To determine whether three variants (388 G>A, 521 T>C, and 463 C>A) of the solute carrier organic anion transporter family member 1B1 (SLCO1B1) are associated with neonatal hyperbilirubinemia.
Data sourceThe China National Knowledge Infrastructure and MEDLINE databases were searched. The systematic review with meta-analysis included genetic studies which assessed the association between neonatal hyperbilirubinemia and 388 G>A, 521 T>C, and 463 C>A variants of SLCO1B1 between January of 1980 and December of 2012. Data selection and extraction were performed independently by two reviewers.
Summary of the findingsTen articles were included in the study. The results revealed that SLCO1B1 388 G>A is associated with an increased risk of neonatal hyperbilirubinemia (OR, 1.39; 95% CI, 1.07–1.82) in Chinese neonates, but not in white, Thai, Latin American, or Malaysian neonates. The SLCO1B1 521 T>C mutation showed a low risk of neonatal hyperbilirubinemia in Chinese neonates, while no significant associations were found in Brazilian, white, Asian, Thai, and Malaysian neonates. There were no significant differences in SLCO1B1 463 C>A between the hyperbilirubinemia and the control group.
ConclusionThis study demonstrated that the 388 G>A mutation of the SLCO1B1 gene is a risk factor for developing neonatal hyperbilirubinemia in Chinese neonates, but not in white, Thai, Brazilian, or Malaysian populations; the SLCO1B1 521 T>C mutation provides protection for neonatal hyperbilirubinemia in Chinese neonates, but not in white, Thai, Brazilian, or Malaysian populations.
Determinar se três variantes (388 G>A, 521 T>C, 463 C>A) do membro 1B1da família de transportadores de ânions orgânicos portadores de solutos (SLCO1B1) se associam à hiperbilirrubinemia neonatal.
Fonte de dadosFoi realizada busca na Infraestrutura do Conhecimento Nacional da China e em MEDLINE. A revisão sistemática com metanálise incluiu estudos genéticos que avaliaram a associação entre hiperbilirrubinemia neonatal e as variantes 388 G>A, 521 T>C, 463 C>A de SLCO1B1 entre janeiro de 1980 e dezembro de 2012. Foi realizada seleção e extração de dados por dois analistas, de forma independente.
Sumário dos achadosForam incluídos dez artigos no estudo. Os resultados revelaram que SLCO1B1 388 G>A se associa a um aumento do risco de hiperbilirrubinemia neonatal (OR< 1,39; IC 95%: 1,07 a 1,82) em recém-nascidos chineses, mas não em recém-nascidos caucasianos, tailandeses, latino-americanos ou malaios. A mutação SLCO1B1 521 T>C mostrou baixo risco de hiperbilirrubinemia neonatal em recém-nascido chineses, e não foram encontradas associações importantes no Brasil nem em recém-nascidos caucasianos, asiáticos, tailandeses e malaios. Não houve diferenças significativas da SLCO1B1 463 C>A entre o grupo com hiperbilirrubinemia e o grupo controle.
ConclusãoO estudo mostrou que a mutação 388 G>A do gene SLCO1B1 é fator de risco para desenvolver hiperbilirrubinemia neonatal em recém-nascidos chineses, mas não em populações caucasianas, tailandesas, brasileiras ou malaias; a mutação SLCO1B1 521 T>C fornece proteção de hiperbilirrubinemia neonatal em recém-nascidos chineses, mas não nas populações caucasianas, tailandesas, brasileiras ou malaias.
Hyperbilirubinemia is the most common clinical condition in newborns. Between 8% and 11% of neonates develop significant hyperbilirubinemia, defined as total serum bilirubin (TSB) above the 95th percentile for age (high-risk zone) during the first week of life.1 The levels of TSB rise to the high-risk zone, leading to long-term consequences, including bilirubin-induced encephalopathy and kernicterus.2 Despite the advent of phototherapy and exchange transfusion, kernicterus continues to be reported worldwide, especially in developing countries.3
Therefore, the identification of infants at risk of developing neonatal hyperbilirubinemia has become particularly important.4 There are many factors that could account for the development of neonatal hyperbilirubinemia, including ABO or Rh incompatibilities, deficiency of glucose-6-phosphate dehydrogenase (G6PD) and pyruvate kinase, hereditary spherocytosis, defective hemoglobin synthesis, hypothyroidism, breast milk jaundice, and cephalohematoma, among others.5
Several clinical genetic disorders influence bilirubin physiology. The UDP-glycosyltransferase 1 family, polypeptide A1 (UGT1A1), and solute carrier organic anion transporter family enzymes organic anion transporter polypeptide 2 (OATP2) are responsible for glucuronidation and cellular uptake of bilirubin, respectively, and play an important role in regulating the bilirubin levels.6 OATP2 is located on the basolateral (sinusoidal) membrane of human hepatocytes, and is encoded by the gene of the solute carrier organic anion transporter family member 1B1 (SLCO1B1).
Recent studies have suggested that the variations 388 G>A, 521 T>C, and 463 C>A of the SLCO1B1 gene may predispose subjects to neonatal hyperbilirubinemia by limiting hepatic bilirubin uptake.7 They vary in different populations, with a high prevalence of the 388 G>A (73.4%) and 521 T>C (14.0%) variants occurring in Chinese subjects.8 A 16% prevalence of the 463 C>A variant has been reported in Europeans and Americans.9 Neonatal hyperbilirubinemia is known to occur more frequently and to be more severe in Asians than in whites.10 The authors hypothesized that SLCO1B1 mutation may be one of the risk factors for neonatal hyperbilirubinemia, which possibly accounts for the variability in prevalence rates among different ethnic groups. The role of SLCO1B1 gene in neonatal hyperbilirubinemia is still controversial. Thus, the objective of this systematic review with meta-analysis was to assess the impact of the three variants (388 G>A, 521 T>C, 463 C>A) of SLCO1B1 on hyperbilirubinemia in neonates of different ethnicities.
MethodsThis systematic review with meta-analysis was based on a method recommended by the Human Genome Epidemiology Network (http://www.cdc.gov/genomics/hugenet).
Selection of studiesThe electronic databases of the China National Knowledge Infrastructure and MEDLINE were searched for all case-control or cohort studies that evaluated SLCO1B1 in association with neonatal hyperbilirubinemia between January of 1980 and December of 2012, using the following search strategy: (Hyperbilirubinemia, Neonatal OR (Bilirubin AND Infant, Newborn) OR Jaundice, Neonatal) AND (Organic Anion Transporters OR Polymorphism, Restriction Fragment Length OR SLCO1B1 protein, Organic Anion Transport Polypeptide C OR Genetic Predisposition to Disease OR Polymorphism).
