This was a systematic review of the incidence density and risk factors for central venous catheter-related infections in a neonatal population.
Data sourceThe MEDLINE, Embase, Cochrane, BDENF, SciELO, and LILACS databases were used without date or language restriction. Studies that analyzed risk factors for bloodstream infections in newborns were identified.
Data synthesisA total of 134 articles were found that met the eligibility criteria. Of these articles, 14 were selected that addressed risk factors for central venous catheter-related infection in neonates. Catheter-related bloodstream infections remain an important complication, as shown by the incidence rates reported in the studies included in this review. The observed risk factors indicate that low birth weight, prematurity, and longer catheter permanence are related to a higher incidence of bloodstream infections. It has been observed that low rates of catheter-related infections, i.e., close to zero, are already a reality in health institutions in developed countries, since they use infection surveillance and control programs.
ConclusionCatheter-related bloodstream infections still show high incidence density rates in developing countries. The authors emphasize the need for further longitudinal studies and the need for better strategies to prevent risk factors, aiming at the reduction of catheter-related infections.
Trata-se de uma revisão sistemática sobre a densidade de incidência e de fatores de risco para infecção associada a cateter venoso central em população neonatal.
Fontes dos dadosUtilizou-se os bancos de dados Medline, Embase, Cochrane, Bdenf, Scielo, Lilacs, sem restrição de data ou de idioma. Identificaram-se os estudos que analisaram fatores de risco para infecção da corrente sanguínea em recém-nascidos.
Síntese dos dadosForam encontrados 134 artigos conforme os critérios de elegibilidade. Destes artigos, foram selecionados 14 que abordaram fatores de risco para infecção associada a cateter venoso central em neonatos. A infecção da corrente sanguínea associada a cateter continua a mostrar-se como uma importante complicação, conforme demonstram as taxas de incidência relatadas nos estudos incluídos nesta revisão. Os fatores de risco observados apontam que baixo peso ao nascer, prematuridade e maior tempo de permanência do cateter estão relacionados à maior incidência de infecção da corrente sanguínea. Observou-se que taxas de infecção associada a cateter em valores baixos, próximos a zero, já são uma realidade em instituições de saúde de países desenvolvidos, uma vez que utilizam programas de vigilância e controle de infecção.
ConclusãoA infecção da corrente sanguínea associada a cateter ainda apresenta altas taxas de densidade de incidência em países em desenvolvimento. Destaca-se a necessidade de realização de mais estudos longitudinais e a necessidade de melhores estratégias de prevenção dos fatores de risco para a redução de infecção associada a cateter.
Care procedures for the neonate in intensive care units require the use of advanced technology; the central venous catheter (CVC) is one of the most common among the invasive procedures used in these patients.1–3 Depending on the material and caliber, it can be inserted at the bedside, such as the peripherally inserted central catheter (PICC), and remain for a prolonged period to allow the administration of solutions and medications, sample collection for examinations, blood product transfusion, and monitoring of hemodynamics.3,4 Among the complications related to its use, infections show the highest frequency and the most potential for morbidity and mortality.5
Newborns, especially preterm, are at increased risk of infection and are considered immunocompromised due to their immune system immaturity.6 Their immune response is characterized by a decrease in neutrophil–endothelial adhesion, low levels of complement factors, and immaturity regarding the different subpopulations of lymphocytes and mononuclear phagocytic system cells.6,7
The use of invasive devices implies the impairment of the natural physical barrier consisting of the skin, which allows bloodstream invasion by opportunistic microorganisms. When bacteremia progresses into severe sepsis, it can lead to hemodynamic changes, and even death.8 The risk factors for early sepsis, defined as those that occur within the first 48h of life, are related to the underlying disease and to the quality of the care provided. Regarding late sepsis, which occurs after the first 48h of life, it is related to the indirect contact with the contaminated hospital environment, with low birth weight, the use of invasive devices such as CVC and mechanical ventilation (MV), delayed start of enteral nutrition, prolonged use of parenteral nutrition, and complications of prematurity, such as patent ductus arteriosus and necrotizing enterocolitis, which may require surgical intervention.9,10
According to the National Health Surveillance Network (NHSN),11 the rates of central venous catheter-associated bloodstream infections (CVC BSI) were between 0.6/1000 CVC-day and 2.5/1000 CVC-day. Other studies reported a wide variation in infection rates, ranging from 2 to 49/1000 CVC-day. Newborns weighing<1000g have a higher mortality risk, with attributable mortality of 4–20%.12
