To analyze the implementation of a protocol proposed by the Brazilian National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária – ANVISA) to improve sepsis diagnosis in very low birth weight newborns.
MethodsThis was a prospective study that evaluated the implementation of a protocol involving clinical and laboratory criteria (hematologic scoring system of Rodwell and C-reactive protein serial measurements), recommended by ANVISA, to improve the diagnosis of neonatal sepsis in very low birth weight newborns. The study included all patients who were born and remained in the neonatal intensive care unit until discharge or death, and excluded those with congenital diseases. The main outcomes measured in newborns before (2006-2007) and after implementation of the protocol (2008) were the rates of early and late-onset sepsis, use of antibiotics, and mortality. Means were compared by Student's t-test and categorical variables were compared by the chi-squared test; the significance level for all tests was set at 95%.
ResultsThe study included 136 newborns with very low birth weight. There was no difference between groups regarding general clinical characteristics in the studied periods. There was, however, a decrease in the number of diagnoses of probable early-onset sepsis (p<0.001), use of antimicrobial regimens (p<0.001), and overall mortality and infection-related mortality (p=0.009 and p=0.049, respectively).
ConclusionThe implementation of the protocol allowed improvement of sepsis diagnosis by reducing the diagnosis of probable early-onset sepsis, thus promoting efficient antimicrobial use in this population.
Analisar a aplicação de um protocolo proposto pela Agência Nacional de Vigilância Sanitária (ANVISA) para aprimorar o diagnóstico de sepse em recém-nascidos de muito baixo peso.
MétodosEstudo prospectivo que avaliou a aplicação de protocolo envolvendo critérios clínicos e laboratoriais (escore hematológico de Rodwell e dosagem seriada da proteína C-reativa), recomendado pela ANVISA, para aprimorar o diagnóstico de sepse neonatal em recém-nascidos de muito baixo peso. Participaram do estudo todos os pacientes que nasceram e permaneceram na Unidade Neonatal até a alta ou óbito, e foram excluídos aqueles com doenças congênitas. Os principais desfechos analisados entre os recém-nascidos antes da aplicação do protocolo (2006-2007) e após a aplicação do mesmo (2008) foram as taxas de sepses precoce e tardia, o uso de antimicrobianos e a mortalidade. As médias foram comparadas por meio de teste t e as variáveis categóricas pelo teste Qui-quadrado (X2); o nível de significância para todos eles foi fixado em 95%.
ResultadosForam incluídos no estudo 136 recém-nascidos de muito baixo peso. Não houve diferença entre os grupos em relação às características clínicas gerais nos períodos estudados. Houve, no entanto, redução na quantidade de diagnóstico de sepse precoce provável (p<0,001), de uso de esquemas antimicrobianos (p<0,001) e da mortalidade geral e associada à sepse (p=0,009 e p=0,049, respectivamente).
ConclusãoA utilização do protocolo permitiu aprimorar o diagnóstico de sepse, reduzindo aquele de sepse precoce provável, promovendo, desta forma, o uso racional de antimicrobianos na população estudada.
In spite of advances in obstetric care and intrapartum use of antimicrobial agents for streptococcal infection prophylaxis, neonatal sepsis remains an important cause of morbidity and mortality in newborns (NBs), especially in those who weigh less than 1,500g.1–7 The clinical picture of neonatal sepsis and laboratory diagnosis are usually nonspecific.8,9 Blood cultures, which are considered the gold standard, have widely variable positive results and may be false negative in 20% of cases; moreover, their results are not readily available to define the therapeutic conduct.10–13 Thus, neonatologists often empirically administer antibiotics to symptomatic NBs or those at high risk of sepsis, while awaiting culture results; even when cultures are negative, they tend to not withdraw the use of antimicrobials.7
In 2008, the Brazilian National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária - ANVISA) submitted to public consultation a handbook for the diagnosis of healthcare-related infections (HCRI) in neonatology,14 which established the diagnosis of primary infection of the bloodstream based on the use of hematological clinical criteria (hematologic scoring system of Rodwell), serial measurements of C-reactive protein (CRP), and partial results of blood cultures collected at the time of sepsis diagnosis.15,16 The association of normal leukocyte count with serial measurements of negative CRP may help to rule out this diagnosis within one to three days of clinical evolution, increasing the probability of a correct diagnosis.7,15
This study aimed to analyze the impact of using a protocol recommended by ANVISA to improve the diagnosis of probable sepsis in NBs with very low weight.