The MEDLINE database was searched using the following search strategy: (Hyperbilirubinemia, Neonatal OR (Bilirubin AND Infant, Newborn) OR Jaundice, Neonatal) AND (Organic Anion Transporters OR Polymorphism, Restriction Fragment Length OR SLCO1B1 protein, human OR DNA Mutational Analysis OR Gene Frequency OR Genotype OR Mutation OR Organic Anion Transport Polypeptide C OR Genetic Predisposition to Disease OR Polymorphism, Genetic OR DNA OR Polymorphism, Single Nucleotide). No language restrictions were applied.
Inclusion and exclusion criteriaPolymorphisms related to neonatal hyperbilirubinemia were divided into three groups according to the three variants (388 G>A, 521 T>C, and 463 C>A) of SLCO1B1. Case-control, cohort, and family-based studies presenting original data on associations between the genetic polymorphisms and neonatal hyperbilirubinemia were eligible for inclusion, provided that (i) the cases of neonatal hyperbilirubinemia were included according to the diagnostic criteria utilized in various countries; (ii) the control group consisted of comparable infants without a history of hyperbilirubinemia; (iii) the enrollment of participants was made based on prior knowledge of genotype, and genotyping; (iv) the study reported the ethnic ancestry of participants; and (v) the reported genotype distributions were in Hardy-Weinberg equilibrium. Hardy-Weinberg equilibrium was performed by chi-squared analysis. Exclusion criteria included review articles as well as articles that studied populations aged up to 28 days.
Data extractionTwo investigators (Long J and Zhang SF) extracted data independently. When conflicting evaluations occurred, an agreement was reached after a discussion. Briefly, for all studies, the following data were extracted from the original publications: first author and year of publication; genes and relevant polymorphisms; neonatal hyperbilirubinemia definition; study population; number of genotyped cases and controls; frequencies of genotype; and SLCO1B1 gene polymorphism genotyping information.
Statistical analysisStata software (version 9.0; Stata Corp. LP - College Station, TX, USA) was used to pool data from case–control or cohort studies. These studies mainly provided three genotypes, and these genotype groups were assessed using allelic comparisons and mutant comparisons (heterozygous and homozygous mutant type vs. homozygous wild type). Results were given as odds ratios (OR) with 95% confidence intervals (CI), and a p-value < 0.05 was considered to be statistically significant. The heterogeneity assumption was checked using an I2 statistic. An I2 value of > 50% signified “substantial heterogeneity”, and a random effects model was used. An I2 value of ≤ 50% showed the absence of heterogeneity and defaulted to the fixed effects model approach. Funnel plots and Egger's linear regression test were used to identify potential publication bias, and p < 0.05 was considered indicative of statistically significant publication bias.
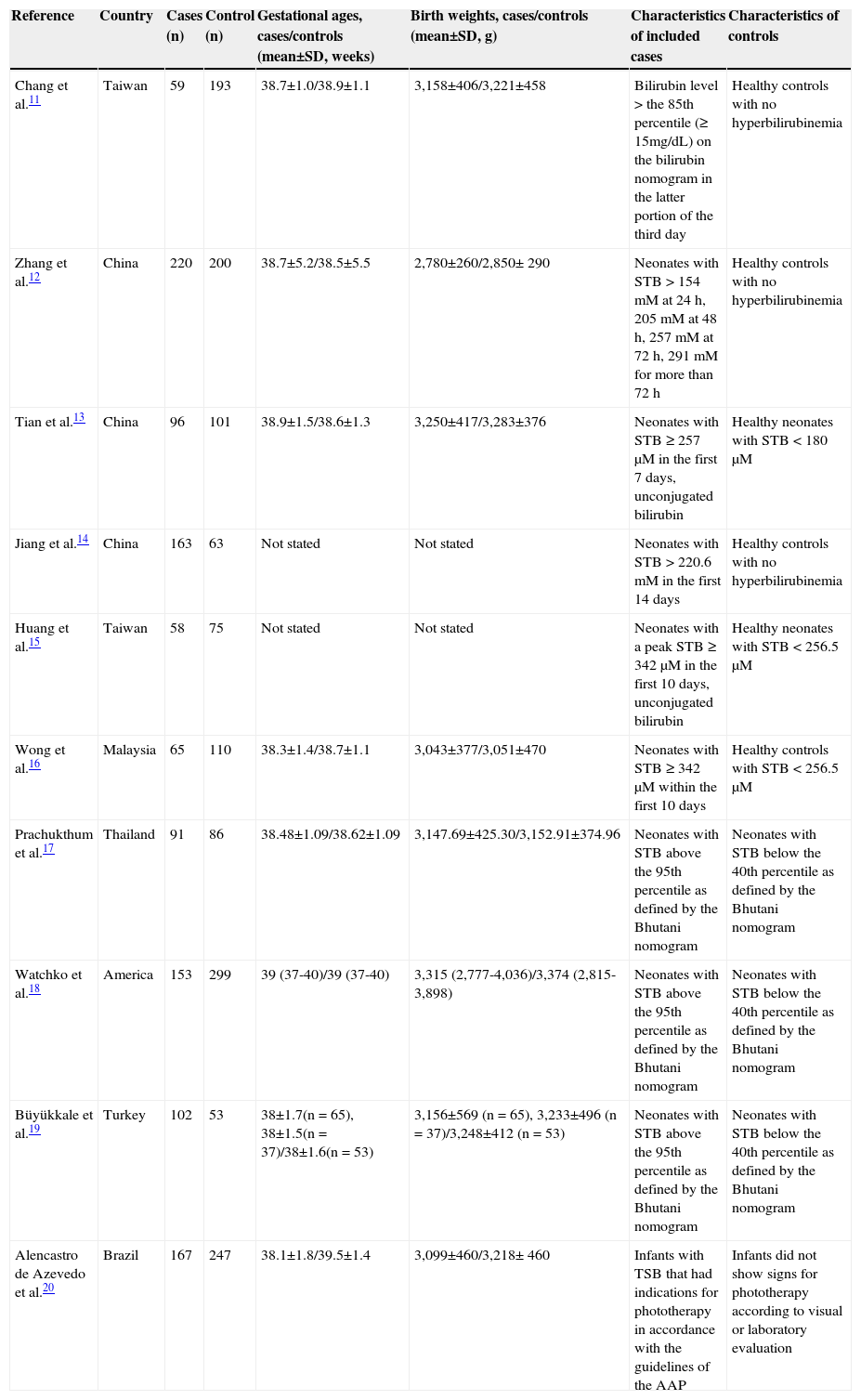
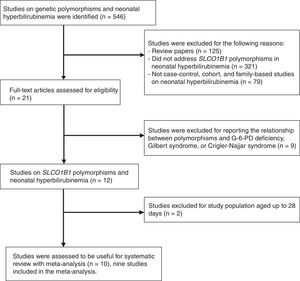
ResultsThe literature search identified 546 articles on the association between genetic polymorphisms and neonatal hyperbilirubinemia. Of these, 536 were subsequently excluded after screening of abstracts or full texts. Ultimately, 10 articles were assessed as useful for the systematic review with meta-analysis,11–20 and nine studies were included in the meta-analysis.11–19 The flow diagram of study identification is shown in Fig. 1. These studies were conducted on six countries (China, Malaysia, Thailand, the United States, Brazil, and Turkey). They included 1,164 cases of neonatal hyperbilirubinemia and 1,416 controls. The characteristics of the included studies are summarized in Table 1.