In Brazil, CVC-related sepsis represents a serious health problem for the neonatal population. The Brazilian National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária [ANVISA]) Bulletin reports the incidence density of primary bloodstream infection in patients submitted to central venous catheter use in Brazilian neonatal intensive care units as ranging from 7.6 to 8.9/1000 CVC-day.13 The rate of neonatal mortality due to sepsis is as high as 68%,14 so surveillance measures are required to direct actions aimed at reducing the rates of healthcare-associated infections (HAIs), since they can provide data that allow comparisons and evaluation of the impact of the control measure, in addition to allowing comparisons with other healthcare services with the same characteristics.8,9
A study of adverse events associated with the use of central venous catheters in a neonatal unit in the state of Rio Grande do Sul, Brazil, observed a higher prevalence of mechanical obstruction in central peripherally-inserted central catheters (PICC) and a higher prevalence of catheter-associated infection (CAI) in the surgically-inserted catheters, with clinical sepsis being the most frequent (16%).15 Moreover, studies indicate that the micro-organisms most often associated with sepsis are gram-positive, mainly coagulase-negative Staphylococcus.14,16–19 Despite the need to use CVC for the treatment of newborns admitted at the neonatal intensive care unit (NICU), its timely removal is usually associated with a lower incidence of infection.20,21 There have been few studies in Brazil on patients’ clinical evolution after catheter removal.22
It is known that in developed countries, evidence-based strategies are used by the teams responsible for neonatal catheter-related infection prevention programs.23 In contrast, in developing countries such as Brazil, in addition to the need to establish such strategies, systematic epidemiological surveillance is performed in few health services.18 There are structural shortcomings, such as overcrowding, lack of or shortage of multiprofessional staff, lack of or misuse of supplies and equipment, and excessive antibiotic use.24 For these reasons, more evidence-based studies are needed to evaluate the risks related to CVC use.25
The aim of this article is to carry out a systematic review of the risk factors for CVC-associated infection in the neonatal population, considering national and international references.
MethodsThe search for the articles was carried out in the following databases: MEDLINE, Embase, Cochrane, BDENF, SciELO, and LILACS, in addition to searching for the references mentioned in the studies that were identified, as well as by searching the gray literature, according to the descriptors and eligibility criteria. Formal search was not considered only if there was no access to any of the databases. The search period started in January 2010 and ended in December 2016, with no date or language restrictions.
The following descriptors were used: “cross infection,” “catheter-related infections,” “catheterization, central venous,” “risk factors,” “newborn,” “infant,” according to the following strategy:
PubMed: ((((“Cross Infection”[Title/Abstract] OR “Infeccion Hospitalaria”[Title/Abstract] OR “Infeccao Hospitalar”[Title/Abstract] OR “Cross Infection”[Title/Abstract] OR “Infeccion Hospitalaria”[Title/Abstract] OR “Infeccao Hospitalar”[Title/Abstract] OR “Catheter-Related Infections”[Title/Abstract] OR “Infecciones Relacionadas con Cateteres”[Title/Abstract] OR “Infeccoes Relacionadas a Cateter”[Title/Abstract])) OR ((“Cross Infection”[Mesh:noexp]) OR “Catheter-Related Infections”[Mesh:noexp]))) AND (((“Catheterization, Central Venous”[Title/Abstract] OR “Cateterismo Venoso Central”[Title/Abstract] OR “Cateterismo Venoso Central”[Title/Abstract] OR “Central Venous Catheters”[Title/Abstract] OR “Cateteres Venosos Centrales”[Title/Abstract] OR “Cateteres Venosos Centrais”[Title/Abstract])) OR ((“Catheterization, Central Venous”[Mesh:noexp]) OR “Central Venous Catheters”[Mesh:noexp]))
BVS: (w:(tw:(((“Cross Infection” OR “Infección Hospitalaria” OR “Infecção Hospitalar” OR “Cross Infection” OR “Infección Hospitalaria” OR “Infecção Hospitalar” OR “Catheter-Related Infections” OR “Infecciones Relacionadas con Catéteres” OR “Infecções Relacionadas a Cateter”) AND (“Catheterization, Central Venous” OR “Cateterismo Venoso Central” OR “Cateterismo Venoso Central” OR “Central Venous Catheters” OR “Catéteres Venosos Centrales” OR “Cateteres Venosos Centrais”)) AND (“Risk Factors” OR “Factores de Riesgo” OR “Fatores de Risco”)) AND (instance:“regional”) AND (limit:(“newborn” OR “infant”) AND la:(“en” OR “es” OR “pt”) AND year_cluster:(“2013” OR “2011” OR “2012” OR “2014” OR “2010” OR “2015”))) AND (instance:“regional”)
The articles included in the review were assessed by two independent examiners and met the following criteria: (A) to have been published by December 2016 in any national or international journal and other specialized medical literature; (B) being available in any language; (C) addressing risk factors associated with bloodstream infections, comparing groups of newborns with and without central venous catheter; (D) being an original study with a case–control or cohort design.