MethodsThis prospective study was conducted to analyze the implementation of a protocol for the diagnosis of neonatal sepsis based on clinical and laboratory criteria, in NBs with birth weight of 1,500g or less from January, 2006 to December, 2008. The period of protocol implementation (January-December 2008) was termed the post-intervention period; the previous period was termed pre-intervention. The study included all patients who were born and remained in the neonatal intensive care unit (NICU) until discharge or death, and excluded those with congenital malformations or diseases, those transferred from other health services, and those who died on the same day as birth.
The study was performed in the NICU of Hospital Universitário Antônio Pedro of the Universidade Federal Fluminense (HUAP/UFF), consisting of 15 beds with a mean of 250 admissions/year. The hospital has a clinical routine that consists of two neonatologists in charge of medical decisions, including the use of antimicrobial agents. Protocol implementation was supervised daily by the clinical routine, and the team's adherence was considered to be very good. During the study period, there were no changes in the health unit staff nor additions of technological features that could differentiate the study periods. There was no obstetric routine for group B streptococcus infection prophylaxis nor prophylaxis in case of premature rupture of membranes and preterm delivery with no apparent cause and treatment of chorioamnionitis.
InterventionCollection of complete blood count (CBC), CRP levels, blood culture, and cerebrospinal fluid at the time of the suspected sepsis (before the antibiotic therapy) were performed, as well as CBC and CRP 48hours after the start of antibiotic therapy. The normalization of the clinical picture, negative blood cultures, negative serum CRP measurements, and normal hematological parameters 48hours after start of antibiotic therapy ruled out the diagnosis of sepsis, and the antibiotic therapy was suspended. NBs were classified as: confirmed sepsis (CS) – clinical picture compatible with sepsis and positive culture; probable sepsis (PS) – clinical picture compatible with sepsis, altered WBC and/or CRP results, negative cultures, followed by treatment with antibiotics for at least seven days; non-infected (NI) – initial clinical suspicion of sepsis, but absence of clinical course consistent with infection, normal WBC and CRP results, and negative cultures.
Maternal variables evaluated were type of delivery, clinical and laboratory signs of maternal infection (presence of fever and antibiotic use), and time of ruptured membranes at birth. The neonatal data were: birth weight, gestational age, gender, length of hospitalization, severity criterion (SNAPPE II), and mortality, as well as variables related to the main neonatal diseases and to the diagnosis and treatment of neonatal sepsis.
Statistical analysisThe comparison of the data collected during the study period was described based on measures of central tendency (continuous variables: mean, standard deviation, median, minimum and maximum values) and percentages (categorical variables). The means of continuous variables of the study groups were compared by Student's t-test and, when necessary, by the nonparametric Mann-Whitney test. Categorical variables were compared by the chi-squared test with Fisher's correction, when necessary. A logistic regression was performed to assess the influence of protocol use on the diagnosis of neonatal sepsis (confirmed and probable). The significance level for all tests was set at 0.05; a 95% confidence interval was calculated, when necessary. The Statistical Package for Social Sciences (SPSS) release 16.0 for Windows was used in all statistical analyses.
The study was approved by the Research Ethics Committee of HUAP/UFF, under protocol No. CAAE: 0040.0.258.000-10.
