The pandemic caused by influenza A(H1N1)pdm09 virus peaked between July and August of 2009 in southern Brazil, with the highest incidence in children and young adults. In the post-pandemic period, there was an increase in the incidence of cases during the winter months of 2011 and 2012 in Brazil, similar to seasonal influenza virus. Since infections due to pandemic influenza are still occurring, the present study aimed to investigate the risk factors for worse outcome in children.
MethodsA retrospective cohort study was performed by reviewing the charts of hospitalized patients younger than 14 years with reverse transcription-polymerase chain reaction (RT-PCR) positive for influenza A(H1N1)pdm09 during the first pandemic wave in six Brazilian tertiary centers. Need for mechanical ventilation was defined as the severity of outcome; age, chronic diseases, bacterial and viral co-detection, chest radiograph findings, and use of oseltamivir were possible predictors.
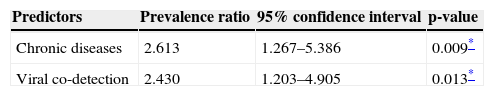
ResultsIn the present study, 120 patients were included. In a multivariate analysis, chronic diseases (prevalence ratio: 2.613, 95% CI: 1.267-5.386) and viral co-detection (prevalence ratio: 2.43, 95% CI: 1.203-4.905) were statistically associated with worse outcome (p<0.05).
ConclusionsThe presence of chronic diseases as predictors reinforces previous finding. Furthermore, viral co-detection was found to be a risk factor. Further studies are necessary to confirm this association.
A pandemia causada pelo vírus Influenza A(H1N1)pdm09 teve seu pico nos meses de julho e agosto de 2009, no Sul do Brasil, sendo a incidência mais alta em crianças e adultos jovens. No período pós-pandêmico, no Brasil, houve aumento de casos nos meses de inverno dos anos de 2011 e 2012, de forma semelhante ao vírus influenza sazonal. Como ainda estão ocorrendo infecções devido ao influenza pandêmico, nosso objetivo foi investigar fatores de risco para pior desfecho em crianças.
MétodosFoi realizado um estudo de coorte retrospectivo analisando as fichas de pacientes menores de 14 anos hospitalizados e com RT-PCR positiva para Influenza A(H1N1)pdm09 durante a primeira onda, em seis centros terciários brasileiros. Definimos a necessidade de ventilação mecânica como desfecho com gravidade e, como possíveis preditores, os fatores idade, doenças crônicas, codetecção bacteriana e viral, achados da radiografia do tórax e uso de oseltamivir.
ResultadosNo presente estudo, foram incluídos120 pacientes. Em uma análise multivariada, doenças crônicas (razão de prevalência: 2,613; intervalo de confiança de 95%: 1,267 a 5,386) e codetecção viral (razão de prevalência: 2,43; intervalo de confiança de 95%: 1,203 a 4,905) se associaram estatisticamente a um pior desfecho (p<0,05).
ConclusõesA presença de doenças crônicas como preditores reforça evidências prévias. Além disso, verificamos que a codetecção viral é fator de risco. São necessários outros estudos para confirmar essa associação.
In March and April of 2009, casualties from influenza-like illness showed a rise in Mexico and the United States. Caused by a new strain of influenza A (H1N1), it rapidly emerged as the new influenza pandemic, which caused a considerable number of deaths by respiratory failure throughout the world in the following months.1,2 As we entered the post-pandemic period in August 2010, the virus continues to circulate in many countries. In 2011, the World Health Organization (WHO) standardized the name of the new virus: influenza A(H1N1)pdm09. In Brazil, infections due to influenza A(H1N1)pdm09 are occurring after the first pandemic wave with a seasonal pattern, mainly in the South and Southeast regions, and are still causing several deaths each year, mostly in winter months.3 Although in most pediatric patients the disease is asymptomatic or mild, with self-limited course, a small proportion of patients die, primarily due to respiratory failure.4,5 Many respiratory viruses also have seasonality in winter months, and a clinical distinction between influenza A(H1N1)pdm09 and other respiratory viruses is difficult, because the signs and symptoms are not specific.6 As a result, the incidence of cases defined as influenza-like illness is usually much higher than real influenza-associated disease.7 As an additional difficulty, laboratory diagnosis of influenza A(H1N1)pdm09 is problematic in most clinical settings, due to lack of sensitivity of rapid tests and direct immunofluorescence assay (DFA), which are the most available tests.8 In an attempt to better guide interventions, several studies were conducted in children with influenza A(H1N1)pdm09, searching for risk factors for worse outcome, defined as intensive care unit (ICU) admission, need for mechanical ventilation (MV), or death.9,10 The most consistent finding as predictor for these outcomes was the presence of one or more chronic diseases (CD), particularly neurologic impairment.11
Others findings, such as chest X-ray with diffuse infiltrate on admission, anemia, and delay in the initiation of antiviral therapy have also been mentioned, but not uniformly observed.12,13 This combination of factors leads to difficulties in the management of suspected cases, since many of these patients are not actually infected by influenza A(H1N1)pdm09 but, based on clinical and laboratory findings, it is not possible rule out infection. Furthermore, predictors to poor outcome in pediatric patients are not completely understood, so most children with influenza-like illness are at risk of unfavorable outcome, and have indication for antiviral therapy.