Characteristics of included studies.
| Reference | Country | Cases (n) | Control (n) | Gestational ages, cases/controls (mean±SD, weeks) | Birth weights, cases/controls (mean±SD, g) | Characteristics of included cases | Characteristics of controls |
|---|---|---|---|---|---|---|---|
| Chang et al.11 | Taiwan | 59 | 193 | 38.7±1.0/38.9±1.1 | 3,158±406/3,221±458 | Bilirubin level > the 85th percentile (≥ 15mg/dL) on the bilirubin nomogram in the latter portion of the third day | Healthy controls with no hyperbilirubinemia |
| Zhang et al.12 | China | 220 | 200 | 38.7±5.2/38.5±5.5 | 2,780±260/2,850± 290 | Neonates with STB > 154 mM at 24 h, 205 mM at 48 h, 257 mM at 72 h, 291 mM for more than 72 h | Healthy controls with no hyperbilirubinemia |
| Tian et al.13 | China | 96 | 101 | 38.9±1.5/38.6±1.3 | 3,250±417/3,283±376 | Neonates with STB ≥ 257μM in the first 7 days, unconjugated bilirubin | Healthy neonates with STB < 180μM |
| Jiang et al.14 | China | 163 | 63 | Not stated | Not stated | Neonates with STB > 220.6 mM in the first 14 days | Healthy controls with no hyperbilirubinemia |
| Huang et al.15 | Taiwan | 58 | 75 | Not stated | Not stated | Neonates with a peak STB ≥ 342μM in the first 10 days, unconjugated bilirubin | Healthy neonates with STB < 256.5μM |
| Wong et al.16 | Malaysia | 65 | 110 | 38.3±1.4/38.7±1.1 | 3,043±377/3,051±470 | Neonates with STB ≥ 342μM within the first 10 days | Healthy controls with STB < 256.5μM |
| Prachukthum et al.17 | Thailand | 91 | 86 | 38.48±1.09/38.62±1.09 | 3,147.69±425.30/3,152.91±374.96 | Neonates with STB above the 95th percentile as defined by the Bhutani nomogram | Neonates with STB below the 40th percentile as defined by the Bhutani nomogram |
| Watchko et al.18 | America | 153 | 299 | 39 (37-40)/39 (37-40) | 3,315 (2,777-4,036)/3,374 (2,815-3,898) | Neonates with STB above the 95th percentile as defined by the Bhutani nomogram | Neonates with STB below the 40th percentile as defined by the Bhutani nomogram |
| Büyükkale et al.19 | Turkey | 102 | 53 | 38±1.7(n = 65), 38±1.5(n = 37)/38±1.6(n = 53) | 3,156±569 (n = 65), 3,233±496 (n = 37)/3,248±412 (n = 53) | Neonates with STB above the 95th percentile as defined by the Bhutani nomogram | Neonates with STB below the 40th percentile as defined by the Bhutani nomogram |
| Alencastro de Azevedo et al.20 | Brazil | 167 | 247 | 38.1±1.8/39.5±1.4 | 3,099±460/3,218± 460 | Infants with TSB that had indications for phototherapy in accordance with the guidelines of the AAP | Infants did not show signs for phototherapy according to visual or laboratory evaluation |
AAP, American Academy of Pediatrics; SD, standard deviation; STB, serum total bilirubin; TSB, total serum bilirubin.
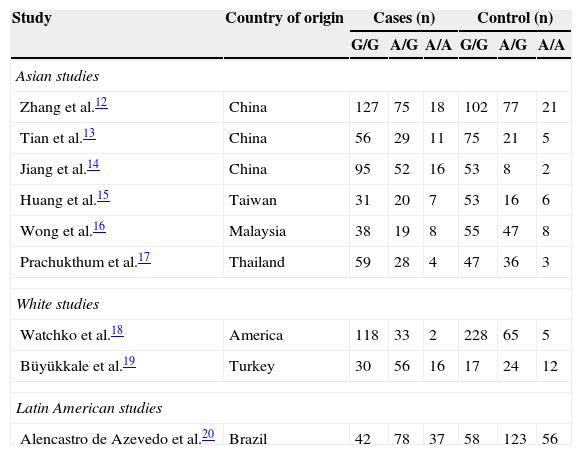
In one study included in the systematic review, there were no statistically significant differences in the risk of neonatal hyperbilirubinemia for the 388 G>A and 521 T>C variants of SLCO1B1.11 Nine studies were included in the meta-analysis, which assessed the association between the SLCO1B1 388 G>A mutation and neonatal hyperbilirubinemia (Table 2).12–20
Genotypic frequencies of SLCO1B1 388 G>A in neonatal hyperbilirubinemic cases and in controls.
| Study | Country of origin | Cases (n) | Control (n) | ||||
|---|---|---|---|---|---|---|---|
| G/G | A/G | A/A | G/G | A/G | A/A | ||
| Asian studies | |||||||
| Zhang et al.12 | China | 127 | 75 | 18 | 102 | 77 | 21 |
| Tian et al.13 | China | 56 | 29 | 11 | 75 | 21 | 5 |
| Jiang et al.14 | China | 95 | 52 | 16 | 53 | 8 | 2 |
| Huang et al.15 | Taiwan | 31 | 20 | 7 | 53 | 16 | 6 |
| Wong et al.16 | Malaysia | 38 | 19 | 8 | 55 | 47 | 8 |
| Prachukthum et al.17 | Thailand | 59 | 28 | 4 | 47 | 36 | 3 |
| White studies | |||||||
| Watchko et al.18 | America | 118 | 33 | 2 | 228 | 65 | 5 |
| Büyükkale et al.19 | Turkey | 30 | 56 | 16 | 17 | 24 | 12 |
| Latin American studies | |||||||
| Alencastro de Azevedo et al.20 | Brazil | 42 | 78 | 37 | 58 | 123 | 56 |
SLCO1B1, solute carrier organic anion transporter family member 1B1.