To select the publications, the title and the abstract were initially evaluated, to confirm whether they contemplated the research question and if they met the previously established inclusion criteria. Subsequently, the evaluation of Methods, Results, and Discussion was performed.
The research question was defined as: “In neonates admitted to the NICU, what are the risk factors identified for the occurrence of the outcome laboratory-confirmed CVC-associated bloodstream infection?
The PICOS26,27 strategy was used, which comprised the following:
- -
Population: newborns admitted to the NICU
- -
Intervention (or exposure): risk factors
- -
Comparison (defined as a standard intervention, the most often used intervention, or no intervention): no risk factors
- -
Outcome: CVC-associated bloodstream infection
- -
Study type: cohort and case–control studies.
As exclusion criteria, studies not assessing neonates, those that did not have a CVC-BSI outcome, and those that did not address risk factors for CVC-BSI were excluded.
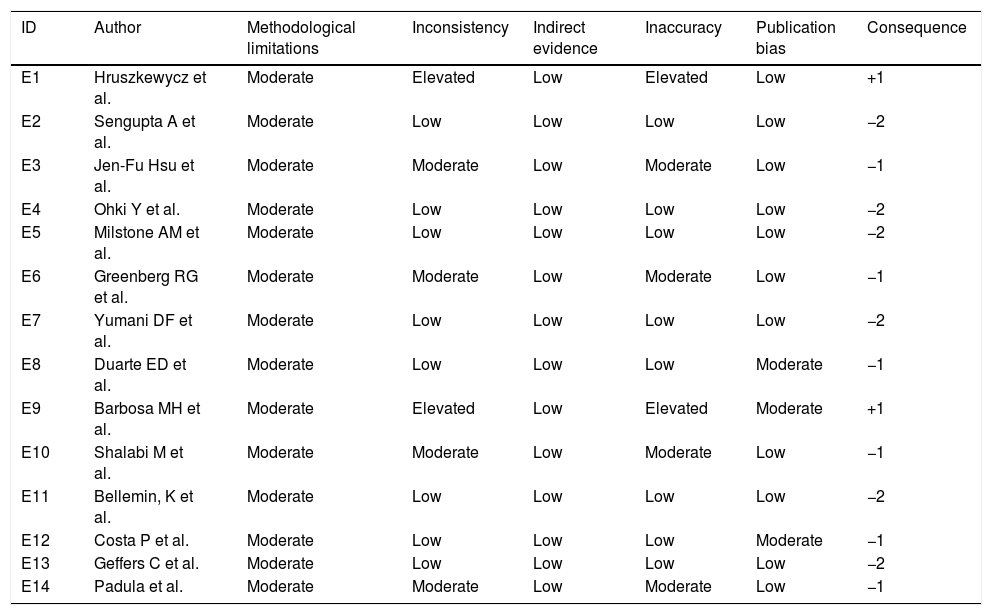
For data extraction, the search and analysis-in-full of the pre-selected articles were carried out by two independent researchers. Data analysis was performed by qualitative evaluation of the studies. The method used for study evaluation was “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) and the strength of evidence of the findings was evaluated according to the “Grading of Recommendation, Assessment, Development, and Evaluation” (GRADE) system,28 as shown in Table 1.
Qualitative evaluation of studies according to the GRADE system.
| ID | Author | Methodological limitations | Inconsistency | Indirect evidence | Inaccuracy | Publication bias | Consequence |
|---|---|---|---|---|---|---|---|
| E1 | Hruszkewycz et al. | Moderate | Elevated | Low | Elevated | Low | +1 |
| E2 | Sengupta A et al. | Moderate | Low | Low | Low | Low | −2 |
| E3 | Jen-Fu Hsu et al. | Moderate | Moderate | Low | Moderate | Low | −1 |
| E4 | Ohki Y et al. | Moderate | Low | Low | Low | Low | −2 |
| E5 | Milstone AM et al. | Moderate | Low | Low | Low | Low | −2 |
| E6 | Greenberg RG et al. | Moderate | Moderate | Low | Moderate | Low | −1 |
| E7 | Yumani DF et al. | Moderate | Low | Low | Low | Low | −2 |
| E8 | Duarte ED et al. | Moderate | Low | Low | Low | Moderate | −1 |
| E9 | Barbosa MH et al. | Moderate | Elevated | Low | Elevated | Moderate | +1 |
| E10 | Shalabi M et al. | Moderate | Moderate | Low | Moderate | Low | −1 |
| E11 | Bellemin, K et al. | Moderate | Low | Low | Low | Low | −2 |
| E12 | Costa P et al. | Moderate | Low | Low | Low | Moderate | −1 |
| E13 | Geffers C et al. | Moderate | Low | Low | Low | Low | −2 |
| E14 | Padula et al. | Moderate | Moderate | Low | Moderate | Low | −1 |
GRADE, Grading of Recommendation, Assessment, Development, and Evaluation.