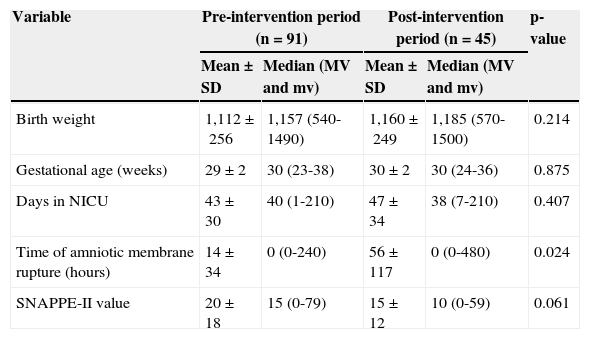
ResultsDuring the study period, 172 NBs with birth weight <1,500g were admitted to the NICU. The study included 136 NBs (79.1%), and excluded 36 (20.9%). Among the excluded NBs, 20 (55.6%) were transferred to or from other services, 11 (30.6%) had malformations and/or congenital infection, and five (13.9%) died on the same day as birth. The NBs included in the study were divided into two groups for comparison: NBs admitted in the pre-intervention (n=91), and NBs admitted in the post-intervention period (n=45). There was no difference between groups regarding the clinical variables studied, except for the time of amniotic membrane rupture (Table 1).
Comparison of clinical characteristics of the groups of newborns studied in the pre- and post-intervention periods – Niterói, RJ, Brazil, 2006-2008.
| Variable | Pre-intervention period (n=91) | Post-intervention period (n=45) | p-value | ||
|---|---|---|---|---|---|
| Mean±SD | Median (MV and mv) | Mean±SD | Median (MV and mv) | ||
| Birth weight | 1,112±256 | 1,157 (540-1490) | 1,160±249 | 1,185 (570-1500) | 0.214 |
| Gestational age (weeks) | 29±2 | 30 (23-38) | 30±2 | 30 (24-36) | 0.875 |
| Days in NICU | 43±30 | 40 (1-210) | 47±34 | 38 (7-210) | 0.407 |
| Time of amniotic membrane rupture (hours) | 14±34 | 0 (0-240) | 56±117 | 0 (0-480) | 0.024 |
| SNAPPE-II value | 20±18 | 15 (0-79) | 15±12 | 10 (0-59) | 0.061 |
| n (%) | n (%) | p-value | |
|---|---|---|---|
| Male gender | 42 (46.2%) | 26 (57.8%) | 0.402 |
| Patent ductus arteriosus | 14 (15.4%) | 11 (24.4%) | 0.169 |
| Pulmonary bronchodysplasia | 7 (7.7%) | 2 (4.4%) | 0.172 |
| Necrotizing enterocolitis | 14 (15.4%) | 4 (8.9%) | 0.220 |
| Surgical delivery | 61 (67.0%) | 33 (73.3%) | 0.293 |
| Maternal fever | 6 (6.6%) | 3 (6.7%) | 0.654 |
| Maternal intrapartum antibiotic use | 25 (27.5%) | 17 (37.8%) | 0.151 |
MV, maximum value; mv, minimum value; NICU, neonatal intensive care unit; SD, standard-deviation.
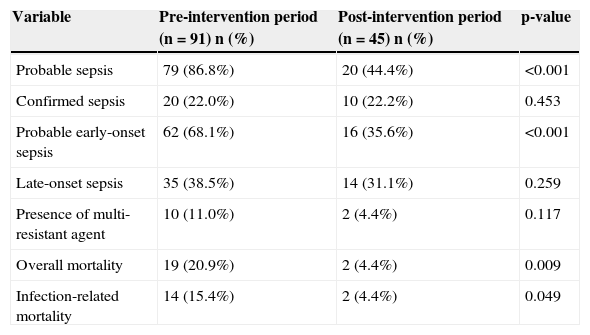
There was no difference between the two groups in relation to the diagnosis of CS; however, regarding the diagnosis of PS, there was a significant reduction in the post-intervention period, particularly for the diagnosis of probable early-onset sepsis (Table 2). The number of positive blood cultures did not differ between periods (10.8% vs. 16.3%, between the pre- and post-intervention periods, respectively). Overall mortality and infection-related mortality decreased in the post-intervention period.
