Henoch–Schönlein purpura is a systemic vasculitis that mainly occurs in children. Renal impairment is a major complication of Henoch–Schönlein purpura, but there is no established predictive marker for renal involvement. Thus, in this study, we investigated the risk factors for renal involvement in children with Henoch–Schönlein purpura.
MethodThe medical records of children newly diagnosed as having Henoch–Schönlein purpura between 2005 and 2020 were reviewed retrospectively. Selected laboratory data were recorded before treatment initiation. The date and the age at diagnosis; sex; and the presence of arthralgia, gastrointestinal and renal involvement were obtained retrospectively.
ResultsThis study included a total of 186 patients with Henoch–Schönlein purpura. Among them, 36.0% had renal involvement; 28.4% had only microscopic hematuria, 53.7% had non-nephrotic range proteinuria, and 17.9% had nephrotic-range proteinuria during follow-up. The mean age was higher (p = 0.016) and female sex was predominant (p = 0.001) in patients with renal involvement than in those without renal involvement. Blood neutrophil/lymphocyte ratio (p = 0.002) and platelet/lymphocyte ratio (p = 0.002) were significantly higher than that of the patients without renal involvement. No statistically significant differences were observed in the hemoglobin concentration, platelet count, presence of arthralgia, and gastrointestinal involvement between patients with and without renal involvement. Logistic regression analysis revealed female sex (odd ratio = 3.213) and neutrophil/lymphocyte ratio (odd ratio = 1.329) as risk factors for renal involvement.
ConclusionsFemale sex and high neutrophil/lymphocyte ratio were risk factors for renal involvement in Henoch–Schönlein purpura.
Henoch–Schönlein purpura (HSP) is a systemic small-vessel vasculitis that mainly occurs in children.1 It mainly affects the skin, joints, gastrointestinal (GI) tract and kidneys, and the severity of renal involvement determines the long-term prognosis.2,3 Approximately 30–50% of HSP patients show symptoms of renal involvement. These symptoms are generally mild, but some patients present nephrotic syndrome or renal failure.4 Whereas other organ involvement in HSP is usually benign and self-limited, HSP nephritis (HSPN) can lead to chronic renal disease and end-stage renal disease.4,5 However, the predictive factors for renal involvement or progression of renal disease in children with HSP are poorly known.
Values from routinely performed laboratory tests have been investigated as useful biomarkers in patients with systemic vasculitis to practically predict and prevent morbidities and complications.6–8 Several recent studies proposed hematologic indices for predicting organ involvement in children with HSP. The relationships between organ involvement and laboratory indices such as leukocytosis, lymphocytopenia, thrombocytopenia, high neutrophil/lymphocyte ratio (NLR), and high platelet/lymphocyte ratio (PLR) have been reported.9–12 However, there is still no canonical laboratory biomarker to predict organ involvement in HSP. Thus, the present study investigated the clinical features of HSP with or without renal involvement and examined the usefulness of laboratory markers for predicting renal involvement.
MethodsStudy design and patient selectionBetween June 2005 and July 2020, we retrospectively reviewed the cases of 288 children diagnosed as having HSP. According to the European League against Rheumatism/Paediatric Rheumatology European Society (EULAR/PRES) criteria for HSP, HSP was defined as palpable purpura (without thrombocytopenia and coagulopathy) and the presence of at least one of the following: (i) abdominal pain (acute, diffuse or colicky); (ii) predominant immunoglobulin A deposition in the biopsy of affected tissue; (iii) arthritis or arthralgia; and (iv) renal involvement (hematuria or proteinuria).13 Patients with underlying disease, medication use before blood sampling, missing laboratory data, renal involvement at the time of HSP diagnosis were excluded from the study. Of the 288 HSP patients, 186 who met the inclusion and exclusion criteria were enrolled in the study. We classified these patients into two groups according to renal involvement (Fig. S1). The patients’ medical records were reviewed for demographic data, clinical symptoms, and laboratory findings. This study was approved by the institutional review board and followed the principles of the Declaration of Helsinki.