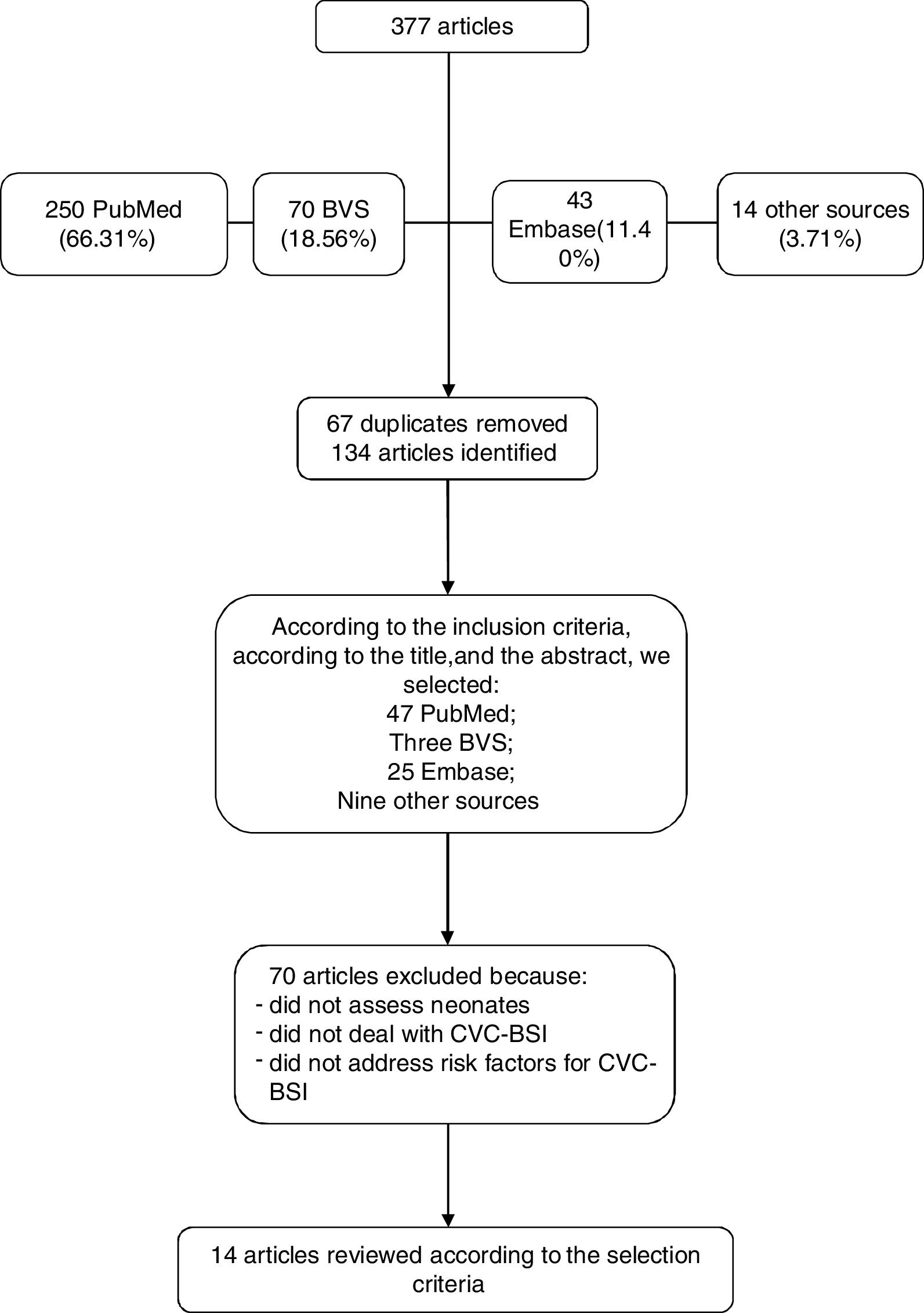
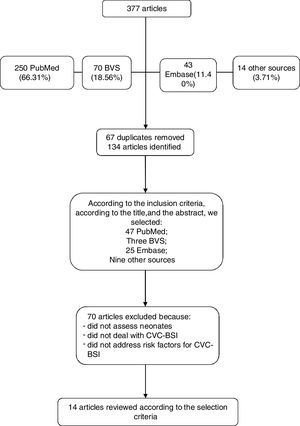
A total number of 250 articles were identified in the PubMed database, of which 77 met the inclusion criteria. The search in the Virtual Health Library database totaled 70 references; however, 67 of those that met the inclusion criteria were the same found in the PubMed database. Forty-three articles were found in the Embase database, of which 25 were selected. Another 14 articles were found in references cited in articles located in the abovementioned databases or through an Internet search, using the same descriptors and the same eligibility criteria.