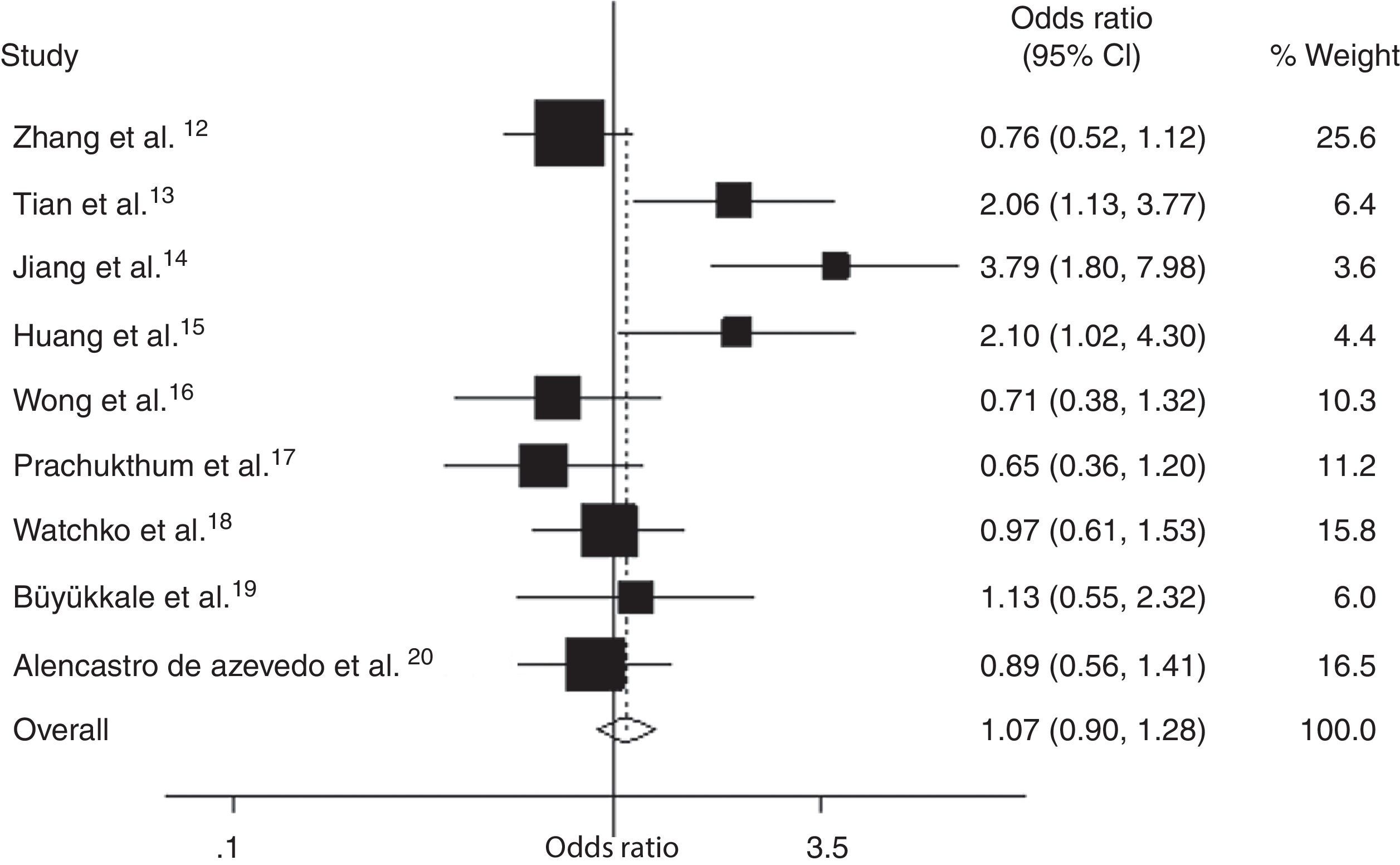
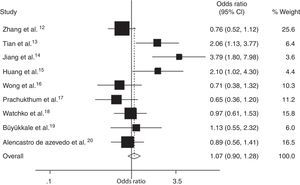
Results of the meta-analysis indicated that there was no statistically significant difference in the risk of neonatal hyperbilirubinemia between SLCO1B1 388 G>A allele carriers (A/A+G/A) and G/G allele carriers (OR, 1.07; 95% CI: 0.90–1.28) (Fig. 2). A significant inter-study heterogeneity was observed (p = 0.00). Egger's test provided no evidence for funnel plot asymmetry in the comparison of the SLCO1B1 388 G>A mutation and neonatal hyperbilirubinemia (t = 2.12, p = 0.07).
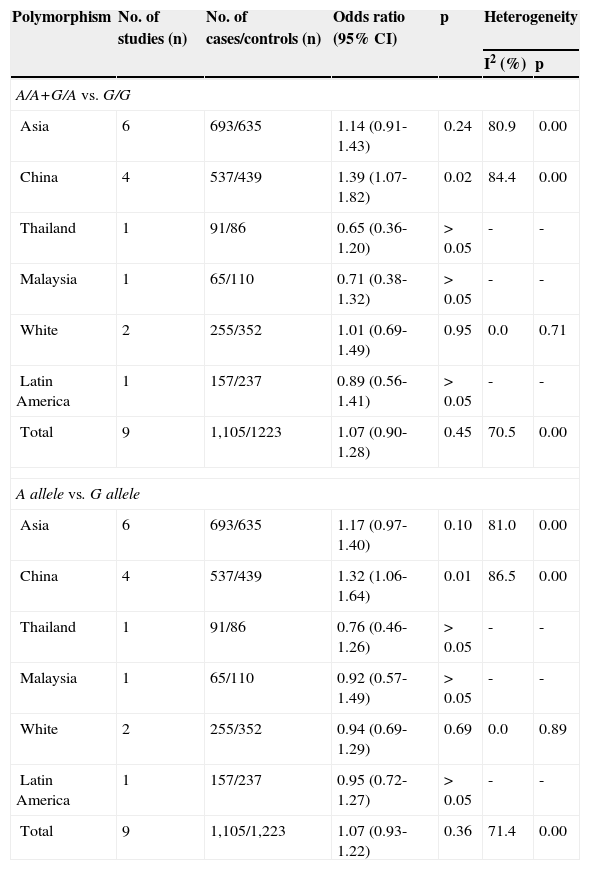
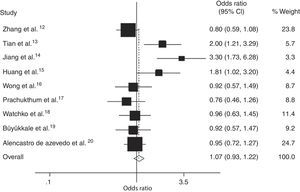
Additionally, in the subgroup analyses based on ethnicity, no significant associations were found in white (OR, 1.01; 95% CI: 0.69–1.49), Asian, Thai, Latin American, or Malaysian populations (Table 3). However, significantly elevated risks were found in the SLCO1B1 388 G>A variant genotypes in Chinese neonates (OR, 1.39; 95% CI: 1.07–1.82). A significant inter-study heterogeneity was also observed in subgroup analysis of Asian populations (p = 0.02).
