To evaluate the prevalence and circulation of rotavirus genotypes before and after the introduction of oral vaccine against human rotavirus, and to check for a possible change in the age of occurence of the infection by RV-A.
MethodsThis was a cross-sectional study conducted between 2002-2011, in the city of Juiz de Fora, state of Minas Gerais, Brazil. A total of 1,144 diarrheal stool specimens were obtained from nonhospitalized children aged between 0 and 5 years, and analyzed by polyacrylamide gel electrophoresis and reverse-transcription polymerase chain reaction for genotype characterization. Data on prevalence and age distribution of rotavirus cases were analyzed through the chi-squared test (p < 0.05), using SPSS, release 13.0.
ResultsRotavirus infection was detected in 9.35% (107/1,144) samples, with prevalence rates ranging from 11.12% (90/809) in the pre-vaccine to 5.07% (17/335) in the post-vaccine period (p = 0.001). Among the samples tested, the most frequently detected genotypes were G1P[6] (6/33 = 18.2%) in the period between 2002 and 2005 and G2P[4] in 2006 (11/33 = 33.3%) and in the period between 2007 and 2011 (5/33 = 15.2%). There was also a significant reduction in the number of cases of rotavirus disease in children aged between 0 and 36 months after the vaccine introduction.
ConclusionsThe study evidenced a significant decrease in the prevalence of rotavirus, mainly in children aged between 0 and 36 months in the 2007-2011 period, as well as a reduction in G1 genotype circulation.
Avaliar a prevalência e a circulação dos genótipos de rotavírus, antes e após a introdução da vacina oral contra rotavírus humano, bem como verificar uma possível mudança na faixa etária de ocorrência da infecção pelo RV-A.
MétodosTrata-se de um estudo transversal realizado no período de 2002 a 2011, em Juiz de Fora, MG. Foram avaliados 1.144 espécimes fecais diarreicos, obtidos de crianças de 0 a cinco anos não hospitalizadas, que foram analisadas por PAGE e RT-PCR. Os dados relativos à prevalência e distribuição etária dos casos de rotavirose foram analisados pelo teste χ2 (p < 0,05), utilizando-se o programa SPSS, versão 13.0.
ResultadosInfecções por rotavírus foram detectadas em 9,35% (107/1.144) das amostras, com prevalências variando de 11,12% (90/809) no período pré-vacinal a 5,07% (17/335) no pós-vacinal (p = 0,001). Dentre as amostras caracterizadas, os genótipos mais frequentemente detectados foram G1P[6] (6/33 = 18,2%) no período 2002-2005 e G2P[4] no ano de 2006 (11/33 = 33,3%) e no período 2007-2011 (5/33 = 15,2%). Observou-se, ainda, uma redução significativa no número de casos de rotavirose em crianças de 0 a 36 meses, após a introdução da vacina.
ConclusõesO estudo revelou queda significativa na prevalência de rotavírus, principalmente na faixa etária de 0 a 36 meses, no período 2007-2011, bem como redução na circulação do genótipo G1.
Group A rotaviruses (RV-A) are important viral pathogens associated with acute diarrheal disease (ADD) in children. Around the world, they are responsible for 125 million episodes of diarrhea, 25 million physician consultations, 2.4 million hospitalizations, and 611,000 deaths per year; 29% of all diarrhea deaths involve children younger than 5 years.1,2
In developing countries, RV-A are directly related to child mortality and high morbidity, considering the large number of hospitalizations by diarrhea and dehydration, impacting the family, society, public health care expenses, productivity, and psychosocial and environmental aspects.1 In Latin America, according to available data were recorded ten million cases of diarrhea, two million doctor consultations, 75,000 hospitalizations, and 15,000 deaths annually caused by RV-A.3
In Brazil, before vaccination, RV-A were associated with 3.5 million episodes of ADD, 650,000 outpatient visits to health care facilities, 92,000 hospitalizations, and 850 deaths per year in children aged < 5 years.4 Studies performed in the secondary and tertiary levels of health care with individuals of the same age group demonstrated that the prevalence of diarrhea disease by RV-A ranged from 20.7% to 30.9%,5 and this virus was also considered an important cause of hospitalization.