A total of 134 articles met the eligibility criteria. Among those, those that addressed risk factors for the pre-defined outcome were selected, i.e., CVC-associated infection in newborns; therefore, 14 articles were included in this systematic review, as shown in Fig. 1 flowchart.
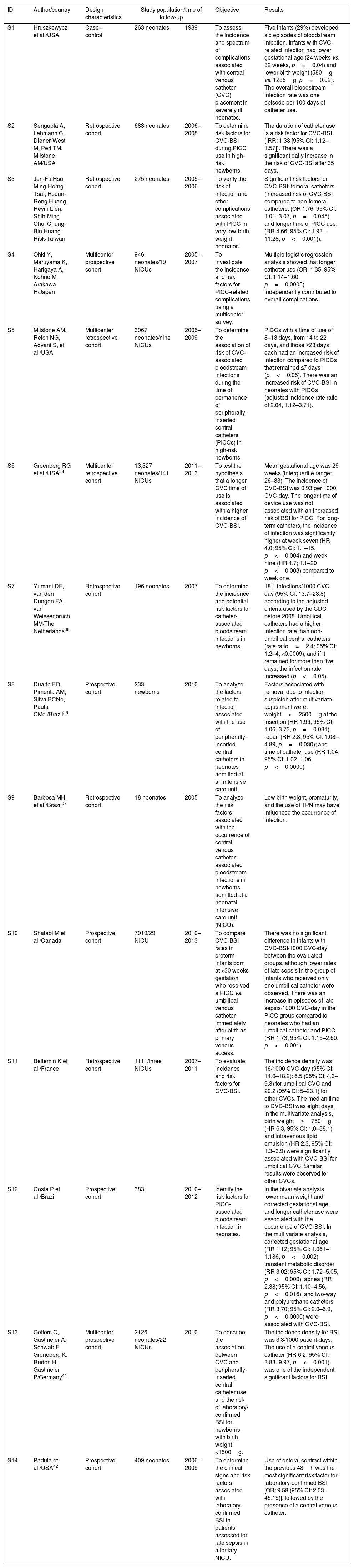
Of these, 11 represented studies carried out in Europe, North America, and Asia, whereas three were from South America; no articles were found that were carried out in other Latin American countries. Table 2 shows the identification of the studies, their design, included population, analyzed outcomes, and results.
Risk factors for CVC-related infection in the neonatal population in original studies.
| ID | Author/country | Design characteristics | Study population/time of follow-up | Objective | Results | |
|---|---|---|---|---|---|---|
| S1 | Hruszkewycz et al./USA | Case–control | 263 neonates | 1989 | To assess the incidence and spectrum of complications associated with central venous catheter (CVC) placement in severely ill neonates. | Five infants (29%) developed six episodes of bloodstream infection. Infants with CVC-related infection had lower gestational age (24 weeks vs. 32 weeks, p=0.04) and lower birth weight (580g vs. 1285g, p=0.02). The overall bloodstream infection rate was one episode per 100 days of catheter use. |
| S2 | Sengupta A, Lehmann C, Diener-West M, Perl TM, Milstone AM/USA | Retrospective cohort | 683 neonates | 2006–2008 | To determine risk factors for CVC-BSI during PICC use in high-risk newborns. | The duration of catheter use is a risk factor for CVC-BSI (IRR: 1.33 [95% CI: 1.12–1.57]). There was a significant daily increase in the risk of CVC-BSI after 35 days. |
| S3 | Jen-Fu Hsu, Ming-Horng Tsai, Hsuan-Rong Huang, Reyin Lien, Shih-Ming Chu, Chung-Bin Huang Risk/Taiwan | Retrospective cohort | 275 neonates | 2005–2006 | To verify the risk of infection and other complications associated with PICC in very low-birth weight neonates. | Significant risk factors for CVC-BSI: femoral catheters (increased risk of CVC-BSI compared to non-femoral catheters: (OR 1.76, 95% CI: 1.01–3.07, p=0.045) and longer time of PICC use: (RR 4.66, 95% CI: 1.93–11.28; p<0.001)). |
| S4 | Ohki Y, Maruyama K, Harigaya A, Kohno M, Arakawa H/Japan | Multicenter prospective cohort | 946 neonates/19 NICUs | 2005–2007 | To investigate the incidence and risk factors for PICC-related complications using a multicenter survey. | Multiple logistic regression analysis showed that longer catheter use (OR, 1.35, 95% CI: 1.14–1.60, p=0.0005) independently contributed to overall complications. |
| S5 | Milstone AM, Reich NG, Advani S, et al./USA | Multicenter retrospective cohort | 3967 neonates/nine NICUs | 2005–2009 | To determine the association of risk of CVC-associated bloodstream infections during the time of permanence of peripherally-inserted central catheters (PICCs) in high-risk newborns. | PICCs with a time of use of 8–13 days, from 14 to 22 days, and those ≥23 days each had an increased risk of infection compared to PICCs that remained ≤7 days (p<0.05). There was an increased risk of CVC-BSI in neonates with PICCs (adjusted incidence rate ratio of 2.04, 1.12–3.71). |
| S6 | Greenberg RG et al./USA34 | Multicenter retrospective cohort | 13,327 neonates/141 NICUs | 2011–2013 | To test the hypothesis that a longer CVC time of use is associated with a higher incidence of CVC-BSI. | Mean gestational age was 29 weeks (interquartile range: 26–33). The incidence of CVC-BSI was 0.93 per 1000 CVC-day. The longer time of device use was not associated with an increased risk of BSI for PICC. For long-term catheters, the incidence of infection was significantly higher at week seven (HR 4.0; 95% CI: 1.1–15, p<0.004) and week nine (HR 4.7; 1.1–20 p<0.003) compared to week one. |
| S7 | Yumani DF, van den Dungen FA, van Weissenbruch MM/The Netherlands35 | Retrospective cohort | 196 neonates | 2007 | To determine the incidence and potential risk factors for catheter-associated bloodstream infections in newborns. | 18.1 infections/1000 CVC-day (95% CI: 13.7–23.8) according to the adjusted criteria used by the CDC before 2008. Umbilical catheters had a higher infection rate than non-umbilical central catheters (rate ratio=2.4; 95% CI: 1.2–4, <0.0009), and if it remained for more than five days, the infection rate increased (p<0.05). |