Subgroup analysis of SLCO1B1 388 G>A in neonatal hyperbilirubinemic cases and in controls.
| Polymorphism | No. of studies (n) | No. of cases/controls (n) | Odds ratio (95% CI) | p | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2 (%) | p | |||||
| A/A+G/A vs. G/G | ||||||
| Asia | 6 | 693/635 | 1.14 (0.91-1.43) | 0.24 | 80.9 | 0.00 |
| China | 4 | 537/439 | 1.39 (1.07-1.82) | 0.02 | 84.4 | 0.00 |
| Thailand | 1 | 91/86 | 0.65 (0.36-1.20) | > 0.05 | - | - |
| Malaysia | 1 | 65/110 | 0.71 (0.38-1.32) | > 0.05 | - | - |
| White | 2 | 255/352 | 1.01 (0.69-1.49) | 0.95 | 0.0 | 0.71 |
| Latin America | 1 | 157/237 | 0.89 (0.56-1.41) | > 0.05 | - | - |
| Total | 9 | 1,105/1223 | 1.07 (0.90-1.28) | 0.45 | 70.5 | 0.00 |
| A allele vs. G allele | ||||||
| Asia | 6 | 693/635 | 1.17 (0.97-1.40) | 0.10 | 81.0 | 0.00 |
| China | 4 | 537/439 | 1.32 (1.06-1.64) | 0.01 | 86.5 | 0.00 |
| Thailand | 1 | 91/86 | 0.76 (0.46-1.26) | > 0.05 | - | - |
| Malaysia | 1 | 65/110 | 0.92 (0.57-1.49) | > 0.05 | - | - |
| White | 2 | 255/352 | 0.94 (0.69-1.29) | 0.69 | 0.0 | 0.89 |
| Latin America | 1 | 157/237 | 0.95 (0.72-1.27) | > 0.05 | - | - |
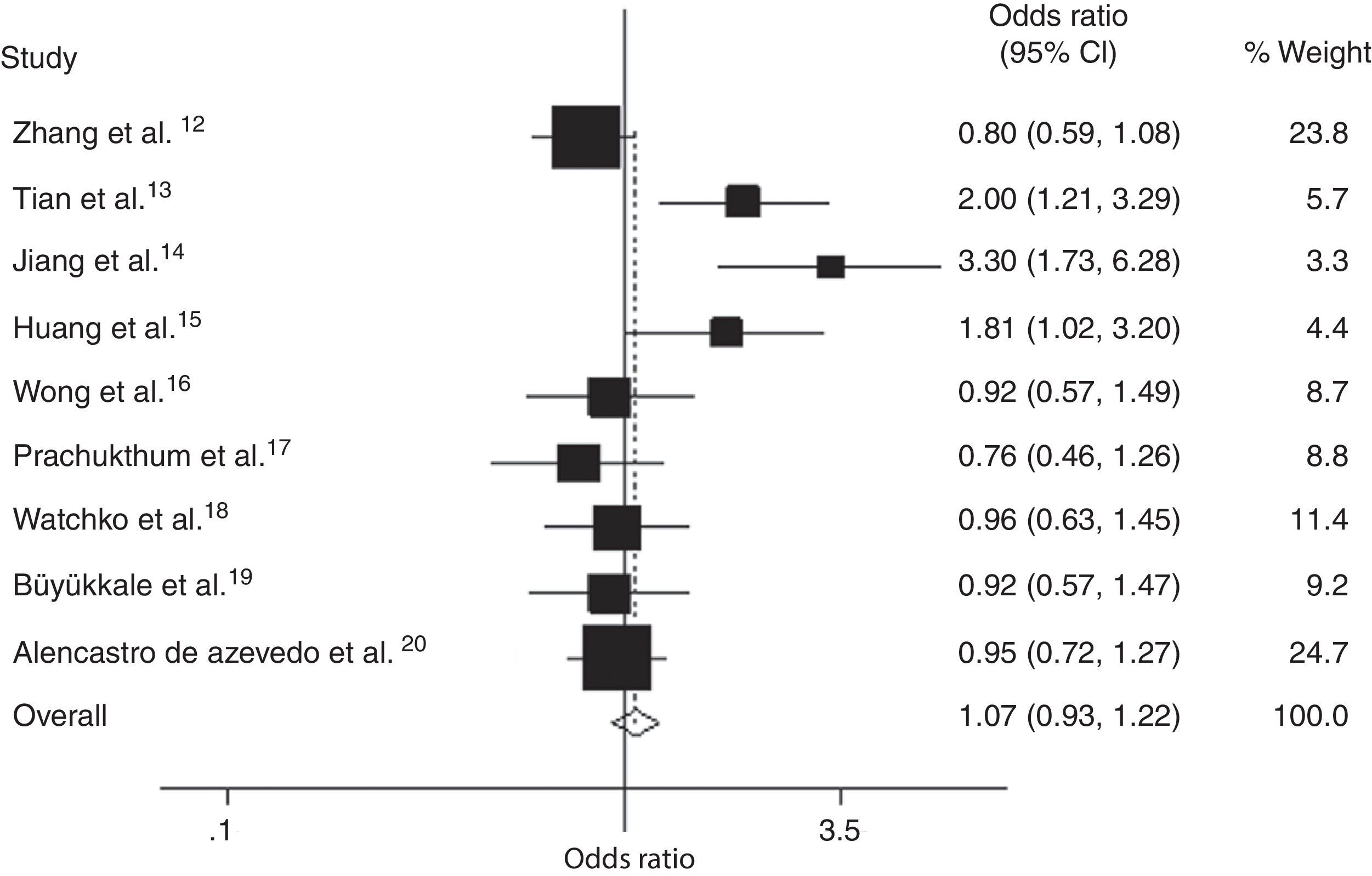
| Total | 9 | 1,105/1,223 | 1.07 (0.93-1.22) | 0.36 | 71.4 | 0.00 |
CI, confidence interval; SLCO1B1, solute carrier organic anion transporter family member 1B1.
Meta-analysis comparing the A allele to the G allele in the SLCO1B1 388 G>A mutation also showed an increased risk of neonatal hyperbilirubinemia (OR, 1.32; 95% CI: 1.06–1.64) in Chinese neonates, but not in white, Thai, Latin American, or Malaysian populations (Fig. 3 and Table 3). Significant inter-study heterogeneity was also observed in subgroup analyses of Asian and Chinese neonates, but not in white populations. Egger's test provided no evidence for funnel plot asymmetry in the comparison of the SLCO1B1 388 G>A mutation and neonatal hyperbilirubinemia (t = 2.29, p = 0.06).