RV-A infection is self-limited and can be symptomatic or asymptomatic. The clinical picture of the disease varies from mild to severe and can lead to dehydration. It affects individuals in all age groups, but predominantly infants.6
RV-A belong to the genus Rotavirus, family Reoviridae, whose genome consists of 11 segments of double-stranded RNA. Genotypes are classified according to a binary system through the determination of gene sequences that encode the VP7 (G types) and VP4 (P types) proteins.6 However, a more complete classification system was recently suggested, based on the sequence of all genomic segments of the virus.7 The most common G and P combinations worldwide are: G1P[8], G2P[4], and G9P[8].5
Improved sanitation and hygiene habits are desirable for the prevention of diarrheal diseases, but not enough to prevent infection by RV-A. Thus, studies have focused on the development of a vaccine, aiming to reduce the number of severe cases of the disease and consequent hospitalizations and deaths in all socioeconomic levels. In the last decade, several candidates were tested without success until development of Rotarix monovalent vaccine® (GlaxoSmithKline – Rixensart, Belgium) produced using an human-attenuated RV-A G1P[8].1
The Rotarix vaccine® was introduced in the National Immunization Program of Brazil in March of 2006,8 and was also implemented in other 11 Latin American countries.5 Although the immunity conferred by the vaccine protects against severe disease, reinfections by different genotypes of RV-A can occur throughout life, as these viruses have a high genetic diversity.9
In this context, genotypic characterization studies are important to understand the impact of vaccination on the RV-A genotypes circulating in the population and to provide subsidies for reassessment of the formulations in the search for a more appropriate vaccine. Thus, the objective of this study was to evaluate the prevalence of RV-A infection and circulation of RV-A genotypes before and after the introduction of oral vaccine against human rotavirus, as well as to check for a possible change in the age of occurence of the infection by RV-A.
MethodsThis was cross-sectional study that analyzed 1,144 fecal samples from non-hospitalized children aged 0-5 years, with clinical evidence of ADD, attended at public outpatient clinics and private offices, from 2002 to 2011 in the city of Juiz de Fora, state of Minas Gerais, Brazil.
This study was approved by the Ethics Committee on Human Research of the Universidade Federal de Juiz de Fora. Fecal samples were kept under refrigeration (4°C) and sent to the virology laboratory, where, after registration, they were stored at -20°C, constituting a bank of samples at the Universidade Federal de Juiz de Fora.
Fecal suspensions (10% w/v) were prepared in Tris-HCl-Ca+2 buffer, at pH 7.2, clarified (5,000rpm for 20minutes at 4°C) and submitted to RNA extraction technique,10 followed by polyacrylamide gel electrophoresis (PAGE)11 metodology for RV-A detection.
The genotypic characterization of positive RV-A samples was performed by polymerase chain reaction preceded by reverse transcription (RT-PCR), using consensus primers for the amplification of genes encoding the VP7 and VP4 proteins, followed by multiplex seminested PCR with specific nucleotide primers for the main genotypes G (G1, G2, G3, G4, G8, and G9)12,13 and P P[4], P[6], P[8] e P[9]14 of human RV-A.
Data on the prevalence and age distribution of positive RV-A samples were entered in the Statistical Package for Social Sciences (SPSS), release 13.0. Comparison of the rate of RV-A detection and age groups during the study period was performed by the chi-squared test, considering p-values < 0.05 as statistically significant.