| S8 | Duarte ED, Pimenta AM, Silva BCNe, Paula CMd./Brazil36 | Prospective cohort | 233 newborns | 2010 | To analyze the factors related to infection associated with the use of peripherally-inserted central catheters in neonates admitted at an intensive care unit. | Factors associated with removal due to infection suspicion after multivariate adjustment were: weight<2500g at the insertion (RR 1.99; 95% CI: 1.06–3.73, p=0.031), repair (RR 2.3; 95% CI: 1.08–4.89, p=0.030); and time of catheter use (RR 1.04; 95% CI: 1.02–1.06, p<0.0000). |
| S9 | Barbosa MH et al./Brazil37 | Retrospective cohort | 18 neonates | 2005 | To analyze the risk factors associated with the occurrence of central venous catheter-associated bloodstream infections in newborns admitted at a neonatal intensive care unit (NICU). | Low birth weight, prematurity, and the use of TPN may have influenced the occurrence of infection. |
| S10 | Shalabi M et al./Canada | Prospective cohort | 7919/29 NICU | 2010–2013 | To compare CVC-BSI rates in preterm infants born at <30 weeks gestation who received a PICC vs. umbilical venous catheter immediately after birth as primary venous access. | There was no significant difference in infants with CVC-BSI/1000 CVC-day between the evaluated groups, although lower rates of late sepsis in the group of infants who received only one umbilical catheter were observed. There was an increase in episodes of late sepsis/1000 CVC-day in the PICC group compared to neonates who had an umbilical catheter and PICC (RR 1.73; 95% CI: 1.15–2.60, p<0.001). |
| S11 | Bellemin K et al./France | Retrospective cohort | 1111/three NICUs | 2007–2011 | To evaluate incidence and risk factors for CVC-BSI. | The incidence density was 16/1000 CVC-day (95% CI: 14.0–18.2): 6.5 (95% CI: 4.3–9.3) for umbilical CVC and 20.2 (95% CI: 5–23.1) for other CVCs. The median time to CVC-BSI was eight days. In the multivariate analysis, birth weight≤750g (HR 6.3, 95% CI: 1.0–38.1) and intravenous lipid emulsion (HR 2.3, 95% CI: 1.3–3.9) were significantly associated with CVC-BSI for umbilical CVC. Similar results were observed for other CVCs. |
| S12 | Costa P et al./Brazil | Prospective cohort | 383 | 2010–2012 | Identify the risk factors for PICC-associated bloodstream infection in neonates. | In the bivariate analysis, lower mean weight and corrected gestational age, and longer catheter use were associated with the occurrence of CVC-BSI. In the multivariate analysis, corrected gestational age (RR 1.12; 95% CI: 1.061–1.186, p<0.002), transient metabolic disorder (RR 3.02; 95% CI: 1.72–5.05, p<0.000), apnea (RR 2.38; 95% CI: 1.10–4.56, p<0.016), and two-way and polyurethane catheters (RR 3.70; 95% CI: 2.0–6.9, p<0.0000) were associated with CVC-BSI. |
| S13 | Geffers C, Gastmeier A, Schwab F, Groneberg K, Ruden H, Gastmeier P/Germany41 | Multicenter prospective cohort | 2126 neonates/22 NICUs | 2010 | To describe the association between CVC and peripherally-inserted central catheter use and the risk of laboratory-confirmed BSI for newborns with birth weight <1500g. | The incidence density for BSI was 3.3/1000 patient-days. The use of a central venous catheter (HR 6.2; 95% CI: 3.83–9.97, p<0.001) was one of the independent significant factors for BSI. |
| S14 | Padula et al./USA42 | Prospective cohort | 409 neonates | 2006–2009 | To determine the clinical signs and risk factors associated with laboratory-confirmed BSI in patients assessed for late sepsis in a tertiary NICU. | Use of enteral contrast within the previous 48h was the most significant risk factor for laboratory-confirmed BSI [OR: 9.58 (95% CI: 2.03–45.19)], followed by the presence of a central venous catheter. |
ID, identification; NICU, neonatal intensive care unit; TPN, total parenteral nutrition; PICC, peripherally-inserted central catheter; CVC-BSI, central venous catheter-associated bloodstream infection; BSI, bloodstream infection; CDC, Centers for Disease Control and Prevention.