Investigation and definitionsThe following laboratory data were recorded before treatment initiation: white blood cell (WBC), neutrophil, and lymphocytes count; hemoglobin concentration; red cell distribution width (RDW); platelet count (Plt) and mean platelet volume (MPV); C-reactive protein (CRP), creatinine concentrations; and urinalysis results. NLR and PLR were calculated using these data. Data on the age at diagnosis; sex; and presence of arthritis or arthralgia, GI involvement, and renal involvement were obtained retrospectively.
Arthralgia was defined as limited movement in the joint or painful periarticular soft-tissue edema. GI involvement was defined as abdominal pain, vomiting, or GI bleeding, such as hematochezia, melena, hematemesis, or occult blood in the stool. Renal involvement was defined as follows: (i) hematuria (>5 red blood cells per high-power microscopic field or ≥2+ on dipstick), (ii) proteinuria (protein concentration in spot urine ≥30 mg/dL or spot urine protein/creatinine ratio [PCR] >0.5 in children <2 years of age and >0.2 in children ≥2 years of age). Nephrotic-range proteinuria was defined as a spot urine PCR > 2.0 (1).
Statistical analysisQuantitative variables are expressed as mean ± standard deviation and categorical variables as absolute and relative frequencies. Continuous data were analyzed using Student’s t-test, and categorical data