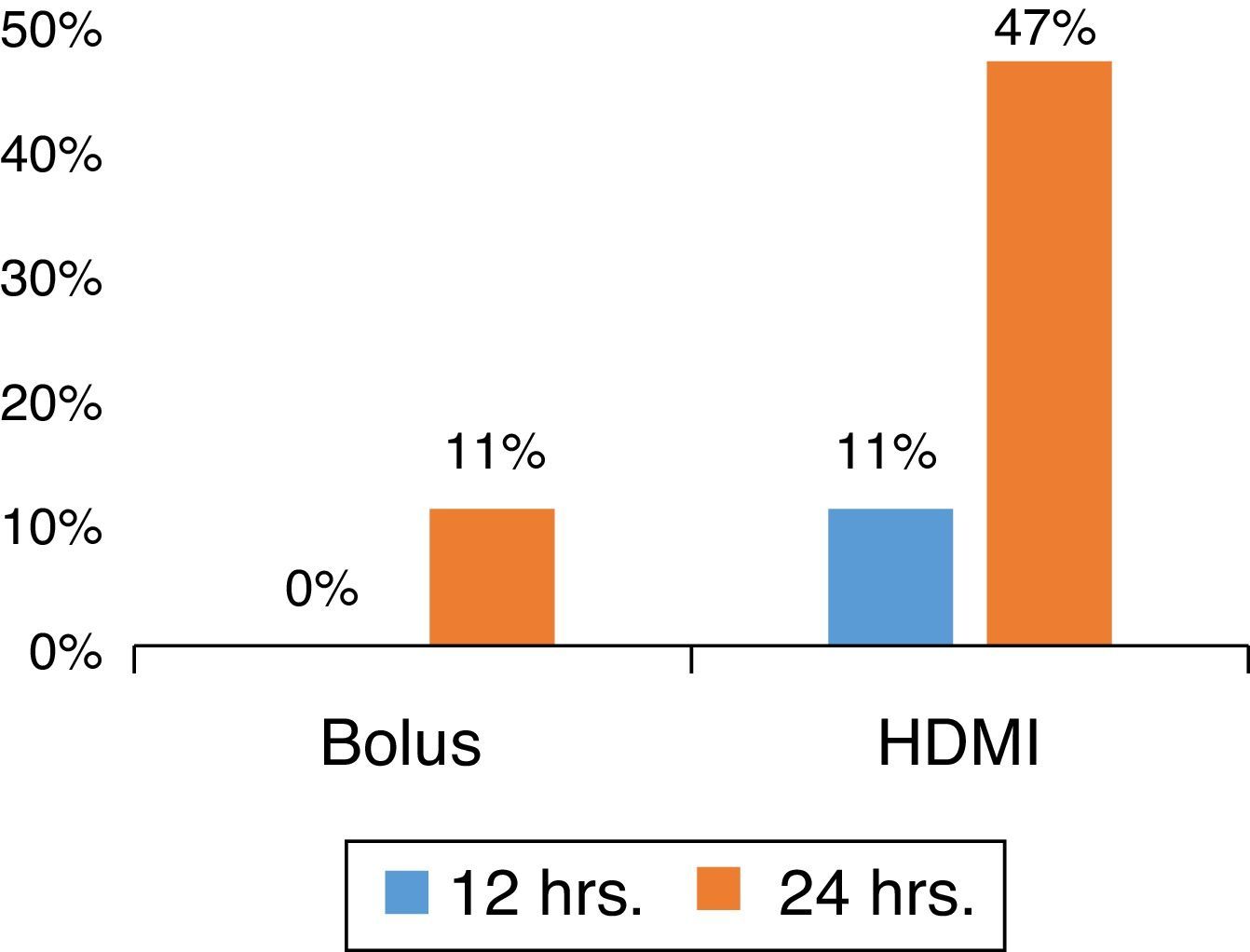
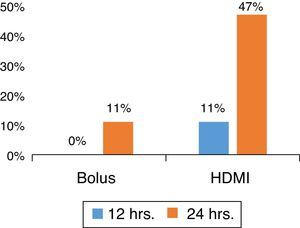
MgSO4 HDMI vs. BolusIn a prospective, randomized ED study for severe asthma, with patients without underlying co-morbidity or infectious etiology, the authors determined that HDMI was superior to MgSO4 bolus (Fig. 1) in shortening the ED length of stay while reducing costs.40
Patients were randomized to receive intravenous MgSO4 50mg/kg bolus (over 1h) or HDMI (50/mg/kg/h for 4h), diluted in 0.9% saline solution at a concentration of 10mg/mL. The HDMI group presented a lower length of stay (HDMI, 34±19h; bolus, 48±19h; p=0.031; 95% CI: 1.3–26.5). Moreover, at 24h, nine out of 19 patients (47%) in the HDMI group were discharged, versus two out of 21 (10%) in the bolus group (p=0.012), with an absolute risk reduction (ARR) 37% (95% CI: 11–63). HDMI was superior to a bolus as an early adjunctive treatment, with a NNT of 3 (95% CI: 1.6–9.5) to facilitate a discharge at 24h from the ED.39 Interim analysis at 12 and 36h presented the same trends favoring the HDMI group; two-thirds of the patients in this group were discharged at 36h (p=0.009; ARR: 42%; 95% CI: 14–70%; NNT: 3; 95% CI: 1.4–7.3). The use of HDMI in the ED management of asthma was cost-effective in the present institution.39
Side effects and potential challengesMgSO4-induced muscle weakness, with the consequent risk of respiratory failure, and potential vasodilatation, with subsequent hypotension, are of concerns when utilized in the context of asthma.41,42 Although many earlier studies showed minimal or no adverse effects, the fear of these side effects is pervasive. Minor side effects were described in 16% of patients: epigastric warmth, tingling, numbness, and pain at the site of infusion, all of them appearing within 5min of the initiation and disappearing shortly afterwards.27 Of note, that study appears to have used a bolus of 100mg/kg over 35min.27 Schuh et al. found contradictory behavior in an online survey of ED physicians. While more than 80% of responders agreed that there were data to support use of MgSO4, it was utilized in less than 20% of the time; 24% of surveyed physicians recalled observing at least one episode of hypotension requiring intervention, and 23% had concerns about its side effects. An online survey, a methodology that suffers from self-selection bias and subjective recall bias, emphasized the general predisposition of physicians to develop opinions when there is lack of data.34