These factors may explain why, during the pandemic, local public health authorities in Brazil recommended priority of antiviral treatment for children younger than 2 years, and for those with CD. In 2012, these recommendations changed, and treatment was suggested for most children with influenza-like illness.14 In this context, it is important to continue to investigate the risk factors for respiratory failure, in order to obtain more accurate recommendations for vaccination and treatment. This study aimed to identify the risk factors for need of MV and to describe deaths that occurred in children due to influenza A(H1N1)pdm09 infections during the first pandemic wave in Porto Alegre, southern Brazil.
MethodsA retrospective cohort study was performed through the review of medical charts of children admitted to six tertiary pediatric centers in Porto Alegre, Brazil, during the first pandemic wave in 2009, from July 2 to October 15. Porto Alegre is the southernmost state capital of Brazil, with a population of 1.5 million people. All pediatric hospitalizations in Porto Alegre occurred at one of the six hospitals included in this study. All socioeconomic groups were considered in these six institutions, with one private hospital, two partially private, and three public hospitals. All patients younger than 14 years who had laboratory-confirmed infection with influenza A(H1N1)pdm09 determined by reverse transcription-polymerase chain reaction (RT-PCR), were eligible. Local guidelines recommended testing all children admitted with influenza-like illness during the pandemic. Those whose medical charts were incomplete to allow data collection of possible predictors and outcome and those whose medical charts were not found were excluded. All patients names were provided by the Rio Grande do Sul Health Department, the division responsible for the active surveillance and testing of influenza A(H1N1)pdm09. All charts were reviewed by the same investigator. Need for invasive or non-invasive MV attributed to influenza A (H1N1)pdm09 was defined as the main outcome, as a surrogate for outcome severity, and the possible predictors among patients who required MV and those who did not were compared. The independent variables investigated as possible predictors for the outcome were gender, age, CD, bacterial and viral co-detection during clinical course, initiation of oseltamivir before and after 48hours of signs and symptoms, and abnormalities in report of chest X-ray at admission (diffuse interstitial infiltrate, consolidation, or both). “Use of oseltamivir” was considered only in patients who actually received medication in the correct dose for 5 days. Correct doses were, for those older than 1 year, 75, 60, 45, and 30 (mg) twice daily for weight rangers greater than 40kg, 23-40kg, 15-23kg, and less than 15kg, respectively. In children younger than 1 year of age, the doses considered correct were 25, 20, and 12mg twice daily for ages 6-11, 3-5, and less than 3 months, respectively.15
It was decided to define bacterial and viral co-detection instead of co-infection since the causal link between pathogen detection and disease is not always possible. Bacterial co-detection was defined as a positive culture for a possible pathogen in respiratory secretions, blood, or other sterile fluid. Viral co-detection was defined as the finding of one or more different viruses detected in respiratory secretion samples determined by RT-PCR or DFA. Performing DFA can detect respiratory syncytial virus (RSV), parainfluenza 1 to 3, adenovirus, and influenza A and B. Tests were performed in the same specimens by RT-PCR in the majority of cases, or, at most, 48hours apart. Chronic diseases were defined as diagnosis of chronic cardiac and respiratory disorders, neurologic impairment, chronic renal insufficiency, malignancy, and immunosuppression based on the diagnosis provided on the charts. As a secondary objective, patients’ deaths were described in detail, as well as the performance of DFA for influenzaA(H1N1)pdm09, comparing to RT-PCR. With a convenience sample of 130 patients, expecting that 25% of the children will need MV, a risk of 2.5 between the predictor variables and the outcome was detected. Demographics were summarized as mean or median and interquartile range according to their distribution. Poisson regression with robust variance was used to analyze the relationships between the main outcome (use of MV) and the predictor variables (gender, age, CD, presence of viral co-detection, use of oseltamivir, and abnormal chest X-ray).16 The Wald's test was used to assess statistical significance. Initially, all covariates that presented p < 0.10 were included in the multivariate model. The next step was the individual exclusion of the covariates that presented critical p-values (values that were not significant). This step was repeated until all variables remaining in the model presented p < 0.05. For statistical analysis, the cut-off probability for rejecting the null hypothesis was defined as less than 5% (p < 0.05). All analyzes were performed using the Statistical Package for Social Sciences (SPSS) v.13 (SPSS Inc, Chicago, IL).