ResultsTo assess the possible influence of vaccination on the prevalence of rotavirus and the age group affected by the disease, the study period was divided into pre-vaccine (2002-2006) and post-vaccine (2007-2011). Although 2006 was the year when the vaccine was introduced, it was included in the pre-vaccination period (2002-2006), as the majority (187/194 = 96.39%) of the samples were obtained from children who were not eligible for vaccination. However, for the analysis of circulating genotypes, considering that other factors, in addition to vaccination, could have an impact on this dynamic, the study period was divided into 2002-2005, 2006, and 2007-2011.
In this study was observed a positivity rate of 9.35% (107/1,144), with a prevalence of ADD associated with RV-A ranging from 11.12% (90/809) in the period before the introduction of the vaccine to 5.07% (17/335), in the period after its implementation, which was statistically confirmed (p = 0.001).
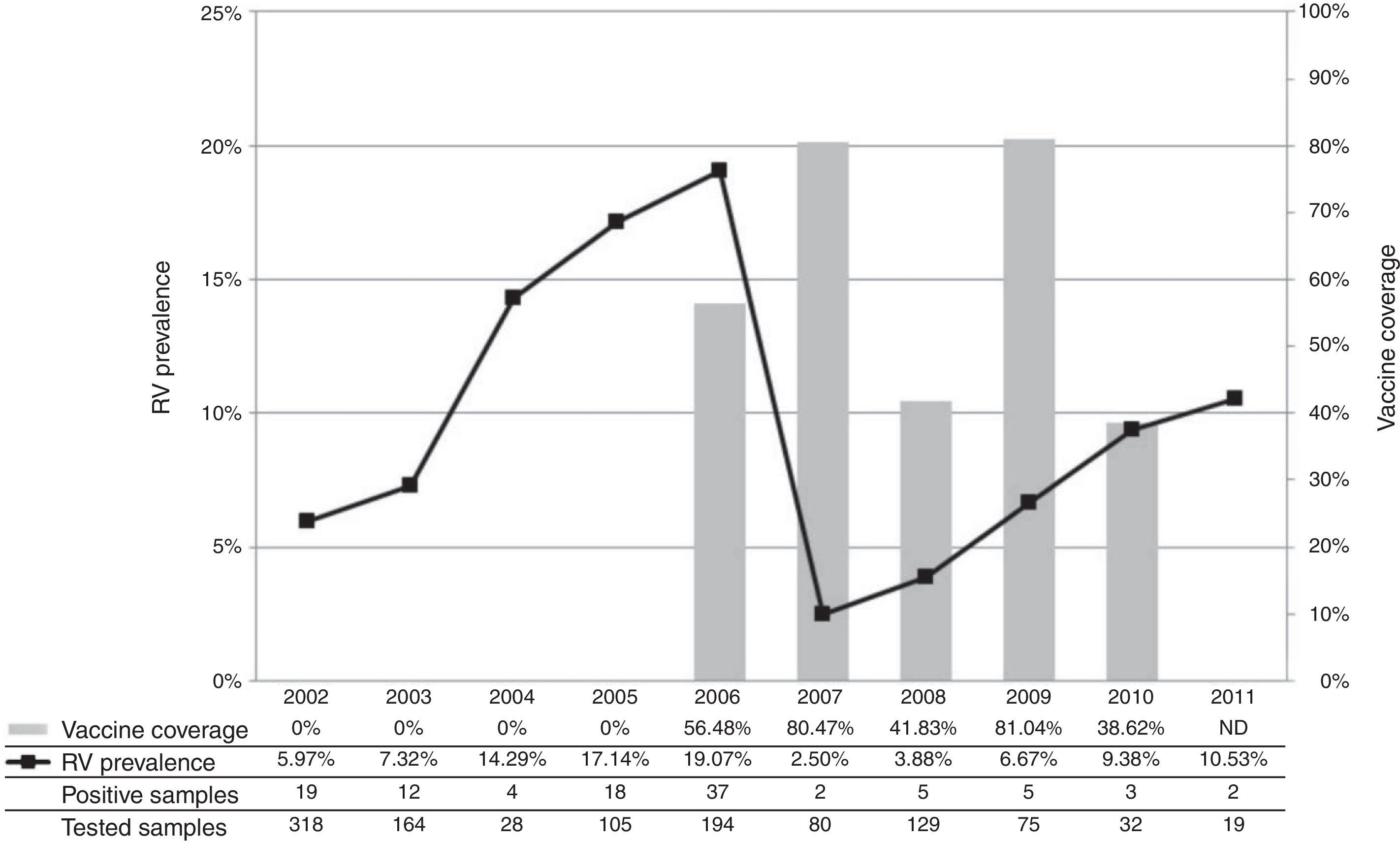
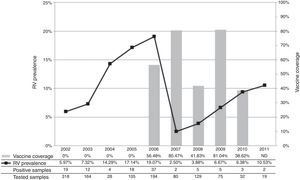
The annual prevalence of RV-A and the comparison with the vaccination coverage achieved in the period are exhibited in Fig. 1, where the curve shows a sharp decline in the incidence of rotavirus disease in 2007.