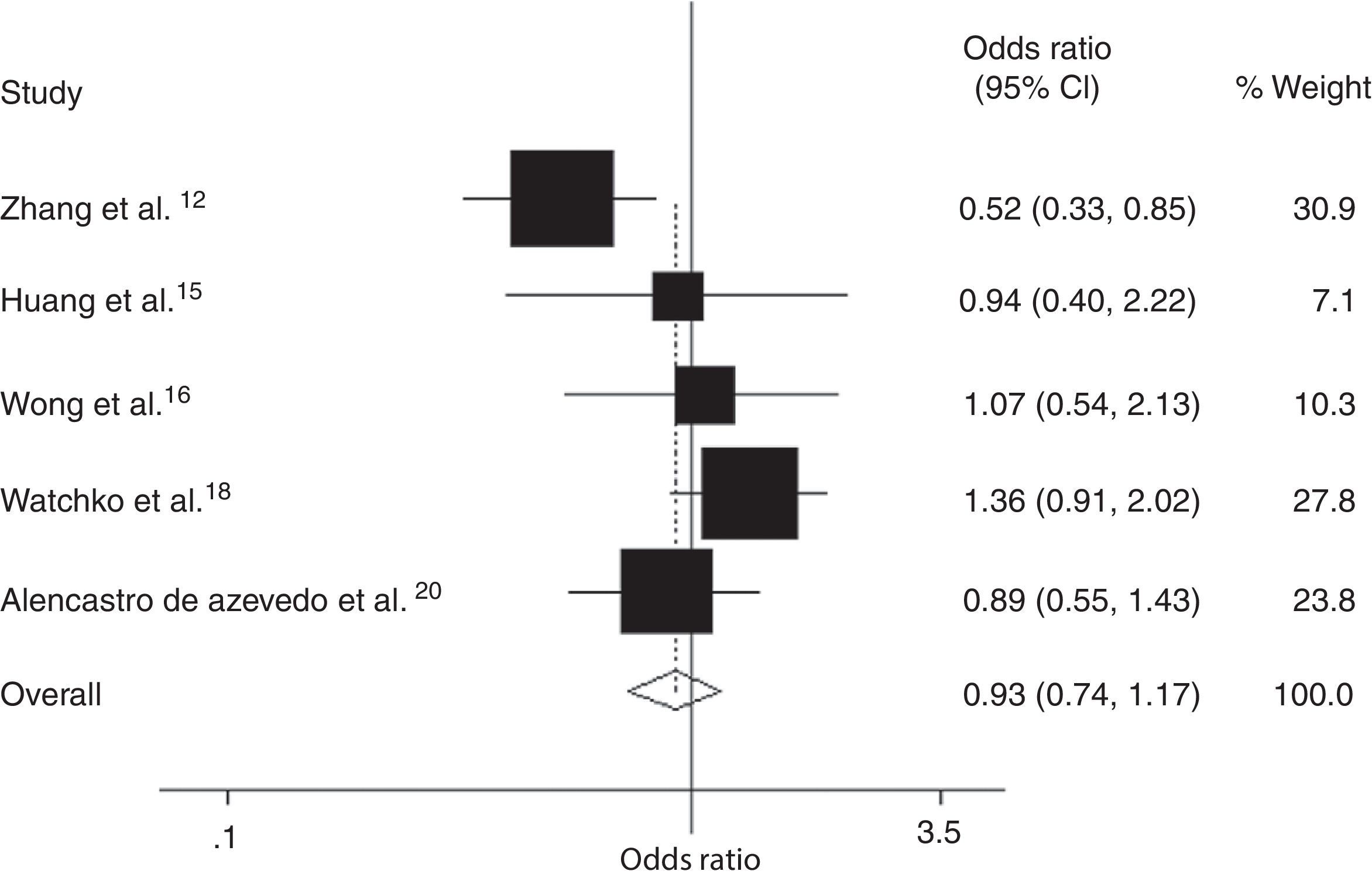
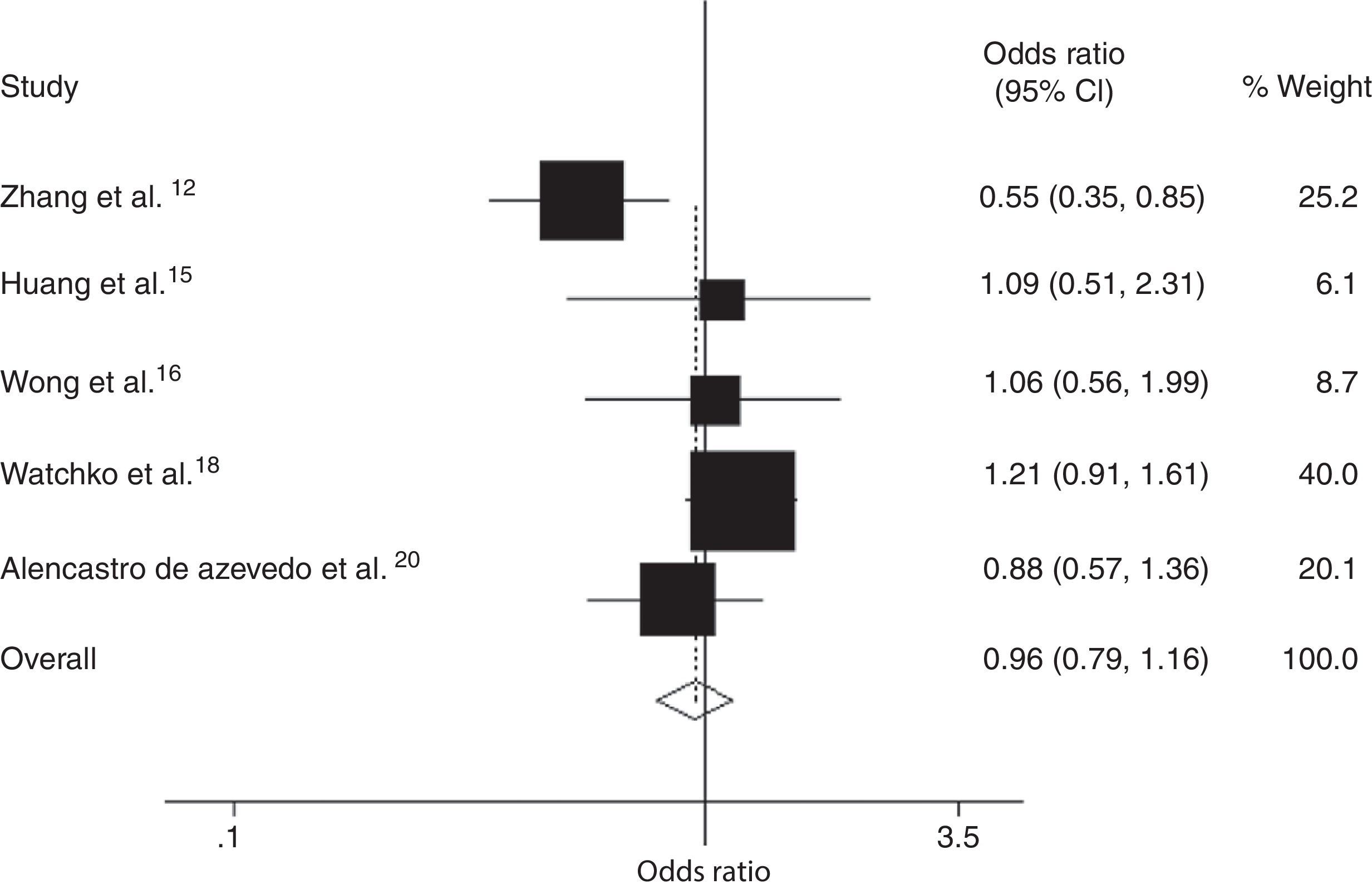
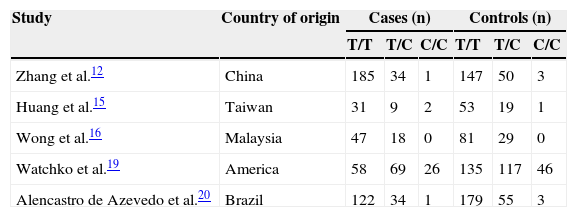
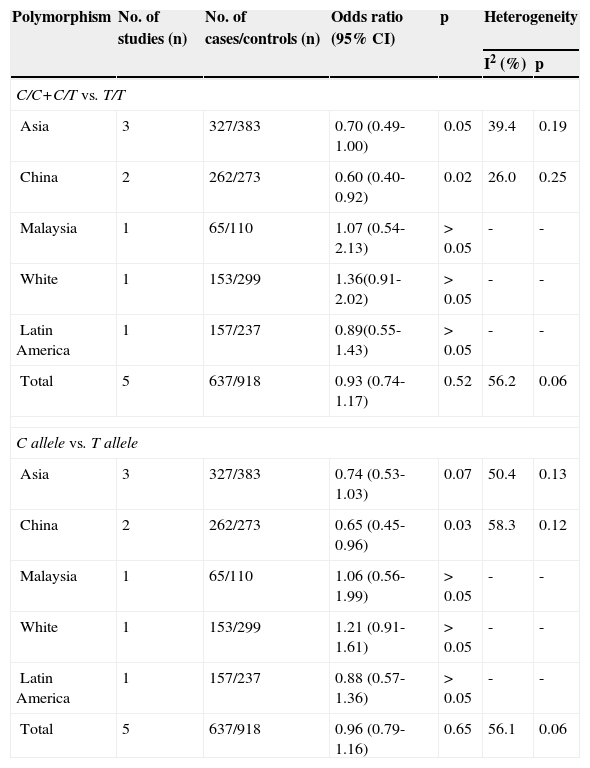
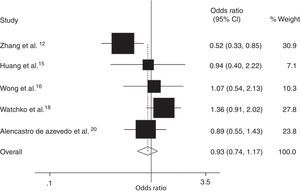
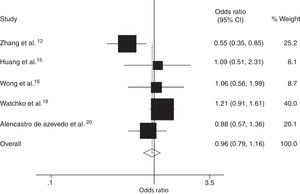
Five case–control studies from three countries, which includes our study, with 637 hyperbilirubinemic case subjects and 918 control subjects, were included in the meta-analysis of the association between the SLCO1B1 521 T>C mutation and neonatal hyperbilirubinemia (Table 4).12,15,16,19,20 Results of the meta-analysis indicated that there was no statistically significant difference in the risk of neonatal hyperbilirubinemia between SLCO1B1 521 T>C allele carriers (C/C+C/T) and T/T allele carriers (Fig. 4); the same was observed when comparing the T allele to the C allele in the SLCO1B1 521 T>C mutation (Fig. 5). In addition, in the subgroup analyses based on ethnicity, low risk of neonatal hyperbilirubinemia was found in Chinese neonates, and no significant associations between SLCO1B1 521 T>C allele carriers (C/C+C/T) and T/T allele carriers were found in Brazilian, white, Asian, Thai, and Malaysian neonates; the same was observed when comparing the T allele to the C allele in the SLCO1B1 521 T>C mutation (Table 5). Egger's test provided no evidence for funnel plot asymmetry in comparisons of SLCO1B1 521 T>C mutations and neonatal hyperbilirubinemia (comparison of C/C+C/T vs. T/T: t = 0.25, p = 0.82; comparison of T allele vs. C allele: t = 0.40, p = 0.71).
Genotypic frequencies of SLCO1B1 521 T>C in neonatal hyperbilirubinemic cases and in controls.
| Study | Country of origin | Cases (n) | Controls (n) | ||||