This study was approved by the institutional review boards of all six hospitals, which did not require informed consent due to the retrospective design of the study. Confidentiality regarding patients’ identity was ensured.
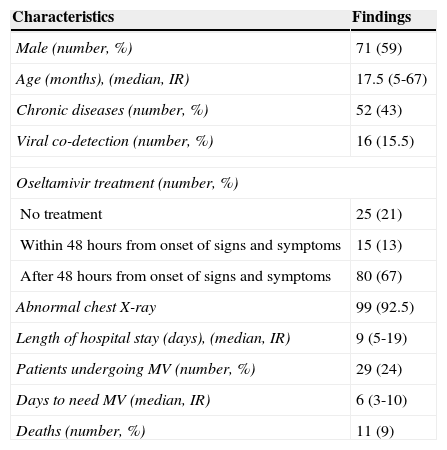
ResultsThe hospital records of 136 children admitted with confirmed influenza A (H1N1)pdm09 between July 2 and October 15 of 2009 in Porto Alegre were reviewed. During this period, the hospitals reported 450 potential cases of influenza A(H1N1)pdm09 in children younger than 14 years of age. Of these, 437 children were tested with RT-PCR, with 136 positive results (31.1% of those tested). Records for 16 children were not found. Table 1 presents the demographic features of the remaining 120 patients. Thirteen patients were from the private hospital, 45 were from partially private hospitals, and 62 were from public institutions. Seventy-one patients were male (59%), and the median age was 17.5 months old. Among the 52 patients with CD, the following diseases were identified, with their respective number of patients: neurologic impairment (17); asthma (10); wheezing infants (seven); preterm infants (five with and two without bronchopulmonary dysplasia); malignancies receiving chemotherapy (five); obliterans bronchiolitis (two); and laryngotracheomalacia, asplenia, corticotherapy in immunosuppressive dose (2mg/kg/day for more than two weeks), short bowel syndrome, and colostomy, with one patient each. Bacterial co-detection occurred in eight patients (6.7%), and this predictor was excluded from the model due to its low prevalence. Blood cell counts were also excluded from the analysis because these data were not available in a substantial proportion of patients at admission. DFA was performed in 103 of the 120 children, and viral co-detection was observed in 16 patients (15.5%).
Characteristics of 120 hospitalized children with influenza A(H1N1)pdm09 during the first pandemic wave in Porto Alegre, Brazil, July 2 to October 15, 2009.
| Characteristics | Findings |
|---|---|
| Male (number, %) | 71 (59) |
| Age (months), (median, IR) | 17.5 (5-67) |
| Chronic diseases (number, %) | 52 (43) |
| Viral co-detection (number, %) | 16 (15.5) |
| Oseltamivir treatment (number, %) | |
| No treatment | 25 (21) |
| Within 48hours from onset of signs and symptoms | 15 (13) |
| After 48hours from onset of signs and symptoms | 80 (67) |
| Abnormal chest X-ray | 99 (92.5) |
| Length of hospital stay (days), (median, IR) | 9 (5-19) |
| Patients undergoing MV (number, %) | 29 (24) |
| Days to need MV (median, IR) | 6 (3-10) |
| Deaths (number, %) | 11 (9) |
IR, interquartile range; MV, mechanical ventilation.