were analyzed using Pearson’s chi-squared test. Logistic regression analysis was used to identify the risk factors for the presence of renal involvement in HSP patients. p-Values < 0.05 were considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY).
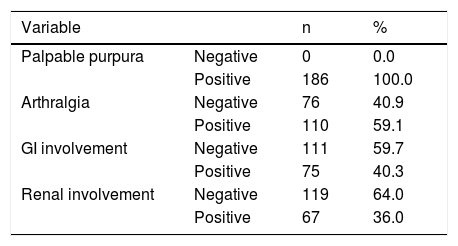
ResultsThis study included a total of 186 children with HSP: 95 were male; 91 were female. The mean age of the HSP patients was 6.6 ± 2.9 years (range 1.5–16.5 years) but most of the cases occurred in those aged 2–12 years. Among these patients, 186 (100.0%) had palpable purpura, 110 (59.1%) had arthralgia, 75 (40.3%) had GI involvement, and 67 (36.0%) had renal involvement (Table 1).
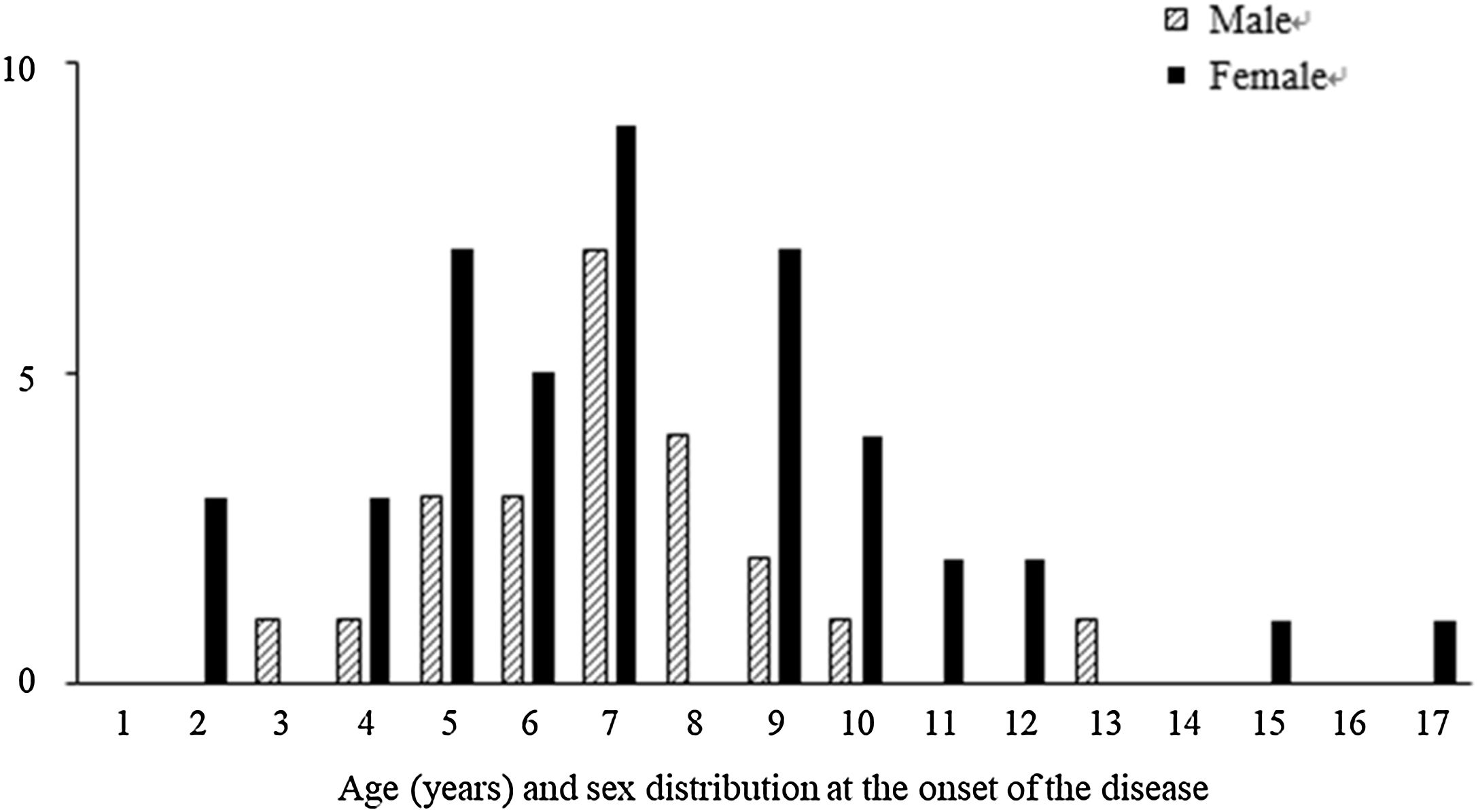
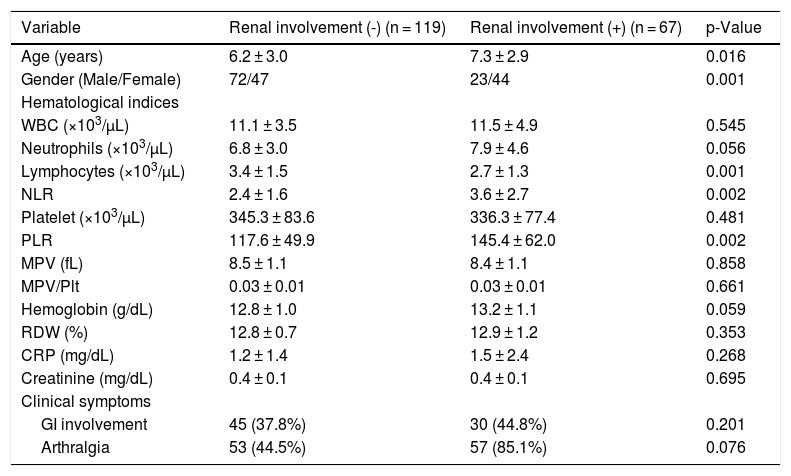
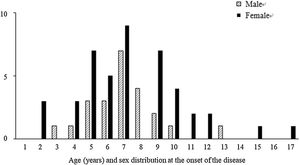
Among the 67 patients with renal involvement, 19 (28.4%) had isolated hematuria, 36 (53.7%) had non-nephrotic proteinuria, and 12 (17.9%) had nephrotic-range proteinuria during follow-up. The mean age was higher (7.3 ± 2.9 vs. 6.2 ± 3.0 years, p = 0.016) and the female sex was predominant (p = 0.001) among patients with renal involvement than those without renal involvement (Table 2). Further, we revealed that male patients with renal involvement were prevalent between 5-8 years of age, and female patients with renal involvement were prevalent between 4-10 years of age (Fig. 1).