Comparison between groups in the pre- and post-intervention periods for the variables related to the diagnosis of neonatal sepsis and mortality – Niterói, RJ, Brazil, 2006-2008.
| Variable | Pre-intervention period (n=91) n (%) | Post-intervention period (n=45) n (%) | p-value |
|---|---|---|---|
| Probable sepsis | 79 (86.8%) | 20 (44.4%) | <0.001 |
| Confirmed sepsis | 20 (22.0%) | 10 (22.2%) | 0.453 |
| Probable early-onset sepsis | 62 (68.1%) | 16 (35.6%) | <0.001 |
| Late-onset sepsis | 35 (38.5%) | 14 (31.1%) | 0.259 |
| Presence of multi-resistant agent | 10 (11.0%) | 2 (4.4%) | 0.117 |
| Overall mortality | 19 (20.9%) | 2 (4.4%) | 0.009 |
| Infection-related mortality | 14 (15.4%) | 2 (4.4%) | 0.049 |
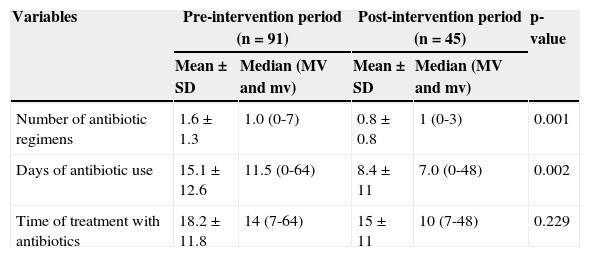
There was a decrease in the number of antimicrobial regimens used (both the first and third regimens) and in the number of days of antibiotic use. However, the mean length of treatment in NBs that required antimicrobial treatment was not different between the groups. There was also a decrease in the need for carbapenem use (Table 3).
Comparison of the number of regimens and treatment duration in groups of newborns studied – Niterói, RJ, Brazil, 2006-2008.
| Variables | Pre-intervention period (n=91) | Post-intervention period (n=45) | p-value | ||
|---|---|---|---|---|---|
| Mean±SD | Median (MV and mv) | Mean±SD | Median (MV and mv) | ||
| Number of antibiotic regimens | 1.6±1.3 | 1.0 (0-7) | 0.8±0.8 | 1 (0-3) | 0.001 |
| Days of antibiotic use | 15.1±12.6 | 11.5 (0-64) | 8.4±11 | 7.0 (0-48) | 0.002 |
| Time of treatment with antibiotics | 18.2±11.8 | 14 (7-64) | 15±11 | 10 (7-48) | 0.229 |
| n (%) | N (%) | X2 | |
|---|---|---|---|
| First antibiotic regimen | 83 (91.2%) | 26 (57.8%) | 0.001 |
| Second antibiotic regimen | 30 (33%) | 9 (20%) | 0.083 |
| Third antibiotic regimen | 20 (22%) | 1 (2.2%) | 0.001 |
| Vancomycin | 13 (14.3%) | 3 (6.7%) | 0.165 |
| Carbapenem | 17 (18.7%) | 3 (6.7%) | 0.049 |
First antibiotic regimen: ampicillin+gentamycin; Second antibiotic regimen: oxacillin+amikacin; Third antibiotic regimen: cefepime+vancomycin.
MV, maximum value; mv, minimum value; SD, standard-deviation.
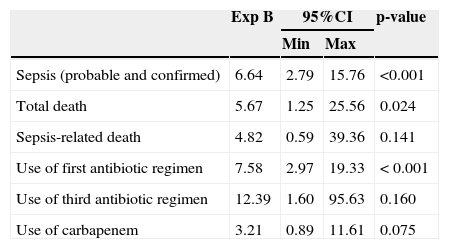
After using binary logistic regression, the “Enter” method, and probability threshold to accept 0.05 variables, having as the dependent variable the protocol implementation period it was observed that NBs in the pre-intervention period were 6.64 times more likely to have a diagnosis of neonatal sepsis than those in the post-intervention period (95% CI: 2.798 to 15.761, p<0.001), and therefore six times more likely to receive antibiotics (Table 4).