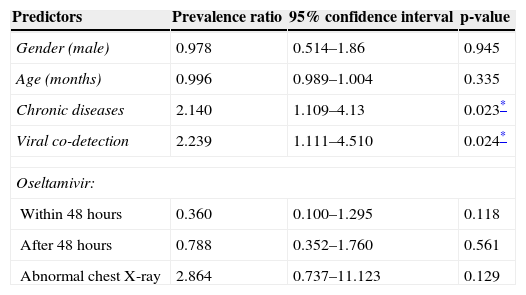
Patients not tested with DFA presented a similar MV/non-MV ratio (five of these patients needed MV). The other viruses found (and respective number of detections) were: RSV (12), influenza B (two), adenovirus (two), and parainfluenza type 3 (two). Two patients had two additional viruses detected. Influenza A(H1N1)pdm09 detection with DFA was achieved in 55.3% (57 patients) of patients tested. Reports of chest X-ray were available for 107 patients. All others predictors had no missing data. Forty-five children were admitted to an ICU, 41 were admitted to a pediatric ward, and 34 were admitted to an emergency unit. MV was required in 29 patients (24.2%), and 11 (9.1%) of them died. Tables 2 and 3 show the results of bivariate and multivariate analyses for risk of MV, respectively.
Univariate analysis of association between need for mechanical ventilation and potential predictors.
| Predictors | Prevalence ratio | 95% confidence interval | p-value |
|---|---|---|---|
| Gender (male) | 0.978 | 0.514–1.86 | 0.945 |
| Age (months) | 0.996 | 0.989–1.004 | 0.335 |
| Chronic diseases | 2.140 | 1.109–4.13 | 0.023* |
| Viral co-detection | 2.239 | 1.111–4.510 | 0.024* |
| Oseltamivir: | |||
| Within 48 hours | 0.360 | 0.100–1.295 | 0.118 |
| After 48 hours | 0.788 | 0.352–1.760 | 0.561 |
| Abnormal chest X-ray | 2.864 | 0.737–11.123 | 0.129 |
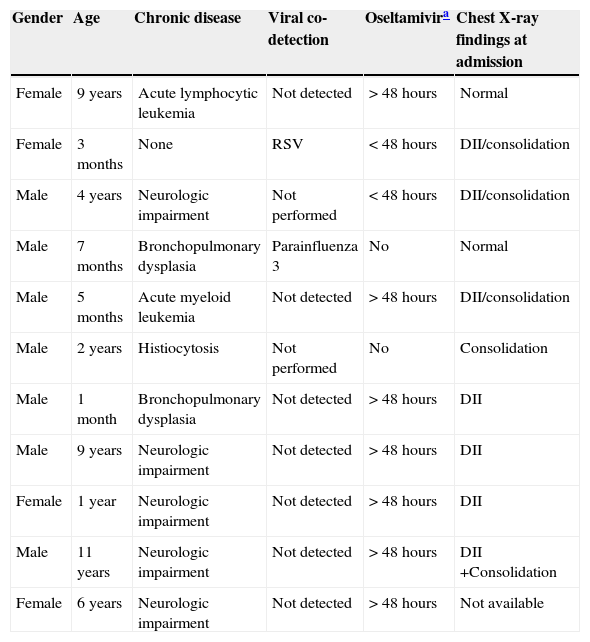
CD (PR 2.613; 95% CI 1.267-5.386; p=0.009) and viral co-detection (PR 2.430; 95% CI 1.203-4.905; p=0.013) were statistically associated with increased risk in multivariate analysis. Table 4 details all pediatric deaths.
Characteristics of 11 children who died with influenza A(H1N1)pdm09 infection in Porto Alegre, Brazil, from July 2 to October 15, 2009.