In four HDMI studies, no significant side effects were observed, except for one patient reporting nausea, two pain at the injection site, and two generalized flushing. No patient experienced significant muscle weakness or the need for respiratory support. Low diastolic blood pressure should be expected during HDMI if measured by automated sphygmomanometer. The authors observed normal troponin levels of 0.05±0.01ng/mL and no EKG changes during HDMI.38 Of note, in a non-invasive method, the changes in tonality between the 4th to the 5th Korotkoff determine the diastolic blood pressure.43 Automated sphygmomanometers have difficult elucidating this change when patients are receiving a high dose β2 adrenergic or HDMI. A study with invasive intra-arterial line may be able to refine this point.
A contemporary problem is that an increased proportion of patients with asthma are obese,44,45 which requires adjustments in the intravenous dose. In this practice, the authors adjust the dosage to ideal body weight when BMI is ≥ 25. Further studies in this area are also needed.
Inhaled MgSO4 in asthmaThe large efficacy of the nebulized β-agonists in the treatment of asthma makes their role undisputed. However, inhaled medications are difficult to deliver to the affected bronchi, even under ideal conditions. Studies have shown that only about 10% of bronchodilators reach the lung and are largely affected by respiratory rate, tidal volumes, dead space ventilation (Vd/Vt), bronchoconstriction, method of delivery, mouth breathing, and particle size and deposition.
The use of inhaled magnesium sulfate has presented inconsistent results. A systematic review showed that clinical trials that assessed the use of inhaled MgSO4 failed to find a beneficial effect, and its use is not widely recommended.46
ConclusionsImprovements in the ED management of severe asthma, a leading diagnosis for admission to hospitals, could have a significant economic impact, in particular in areas with lower socioeconomic resources. A preplanned, organized, and decisive ED initial management is paramount to reverse a condition that can evolve toward respiratory failure. The authors emphasize the role of MgSO4 as an adjunctive therapy in the initial management of asthma, while β2 adrenergic and corticosteroids remain the primary therapy. It is possible that the inconsistent results from previous MgSO4 studies were due to a failure to achieve sustained serum magnesium and spasmolytic effects for β2 adrenergic to reach the site of action. Incorporating HDMI at 50mg/kg/h for 4h in the ED facilitates early discharge, reduces hospitalization rates, and is cost-effective.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Irazuzta JE, Chiriboga N. Magnesium sulfate infusion for acute asthma in the emergency department. J Pediatr (Rio J). 2017;93:19–25.