Comparisons of demographic characteristics and laboratory parameters between patients with and without renal involvement.
| Variable | Renal involvement (-) (n = 119) | Renal involvement (+) (n = 67) | p-Value |
|---|---|---|---|
| Age (years) | 6.2 ± 3.0 | 7.3 ± 2.9 | 0.016 |
| Gender (Male/Female) | 72/47 | 23/44 | 0.001 |
| Hematological indices | |||
| WBC (×103/μL) | 11.1 ± 3.5 | 11.5 ± 4.9 | 0.545 |
| Neutrophils (×103/μL) | 6.8 ± 3.0 | 7.9 ± 4.6 | 0.056 |
| Lymphocytes (×103/μL) | 3.4 ± 1.5 | 2.7 ± 1.3 | 0.001 |
| NLR | 2.4 ± 1.6 | 3.6 ± 2.7 | 0.002 |
| Platelet (×103/μL) | 345.3 ± 83.6 | 336.3 ± 77.4 | 0.481 |
| PLR | 117.6 ± 49.9 | 145.4 ± 62.0 | 0.002 |
| MPV (fL) | 8.5 ± 1.1 | 8.4 ± 1.1 | 0.858 |
| MPV/Plt | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.661 |
| Hemoglobin (g/dL) | 12.8 ± 1.0 | 13.2 ± 1.1 | 0.059 |
| RDW (%) | 12.8 ± 0.7 | 12.9 ± 1.2 | 0.353 |
| CRP (mg/dL) | 1.2 ± 1.4 | 1.5 ± 2.4 | 0.268 |
| Creatinine (mg/dL) | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.695 |
| Clinical symptoms | |||
| GI involvement | 45 (37.8%) | 30 (44.8%) | 0.201 |
| Arthralgia | 53 (44.5%) | 57 (85.1%) | 0.076 |
CRP, C-reactive protein; MPV, mean platelet volume; NLR, blood neutrophil-to-lymphocyte ratio; PLR, blood platelet-to-lymphocyte ratio; Plt, platelet; RDW, red cell distribution width; WBC, white blood cell.
The lymphocyte count was significantly lower (p = 0.001), and the NLR (p = 0.002) and PLR (p = 0.002) were significantly higher in patients with renal involvement than in those without renal involvement. No statistically significant differences were observed in WBC, neutrophil, hemoglobin concentration and Plt counts; MPV, MPV/Plt, and RDW; and CRP, creatinine levels between patients with and without renal involvement (Table 2). There were no significant differences in age, sex, and laboratory parameters according to the severity of renal involvement (data not shown).
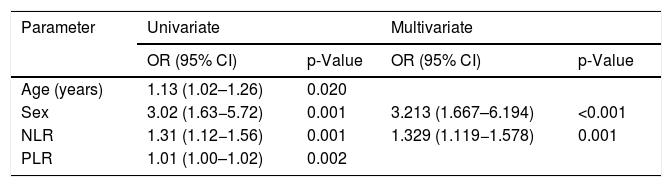
Univariate logistic regression analysis revealed that age at diagnosis (odds ratio [OR] = 1.13, p = 0.020), female sex (OR = 3.02, p = 0.001), NLR (OR = 1.31, p = 0.001), and PLR (OR = 1.01, p = 0.002) were significant risk factors for renal involvement in children with HSP (Table 3). Multivariate logistic regression analysis identified female sex (OR = 3.213, p < 0.001) and NLR (OR = 1.329, p = 0.001) as significant factors.
Logistic regression analysis of risk factors for renal involvement in patients with Henoch–Schönlein purpura.
| Parameter | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age (years) | 1.13 (1.02–1.26) | 0.020 | ||
| Sex | 3.02 (1.63−5.72) | 0.001 | 3.213 (1.667–6.194) | <0.001 |
| NLR | 1.31 (1.12−1.56) | 0.001 | 1.329 (1.119−1.578) | 0.001 |
| PLR | 1.01 (1.00–1.02) | 0.002 | ||
CI, confidence interval; HSP, Henoch–Schönlein purpura; NLR, blood neutrophil-to-lymphocyte ratio; OR, odds ratio; PLR, blood platelet-to-lymphocyte ratio.
We retrospectively reviewed demographic factors, clinical features, and laboratory data and identified the risk factors for renal involvement in children with HSP. Recent studies have reported relationships between laboratory and clinical findings and the occurrence of renal involvement in HSP. In the present study, we identified the risk factors for renal involvement in pediatric HSP. Similar to previous observations, we observed that a high NLR was a risk factor for renal involvement in pediatric HSP. However, sex difference as a risk factor for renal involvement in pediatric HSP showed different results.