| Gender | Age | Chronic disease | Viral co-detection | Oseltamivira | Chest X-ray findings at admission |
|---|---|---|---|---|---|
| Female | 9 years | Acute lymphocytic leukemia | Not detected | >48hours | Normal |
| Female | 3 months | None | RSV | <48hours | DII/consolidation |
| Male | 4 years | Neurologic impairment | Not performed | <48hours | DII/consolidation |
| Male | 7 months | Bronchopulmonary dysplasia | Parainfluenza 3 | No | Normal |
| Male | 5 months | Acute myeloid leukemia | Not detected | >48hours | DII/consolidation |
| Male | 2 years | Histiocytosis | Not performed | No | Consolidation |
| Male | 1 month | Bronchopulmonary dysplasia | Not detected | >48hours | DII |
| Male | 9 years | Neurologic impairment | Not detected | >48hours | DII |
| Female | 1 year | Neurologic impairment | Not detected | >48hours | DII |
| Male | 11 years | Neurologic impairment | Not detected | >48hours | DII +Consolidation |
| Female | 6 years | Neurologic impairment | Not detected | >48hours | Not available |
Similarly to previous studies, this study found that CD predisposes to worse outcome.10,11 Furthermore, a statistically significant association was found between viral co-detection and need for MV, a finding that, to the authors’ knowledge, was only observed in one previous study in children IN WHICH Torres et al. found viral co-detection with RSV as a predictor of death in a multivariable analysis in children admitted during the first pandemic wave in Argentina.17 Esper et al. also found worse outcome in a mixed sample of both adults and children using RT-PCR.18 A minority of reports in pediatric patients mention the prevalence of viral co-detection, suggesting that tests for other viruses were not routinely performed in most situations.10,19–22 Although some studies with other viruses, such as RSV, did not show worse prognosis when two or more viruses were detected, the authors believe that co-detection of influenza A(H1N1)pdm09 with other viruses could lead to more serious damage to the airways. This might be caused by immune response, since Esper et al. have shown that influenza titers were not altered by co-infection.18,23
The limited sensitivity of DFA for most of respiratory viruses is a limitation of the present study, as well as in the study of Torres et al., as tests for other viruses were performed with DFA in both studies; the prevalence of 15.5% observed in the present study may be underestimated.24 The missing data in 17 patients for this predictor, together with the low sensitivity of DFA for viruses tested and the inability of this test to detect other prevalent viruses (such as rRhinovirus) could bias the present results. The finding of 55.3% influenza A(H1N1)pdm09 detection with DFA compared with RT-PCR highlights the low sensitivity of DFA, although most studies show sensitivities higher than that found in the present study.24 Nevertheless, only two studies in hospitalized pediatric population with influenza A(H1N1)pdm09 during the first pandemic wave in Argentina reported a higher prevalence of viral co-detection (19% and 25%).10,17 Another study in outpatients performed in Recife, Brazil, showed 78% of viral co-detection, but the detection of other viruses was performed by multiplex RT-PCR, which could, at least partially, explain the higher prevalence of co-detection.25 It is interesting that both studies with higher prevalence of co-detection than the present study were also performed in South America.
It is also important to highlight the characteristics of pediatric deaths in the present patients, shown in Table 4, as they followed a similar pattern of risk factors found using need for MV as outcome. All but one patient had important CD, reinforcing the association between this risk factor with a worse outcome. The single patient who did not present such conditions was only 3 month-old and had co-detection of RSV. No association of early initiation of oseltamivir with lower incidence of respiratory complications was found. This possible predictor was categorized instead of using it as a continuous variable, mainly due to the difficulties to precisely define the beginning of signs and symptoms in days, which might have resulted in loss of statistical power. However, there is a lack of evidence regarding the efficacy of this drug in preventing serious respiratory complications in most studies.26 Interestingly, some authors suggested, using animal models, that more than one antiviral drug is necessary to prevent complications in influenza A(H1N1)pdm09 infections.27 No association was found between abnormalities on chest X-rays and need for MV, but this analysis had serious limitations because the authors only had access to reports of findings. As this was a retrospective study, it has several limitations, such as loss and lack of accurate data, which led to the exclusion of blood cell count from the analysis, for example, besides all aforementioned limitations. It was not possible to know whether the 16 patients not included biased the findings, since their demographic data was not assessed.
Nevertheless, the authors believe that the present study is relevant because it is one of the first to assess risk factors for respiratory complications in Brazilian children with influenza A(H1N1)pdm09 infections, since there are few reports assessing pediatric hospitalizations during the pandemic in Brazil.28 The present findings highlight that all deaths occurred in the group indicated for antiviral treatment by the local public health authorities’ recommendations during the pandemic, reinforcing that high risk groups of children are more predictable than those of adults, in which more than 30% of deaths usually occur in patients without an identifiable predictor of poorer outcome.29 This information is very helpful to guide future preventive and therapeutic interventions. Viral co-detection may be a new identified predictor of complications, although more studies are necessary to confirm this association, preferably using more sensitive techniques such as RT-PCR.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the Rio Grande do Sul Health Departmentfor supplying the names of patients with confirmed infection. They also thank all participanting institutions for allowing data collection.
Please cite this article as: Scotta MC, Mattiello R, Maróstica PJ, Jones MH, Martins LG, Fischer GB. Risk factors for need of mechanical ventilation in children with Influenza A(H1N1)pdm09. J Pediatr (Rio J). 2013;89:444–9.