The first study (S1) was carried out in a university hospital, aiming to evaluate the incidence and complications associated with CVCs inserted in NICU infants. In their results, only 13 (4.9%) of 263 newborns had infection, five of which were central-venous catheter-associated bloodstream infections (CVC-BSI). The risk factors for CVC-BSI were gestational age (p=0.04) and low birth weight (p=0.02). The infection rate was calculated with a denominator of 100 patients-day, different from that recommended by the National Healthcare Safety Network (NHSN) of the Centers for Disease Control (CDC), which is 1000 CVC-day. As 29% of the inserted CVCs were associated with sepsis, the study demonstrates that catheter use requires extreme caution and preparation by the care team to reduce the infection risk.29
The increase in the daily risk of CVC-BSI after 35 days of PICC insertion in an NICU was recorded in the retrospective cohort study S2. It used a statistical model that allowed the evaluation of potential changes in the risk factor for CVC-BSI over time. The infection rate (2/1000 CVC-day) was based on NHSN criteria, and the sample of 683 newborns is one of the largest found to evaluate the duration of PICC in NICU neonates.30
Another retrospective cohort (S3) with 275 newborns showed that CVC-BSI was the most common complication associated with catheter (incidence: 8.3/1000 CVC-day). Femoral access for catheter insertion (increased risk of CVC-BSI compared to non-femoral catheters: 1.76, 95% CI, 1.01–3.07, p=0.045) and a longer duration of PICC (p<0.001) were found to be risk factors.31
The S4 multicenter study, carried out in Japan, found that time of PICC use was a risk factor for catheter-associated BSI (OR 1.35, 95% CI: 1.14–1.60, p=0.005). The study shows an incidence of catheter-associated BSI of 1.6/1000 CVC-day, with most PICCs remaining for a maximum of two weeks. Infection rates were compared when the catheter was inserted using the maximal barrier precaution (MBP), standard precautions, or no specific precaution, but no statistically significant differences were found.32 However, a retrospective USA multicenter study (S5), with a six-year evaluation period and a sample of 3967 neonates, which followed the diagnostic criteria recommended by the NHSN, observed that the permanence of PICC for more than two weeks resulted in a higher risk for infection than those with less than two weeks of permanence (p<0.0005).33
Another multicenter retrospective study (S6), with 13,327 infants from 141 NICUs, reported an incidence of CVC-BSI of 0.93 per 1000 CVC-day. The mean gestational age was 29 weeks (interquartile range: 26–33). The longer duration of PICC was not associated with an increased risk of CVC-BSI. For long-term catheters, the incidence of infection was significantly higher at weeks seven and nine when compared to the first week after catheter insertion.34
The S7 study, carried out in a university hospital in the Netherlands, used a sample of 196 neonates to determine the incidence and potential risk factors for CVC-BSI in newborns. There were 18.1 infections/1000 CVC-day (95% CI: 13.7–23.8) according to the diagnostic criteria used by the CDC before 2008. Umbilical catheters had a higher infection rate than non-umbilical central catheters (rate ratio of 2.4, 95% CI: 1.2–4.9) and if left for more than five days, an increase in infection rate was observed (p<0.05).35
In Brazil, the S8 study recruited a sample of 233 neonates and observed, after multivariate adjustment, the following factors associated with CVC removal due to suspected infection: weight less than 2500g at the time of catheter insertion, catheter repair, and permanence.36 The S11 study, a retrospective cohort with 18 neonates, found low birth weight, prematurity, and parenteral nutrition as factors that may have influenced the occurrence of infection.37
Study S10 shows a comparison of the CVC-BSI rates in preterm infants born with less than 30 weeks gestation who had a PICC inserted vs. umbilical venous catheter immediately after birth as primary venous access. Infants who received a PICC on the first day after birth were combined with two additional groups of infants (those who received an umbilical catheter on day one, and those who had this catheter and switched to a PICC after four days or more). No significant difference was verified in the infants with CVC-BSI/1000 CVC-day among the assessed groups, despite lower rates of late sepsis in the group of infants who received only an umbilical catheter.38