Binary logistic regression - dependent variable: protocol implementation period – Niterói, RJ, Brazil, 2006-2008.
| Exp B | 95%CI | p-value | ||
|---|---|---|---|---|
| Min | Max | |||
| Sepsis (probable and confirmed) | 6.64 | 2.79 | 15.76 | <0.001 |
| Total death | 5.67 | 1.25 | 25.56 | 0.024 |
| Sepsis-related death | 4.82 | 0.59 | 39.36 | 0.141 |
| Use of first antibiotic regimen | 7.58 | 2.97 | 19.33 | < 0.001 |
| Use of third antibiotic regimen | 12.39 | 1.60 | 95.63 | 0.160 |
| Use of carbapenem | 3.21 | 0.89 | 11.61 | 0.075 |
It is noteworthy that the sample number in this study (136 NBs) was determined by convenience, i.e., it was according to the number of infants born during the study period. However, this number allowed for the assessment of the decrease in the incidence of sepsis in up to 80%, with 80% power (type β error of 20%), and a significance level of 5%.
DiscussionAfter implementing the ANVISA manual as a protocol to improve the diagnosis of neonatal sepsis, there was a decrease in the diagnosis of probable early-onset sepsis and, consequently, a decrease in the use of antimicrobials, especially of the first regimen (ampicillin + gentamicin). The decrease in the diagnosis of probable early-onset sepsis and therefore, in the need to treat these NBs with suspected sepsis, was the most significant result of this study.
The difficulty in ruling out the diagnosis of sepsis in NBs has been considerably discussed, especially in those with very low birth weight, due to lack of specificity of current parameters for this diagnosis, namely: clinical presentation, presence of risk factors, and laboratory results.7,15,16 These factors lead to the overuse of antibiotics and to their potential consequences, such as toxicity and increased bacterial resistance.
Antibiotics are the most often prescribed drugs in the NICU, and their rational use is crucial. The use of broad-spectrum antimicrobials for a prolonged period of time is associated with increased risk of invasive candidiasis, necrotizing enterocolitis (NEC), late-onset sepsis, and increased bacterial resistance.17–22 In this NICU, aminoglycosides are used to treat both early-onset and late-onset sepsis. Medical literature reports that adverse events in the short and long-term, such as cochlear and vestibular damage, may occur with the use of aminoglycosides. In 2010, the National Patient Safety Agency (NPSA) reported 507 adverse events involving the use of gentamicin in NBs within one year.23 Thus, any possibility to reduce the use of antibiotics in NBs, a population at high risk for neonatal sepsis, is essential.
This study, however, showed no decrease in the diagnosis of late-onset sepsis, as this diagnosis is based on altered clinical and laboratory tests, leaving no doubts regarding treatment for the clinical staff.
Blackburn et al. questioned the safety of the withdrawing antibiotic therapy in neonates with probable early-onset sepsis after 36hours of suspected diagnosis, considering the low sensitivity of blood cultures. There have been reports of positive blood cultures in the neonatal period in NBs with suspected sepsis of 12% for bacteria or fungi, and of only 2% on the first day of life.24 The present study demonstrated the effectiveness and safety of withdrawing the antimicrobial therapy based on the protocol proposed by ANVISA, as none of the NBs evaluated through the protocol needed to return to antibiotic therapy or had reported clinical worsening.
Even though the time of prolonged rupture of membranes was four times higher in the post-intervention group, the use of the protocol decreased the need for antibiotic use in this population.
Mortality rates (overall and infection-related) decreased in the post-intervention period. There was no difference in severity among the NBs treated in the periods studied nor regarding the presence of major morbidities, including the rate of CS. As there were no other changes in relation to clinical management, acquisition of new technologies, or alterations in the severity profile of cases treated in this unit, the decrease in mortality observed may be related to a reduction in unnecessary antimicrobial use as a result of improvement in the diagnosis of probable early-onset sepsis, the only parameter that was different between groups.
ConclusionThis study demonstrated that the implementation of the protocol suggested by ANVISA decreased the number of diagnoses of probable early-onset sepsis and the need for antimicrobial use in NBs with very low birth weight.
Conflicts of interestsThe authors declare no conflicts of interest.
Please cite this article as: Pinto MC, Bueno AC, Vieira AA. Implementation of a protocol proposed by the Brazilian National Health Surveillance Agency for antibiotic use in very low birth weight infants. J Pediatr (Rio J). 2013;89:450–5.