|---|---|---|---|---|---|---|---|
| T/T | T/C | C/C | T/T | T/C | C/C | ||
| Zhang et al.12 | China | 185 | 34 | 1 | 147 | 50 | 3 |
| Huang et al.15 | Taiwan | 31 | 9 | 2 | 53 | 19 | 1 |
| Wong et al.16 | Malaysia | 47 | 18 | 0 | 81 | 29 | 0 |
| Watchko et al.19 | America | 58 | 69 | 26 | 135 | 117 | 46 |
| Alencastro de Azevedo et al.20 | Brazil | 122 | 34 | 1 | 179 | 55 | 3 |
CI, confidence interval; SLCO1B1, solute carrier organic anion transporter family member 1B1.
Subgroup analysis of SLCO1B1 521 T>C in neonatal hyperbilirubinemic cases and in controls.
| Polymorphism | No. of studies (n) | No. of cases/controls (n) | Odds ratio (95% CI) | p | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2 (%) | p | |||||
| C/C+C/T vs. T/T | ||||||
| Asia | 3 | 327/383 | 0.70 (0.49-1.00) | 0.05 | 39.4 | 0.19 |
| China | 2 | 262/273 | 0.60 (0.40-0.92) | 0.02 | 26.0 | 0.25 |
| Malaysia | 1 | 65/110 | 1.07 (0.54-2.13) | >0.05 | - | - |
| White | 1 | 153/299 | 1.36(0.91-2.02) | >0.05 | - | - |
| Latin America | 1 | 157/237 | 0.89(0.55-1.43) | >0.05 | - | - |
| Total | 5 | 637/918 | 0.93 (0.74-1.17) | 0.52 | 56.2 | 0.06 |
| C allele vs. T allele | ||||||
| Asia | 3 | 327/383 | 0.74 (0.53-1.03) | 0.07 | 50.4 | 0.13 |
| China | 2 | 262/273 | 0.65 (0.45-0.96) | 0.03 | 58.3 | 0.12 |
| Malaysia | 1 | 65/110 | 1.06 (0.56-1.99) | >0.05 | - | - |
| White | 1 | 153/299 | 1.21 (0.91-1.61) | >0.05 | - | - |
| Latin America | 1 | 157/237 | 0.88 (0.57-1.36) | >0.05 | - | - |
| Total | 5 | 637/918 | 0.96 (0.79-1.16) | 0.65 | 56.1 | 0.06 |
CI, confidence interval; SLCO1B1, solute carrier organic anion transporter family member 1B1.