Annual prevalence of RV-A in the period 2002-2011 and vaccine coverage.
ND, no data available.17
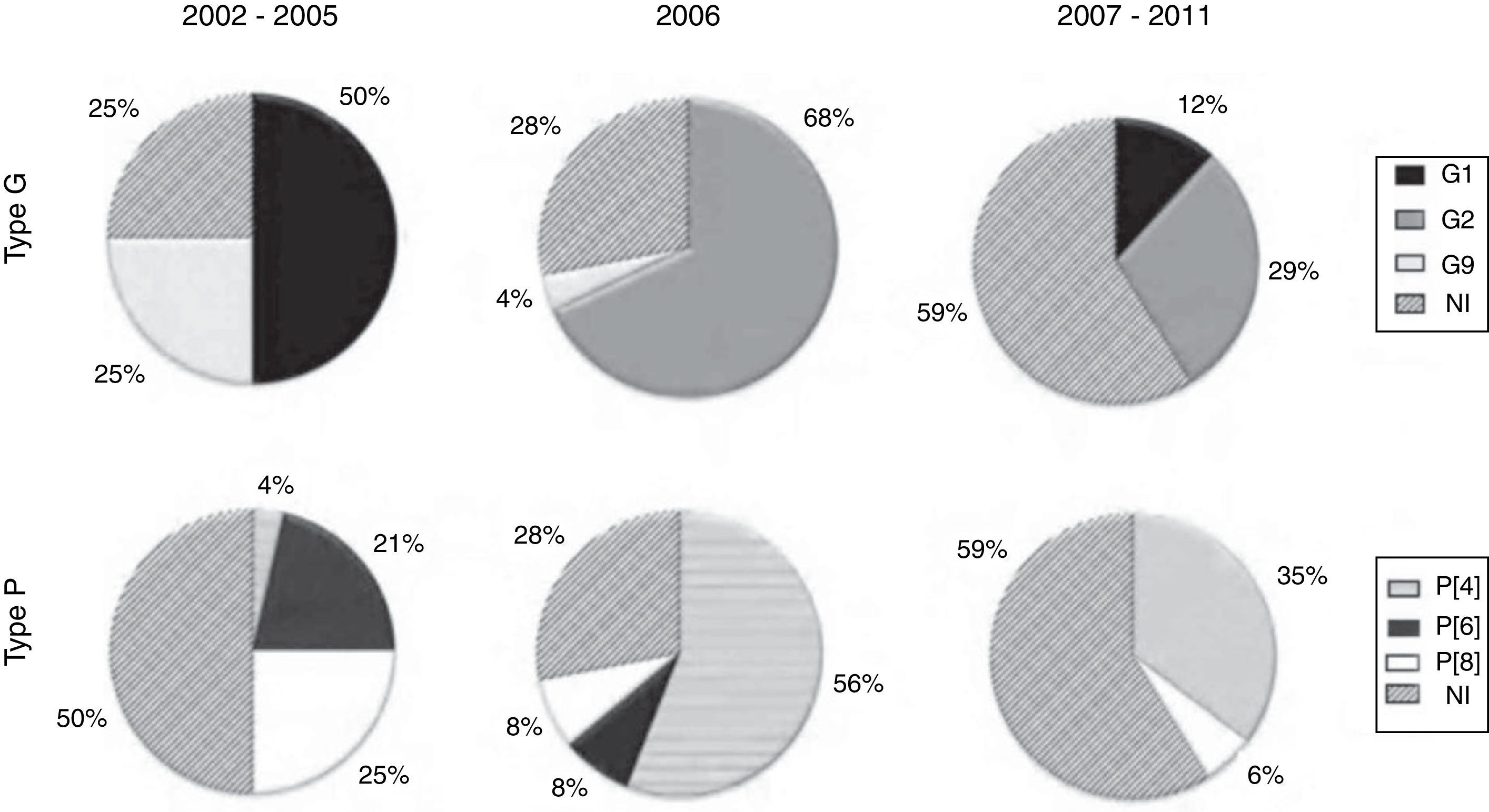
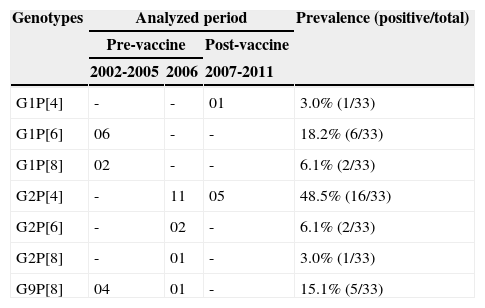
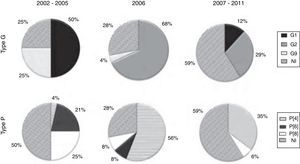
Of the 70 positive RV-A samples submitted to molecular characterization, 52 (74.3%) were genotyped. Of these, 63.5% (33/52) were completely characterized, and a total of seven combinations of G and P genotypes (Table 1) were identified, highlighting G1P[6] in the period between 2002 and 2005 and G2P[4] from the year 2006 onward. Conversely, 36.5% of the samples (19/52) were partially characterized, highlighting the genotypes G1 (7/19 = 36.8%) in 2002-2005 and P[4] (4/19 = 21.1%) in 2006. Even after several attempts, it was not possible to characterize 25.7% (18/70) of the samples, most of them obtained in the period between 2007 and 2011.
Complete genotypic characterization of RV-A samples, in the period 2002-2011.
| Genotypes | Analyzed period | Prevalence (positive/total) | ||
|---|---|---|---|---|
| Pre-vaccine | Post-vaccine | |||
| 2002-2005 | 2006 | 2007-2011 | ||
| G1P[4] | - | - | 01 | 3.0% (1/33) |
| G1P[6] | 06 | - | - | 18.2% (6/33) |
| G1P[8] | 02 | - | - | 6.1% (2/33) |
| G2P[4] | - | 11 | 05 | 48.5% (16/33) |
| G2P[6] | - | 02 | - | 6.1% (2/33) |
| G2P[8] | - | 01 | - | 3.0% (1/33) |
| G9P[8] | 04 | 01 | - | 15.1% (5/33) |
The characterization of the RV-A samples evidenced the predominance of the G1 genotype in 2002-2005, with a significant decline in the 2007-2011 period; however, no samples of this genotype were detected in the year of vaccine introduction (2006). G2 samples, which were not detected in the 2002-2005 period, prevailed from 2006 onwards, while G9 samples were detected only in the 2002-2005 period (Fig. 2).
Regarding the P genotype, Fig. 2 shows that the P[8] and P[4] types were detected throughout the study period; P [8] was the predominant type in the 2002-2005 period. The P[4] type, however, was seldom detected in 2002-2005, but prevailed in the year of vaccine introduction (2006), showing smaller proportions in the 2007-2011 period. The P[6] type was detected only in the 2002-2005 period (Fig. 2).