HSP occurs worldwide and mainly affects children between 3 and 10 years of age in up to 90% of cases.1 The estimated incidence of HSP is 14–20 per 100,000 children per year, and it is more common in males more than in females, with a male to female ratio of 1.2–1.8:1. In the present study, similar to the previous reports, the mean age of the 186 patients was 6.6 years (range 1.5–16.5 years), and the male/female ratio was 1.04. Some studies have reported older age as a risk factor associated with renal involvement in pediatric HSP.14–16 Sano et al.17 concluded that age older than 4 years was an independent risk factor for renal involvement, while Kaku et al.18 reported that age over 7 years at onset was associated with an increased risk of renal involvement. Our results also showed a significantly higher age at diagnosis in HSP with renal involvement compared to that in patients without renal involvement. The OR for renal involvement in pediatric HSP increased by 13% for every 1-year increase in age at onset in this study. On the other hand, unlike previous studies, female sex is also a significant risk factor for renal involvement in children with HSP in this study. The incidence of HSP was higher in male patients, consistent with previous reports; however, renal involvement was more common in female patients; who had a 3.21-fold increased risk. These results are similar to those reported by Kiliç and Demir;19 however, other studies reported HSPN to be more common in male patients.14,16,20 Thus, sex differences in the association of risk factors for renal involvement in pediatric HSP remain controversial.
NLR is a simple and stand-alone method for evaluating systemic inflammation. Neutrophils play a central role in the innate immune response and lymphocytes play a major role in the inflammatory response. Therefore, a high NLR indicates an imbalance of the inflammatory response and may be a surrogate marker of disease severity in infectious diseases. Several studies reported that a high NLR is also associated with a poor prognosis of diseases; such as rheumatoid arthritis, cancer, and ulcerative colitis.11,21–23 Recent studies have reported that high neutrophil counts and NLR may be related to renal involvement and are useful as a predictive marker of renal involvement in HSP patients.9–11,24 Similar to previous studies, the present study also identified the negative impact of a high NLR in HSP with renal involvement.
Renal impairment is the most serious long-term complication of HSP and the severity of renal involvement affects the long-term prognosis of HSP.1 Some studies have reported an incidence of renal involvement of up to 30–50% in children with HSP with 1–2% of them experiencing progression to end-stage renal disease.1,4 In this study, 67 (36.0%) of HSP patients had renal involvement, a rate similar to that mentioned above, ranging from isolated hematuria to nephrotic-range proteinuria during follow-up. Whereas HSP nephritis is mostly benign and self-limiting, it can also lead to chronic renal disease. In this study, of 12 HSP patients with nephrotic-range proteinuria, 7 had completely resolved proteinuria during follow-up. But, the other 5 patients have still observed proteinuria during follow-up, with the longest follow-up period of 96 months, and there were no patients with the end-stage renal disease yet. Although, renal involvement should be closely monitored. Renal involvement is unpredictable in patients at the individual level; hence many authors have investigated its risk factors and possible predictive values for renal involvement in HSP. Clinical symptoms such as severe GI involvement, persistent purpura, and relapse have been suggested as the risk factors for renal involvement in children with HSP.14,15,25 In this study, the clinical symptoms were not associated with HSP with renal involvement. Various laboratory parameters have also been studied as predictive markers for renal involvement in HSP.11,12,14,24 Leukocyte, lymphocyte, and platelet counts, NLR, PLR, MPV, and RDW have been studied for their associations with renal involvement; among these, only NLR (p = 0.001) was a significant risk factor in the present study.
Our study has some limitations. The major limitations of this study are its retrospective design and inclusion of children attending a single center. In addition, we only evaluated laboratory values during the acute phase of the disease; hence, we could not confirm the changes in laboratory data following renal involvement before, during, and after. Therefore, multicenter prospective studies including larger numbers of patients are needed to confirm and generalize our findings.
In conclusion, renal involvement in HSP was independent of GI or joint involvement. Female sex and high NLR were potential risk factors for renal involvement in HSP patients and a very close follow-up is required in patients with these risk factors.
FundingThis study was supported by a grant (CRI18024-1 and BCRI20076) from Chonnam National University Hospital Biomedical Research Institute, Gwangju, Republic of Korea.
Conflicts of interestThe authors declare no conflicts of interest.