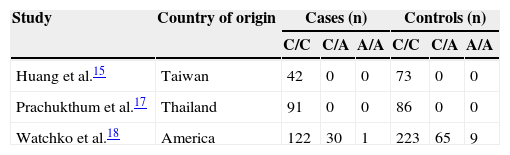
Three case–control studies from three countries, with 286 hyperbilirubinemic cases and 456 controls, were included in the meta-analysis of the association between the SLCO1B1 463 C>A mutation and neonatal hyperbilirubinemia (Table 6).15,17,18 No carriage of the C to A substitution at nucleotide 463 was detected in two studies, and only one study,18 which involved American subjects, showed 31 of 153 (20.26%) neonates in the hyperbilirubinemic group (one homozygous and 30 heterozygous) compared to 74 of 299 (24.75%) in the control groups (nine homozygous and 65 heterozygous). In that study18 there were no statistically significant differences in the risk of neonatal hyperbilirubinemia between SLCO1B1 463 C>A allele carriers (A/A+C/A) and C/C allele carriers (OR, 0.77; 95% CI: 0.48–1.23); the same was observed when comparing the A allele to the C allele in the SLCO1B1 463 C>A mutation (OR, 0.72; 95% CI: 0.47–1.11).
Genotypic frequencies of SLCO1B1 463 C>A in neonatal hyperbilirubinemic cases and in controls.
| Study | Country of origin | Cases (n) | Controls (n) | ||||
|---|---|---|---|---|---|---|---|
| C/C | C/A | A/A | C/C | C/A | A/A | ||
| Huang et al.15 | Taiwan | 42 | 0 | 0 | 73 | 0 | 0 |
| Prachukthum et al.17 | Thailand | 91 | 0 | 0 | 86 | 0 | 0 |
| Watchko et al.18 | America | 122 | 30 | 1 | 223 | 65 | 9 |
SLCO1B1, solute carrier organic anion transporter family member 1B1.
The present systematic review with meta-analysis indicated that there was no statistically significant difference in the risk of neonatal hyperbilirubinemia in those with the SLCO1B1 388 G>A mutation. In subgroup analyses based on ethnicity, no significant associations were found in white, Asian, Thai, Brazilian, and Malaysian populations, but significant associations were present in Chinese neonates. Meta-analysis of five case-control studies indicated that there was no statistically significant difference in the risk of neonatal hyperbilirubinemia for those with the SLCO1B1 521 T>C mutation. In subgroup analyses based on ethnicity, no significant associations were found in white, Asian, Brazilian, and Malaysian populations, but a low risk was found in Chinese neonates. Three case-control studies from three countries assessed the association between the SLCO1B1 463 C>A mutation and neonatal hyperbilirubinemia. No carriage of the C to A substitution at nucleotide 463 was detected among three studies, and only one study of American infants reported the variant SLCO1B1 at nt 463 in hyperbilirubinemic and in control infants (0.156 and 0.155, respectively), with no statistically significant difference between the groups.
Egger's test provided no evidence for funnel plot asymmetry in the comparison of the SLCO1B1 388 G>A and 521 T>C mutations and neonatal hyperbilirubinemia. Three studies focused on the relationship between the SLCO1B1 463 C>A mutation and neonatal hyperbilirubinemia: two studies did not detect a carriage of the C to A substitution, and one study showed a non-significant increase in the risk of neonatal hyperbilirubinemia. No significant inter-study heterogeneity was observed in the analyses. Therefore, it is believed that the results of the present meta-analysis are reliable.
In the nine included studies, which analyzed the association between the SLCO1B1 388 G>A mutation and neonatal hyperbilirubinemia, only three studies from China showed a positive relationship.13–15 In five included studies that analyzed the association between the SLCO1B1 521 T>C mutation and neonatal hyperbilirubinemia, only one study from China showed a negative relationship.12 In other populations, no statistically significant difference was observed. The genetics of racial differences might explain the variability in prevalence of neonatal hyperbilirubinemia among different ethnic groups.
An in vitro expression study demonstrated that SLCO1B1 388 G>A variations are consistently associated with reduced transport activity of SLCO1B1.21 Evidence suggests that transient hyperbilirubinemia may be caused by potent SLCO1B1 inhibitors, such as indinavir, saquinavir, cyclosporine A, and rifamycin.22 Following rifampicin administration (450mg/day) for five consecutive days, serum bilirubin levels were significantly increased, and unconjugated bilirubin, direct bilirubin, and total bilirubin levels were increased by 24.9%, 31.5%, and 26.8%, respectively.23 Thus, modulation of the transporting activity of SLCO1B1 could alter the transportation and subsequent elimination of serum bilirubin. The SLCO1B1*1B (C388 G–C521T) haplotype has been associated with increased OATP1B1 transport activity in vitro in studies performed with bromosulfophthalein and estrone-3-sulfate.21,24 SLCO1B1 may be a useful therapeutic target for neonatal hyperbilirubinemia, but further studies are needed to explore this hypothesis, and to confirm whether some SLCO1B1 activators could improve the transporting activity of the SLCO1B1 gene, which could enhance uptake of bilirubin from blood to bile and decrease serum bilirubin levels.
In addition, there were also some limitations in this study. Firstly, the diagnostic criteria for neonatal hyperbilirubinemia were not consistent. Secondly, there were studies included with a relatively small population of subjects, which might have some effect on the power of our analysis. Thirdly, various other factors may also have contributed to neonatal hyperbilirubinemia, such as environmental factors, which were not explained in the included studies.
ConclusionThe present systematic review with meta-analysis shows that the 388 G>A mutation of the SLCO1B1 gene is a risk factor for developing neonatal hyperbilirubinemia in Chinese neonates, but not in white, Thai, Brazilian, or Malaysian populations; the SLCO1B1 521 T>C mutation provides protection for neonatal hyperbilirubinemia in Chinese neonates, but not in white, Thai, Brazilian or Malaysian neonates. Since other factors involved in neonatal hyperbilirubinemia might impact on the association, further study is needed to assess the effects of genetic variations after adjusting for the effect of other factors.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Liu J, Long J, Zhang S, Fang X, Luo Y. The impact of SLCO1B1 genetic polymorphisms on neonatal hyperbilirubinemia: a systematic review with meta-analysis. J Pediatr (Rio J). 2013;89:434–43.