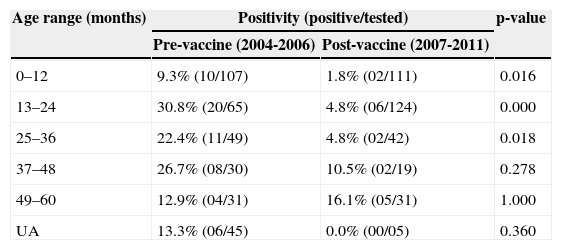
The analysis of distribution of positive RV-A samples, according to age, in the pre- and post-vaccination periods (Table 2) demonstrated a significant reduction in the number of cases of rotavirus disease in children aged between 0 and 36 months in the post-vaccination period. Although all fecal samples were collected from children aged between 0 and 5 years in 2002 (19/318) and 2003 (12/164), there was no accurate information on age. So these samples were therefore not included in Table 2.
Age range distribution of positive samples for RV-A, in the 2004-2011 period.
| Age range (months) | Positivity (positive/tested) | p-value | |
|---|---|---|---|
| Pre-vaccine (2004-2006) | Post-vaccine (2007-2011) | ||
| 0–12 | 9.3% (10/107) | 1.8% (02/111) | 0.016 |
| 13–24 | 30.8% (20/65) | 4.8% (06/124) | 0.000 |
| 25–36 | 22.4% (11/49) | 4.8% (02/42) | 0.018 |
| 37–48 | 26.7% (08/30) | 10.5% (02/19) | 0.278 |
| 49–60 | 12.9% (04/31) | 16.1% (05/31) | 1.000 |
| UA | 13.3% (06/45) | 0.0% (00/05) | 0.360 |
UA, undetermined age.
The introduction of vaccine in the National Immunization Program was a breakthrough, and can be an effective measure to decrease the incidence of severe rotavirus disease and infant mortality.15 However, in order to assess the impact of the measure on the disease occurrence and the fluctuations of different RV-A genotypes, it is necessary to perform continuous longitudinal epidemiological surveillance after the vaccine introduction.
Vaccination was started in 2006, reaching only 56.48% of the target population in the city. In addition to low immunization coverage, it is relevant to observe that, in this study, the majority of samples were obtained from children who were outside the age group considered eligible for vaccination, which partly explains the fact that the prevalence of RV-A was similar to that observed in 2005.
In 2007, however, there was a significant decrease in the rate of virus detection, corroborating data from other researchers in Brazil in the same year. This decrease was concomitant with an increase in the vaccination coverage in the municipality, which reached 80.47% of eligible children during that year.16–18
The comparative analysis between the pre- and post-vaccination periods demonstrated a significant reduction in the prevalence of RV-A in 2007-2011. This finding corroborates other Brazilian studies, which reported a similar trend17–19 even in the hospital environment, where vaccination was associated with an overall reduction in the number of consultations and hospitalizations by ADD.20 This reduction in the occurrence of the disease caused by RV-A is important, as it implies reduction of comorbidities and of the financial burden to the Brazilian health system.
Despite the observed reduction, a trend of gradual increase in the prevalence of RV-A was observed the period of 2008-2011, even considering the smaller number of samples obtained during these years of study. Partially similar data were reported in a study conducted in the Triângulo Mineiro area, Western Minas Gerais, where the largest decrease in the prevalence of RV-A was observed in 2009, but with an upward trend in 2010, in the city of Uberlândia.21
Before the introduction of vaccine (2002-2005), RV-A G1P[8] were predominant in Brazil.5 In this same period, in the city of Juiz de Fora, the largest circulation of G1 samples was also observed; however, with a predominance of G1P[6] followed by G9P[8] and G1P[8]. Molecular characterization of the samples detected in 2006 showed a predominance of G2P[4] reinforcing reports of studies carried out in different states of Brazil and other countries.17,22–24 In the year when the G1P[8] vaccine was implemented in the Brazilian Unified Health System (Sistema Único de Saúde - SUS), a high prevalence of RV-A was observed, associated with low vaccination coverage and greater circulation of G2 genotype samples. Seldom detected in the country since 1996, such samples re-emerged in 2006, thus confirming the observation that they have a characteristic circulation that occurs at ten-year intervals.22
RV-A G2P[4] samples may have been reintroduced into Brazil in 2005, through states bordering other countries in South America that reported the disease associated with G2 genotype25 and others that have not implemented the vaccination.24 The long period of little or no circulation of this genotype would have created favorable conditions for the accumulation of immunologically susceptible individuals,26 which may explain the high prevalence of infection, even with the use of vaccine.
Studies of cross-reactivity of vaccine performed in Latin America evidenced heterotypic response only against G3, G4, and G9 samples.15 However, the evaluation of the response against G2 samples may have been impaired by the low circulation of this genotype during the screening studies.15 Recent studies20,27 supported this theory, showing evidence of vaccine efficacy against the G2P[4] genotype, especially in children aged between six and 11 months, with declining protection after 12 months of age.
Since 2007, the prevalence of infection has decreased. G2 samples remained predominant, cocirculating mainly with G1 samples, both at a lesser extent than in 2006 and 2005, respectively, according to reports by other authors.24 During this period, in addition to the anti-G1 homotypic protection and heterotypic protection against other genotypes conferred by the vaccine, the number of individuals already sensitized against G2 genotype, who have accumulated in the population, should be considered.17,22,24
Thus, the reemergence of G2 samples can be attributed both to characteristic fluctuations of this genotype, as well as to a possible selective advantage granted by the vaccine. However, the finding that G2 samples were prevalent in this period, even in countries where vaccine had not yet been implemented,23,25,28 reinforces the theory of temporal fluctuation. Therefore, from the perspective of epidemiological surveillance, it is essential to maintain sequential studies to evaluate the genotypic variations of RV-A.
It was not possible to characterize a percentage of the positive samples, despite several attempts at genotyping; most of them were detected in the 2007-2011 period. This difficulty has been previously observed,18 and can be associated with the presence of inhibitors in fecal specimens and/or the involvement of different genotypes of RV-A not included in the PCR reaction, selected by pressure of antibodies elicited by the vaccine.
Studies on the post-vaccine period have focused on the verification of the influence of vaccination on the prevalence of infection and characterization of circulating genotypes, with few reports on a possible change in the age-range of higher occurrence of infection. In this context, this study demonstrated that in the pre-vaccine period, rotavirus infection was observed in children aged 0 to 60 months, especially in the age group between 13 and 24 months. Conversely, in the post-vaccine period, there was a significant reduction in the rate of positivity in children aged 0 to 36 months, even considering the loss of data for the years 2002 and 2003. Similar results have also been observed in hospitalized children, showing a significant reduction in hospitalization for diarrheal disease associated with RV-A, in the age group of 0 to 23 months.29 Taken together, these results suggest that vaccination considerably influenced reduction in infection and severe disease by RV-A in the age group of 0 to12 months, which was previously considered the most vulnerable group. In this respect, it must also be considered that this decrease includes herd immunity induced by mass vaccination against RV-A in unvaccinated children, as recently reported by other authors.30
This study demonstrated a significant decrease in the prevalence of RV-A in the period 2007-2011, especially in the age group between 0 and 36 months, and a decrease in the circulation of non-G2 genotypes, predominant during the 2002-2005 period in the municipality, after the implementation of vaccine.
FundingFAPEMIG and PROPESQ-UFJF.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Assis AS, Valle DA, Antunes GR, Tibiriça SH, Assis RM, Leite JP, et al. Rotavirus epidemiology before and after vaccine introduction. J Pediatr (Rio J). 2013;89:470–6.