Study S11 evaluated the incidence density and risk factors for CVC-BSI. The incidence density was 16/1000 CVC-day (95% CI: 14.0–18.2): 6.5 (95% CI: 4.3–9.3) for umbilical CVC and 20.2% (95% CI: 17.5–23.1) for other CVCs. The median time to CVC-BSI was eight days. After multivariate analysis, birth weight≤750g (HR 6.3, 95% CI: 1.0–38.1) and intravenous lipid emulsion (HR 2.3, 95% CI: 1.3–3.9) were significantly associated with CVC-BSI for umbilical CVC. Similar results were observed for other CVCs.39
In the S12 study, the multivariate analysis disclosed that corrected gestational age (RR 1.12, 95% CI: 1.061–1.186, p<0.002), transient metabolic disorder (RR 3.02, 95% CI 1.72–5.05, p<0.000), apnea (RR 2.38, 95% CI: 1.10–4.56, p<0.016), and two-way and polyurethane catheters (RR 3.70, 95% CI: 2.0–6.9, p<0.0000) were risk factors associated with CVC-BSI. The study considered only PICC catheters for data analysis in neonates from a Brazilian institution.40
The S13 study, a multicenter prospective cohort with 2126 neonates from 22 NICUs in Germany, described the association between the use of CVC and peripheral catheters and the risk of laboratory-confirmed BSI for newborns with birth weight below 1500g. The incidence density for BSI was 3.3/1000 patient-days. The multivariate analysis identified the following significant independent risk factors for BSI: lower birth weight (hazard ratio [HR], 1.1–2.2), vaginal delivery (HR, 1.5), use of central venous catheter (HR, 6.2) or use of peripheral venous catheter (HR, 6.0) within two days before developing BSI, and individual characteristics (HR, 0.0–4.6). After adjusting for other risk factors, the use of peripheral venous catheter and CVC use were significantly associated with the occurrence of BSI in very low birth weight newborns.41
Also in the USA, a retrospective cohort study (S14) with 196 neonates found that the use of enteral contrast within 48h of infection onset was the most significant risk factor for laboratory confirmed BSI (odds ratio [OR], 9.58; 95% CI, 2.03–45.19), followed by the presence of a central venous catheter.42
Therefore, CVC-BSI continues to be an important complication, as shown by the incidence of this infection in the analyzed studies, varying from 0.93 to 18.1 infections/1000 CVC-day. The observed risk factors indicate that low birth weight, prematurity, and longer time of catheter use are related to a higher incidence of CVC-BSI.
DiscussionThe S1, S2, and S3 studies demonstrate that the central venous catheter is an important device for the neonate treatment in the NICU. They also do not disagree regarding the importance of the prevention and identification of risk factors associated with central catheters,29–31 with infection among the main complications associated with the use of invasive devices in the NICU, which may be independently associated with an increased risk of death.22,43
The relatively small sample size obtained from studies carried out in a single NICU may make it difficult to extrapolate the results to other institutions.29,31,35–37 Additionally, the CVC-BSI definition criteria did not follow the NHSN parameters.25,30,35,36
Differences were observed between the analyses performed, from absolute and relative frequency evaluations to the use of statistical models that allowed detecting possible differences between the factors studied and the accuracy of data estimation. Multicenter studies were able to more accurately assess risk estimates because of the large population sample; however, as it is well known, the retrospective nature of some studies may be a disadvantage.
Gestational age and low birth weight were factors associated with CVC-BSI in a US case–control study (S1); however, only six CVC-BSI episodes were evaluated in five neonates.29
Time of catheter use was the main risk factor for catheter-associated infection identified in five of the studies analyzed in this review (S2, S3, S5, S6, S7). In US, European, and Asian institutions, retrospective cohort studies emphasize the time of PICC use in neonates as a risk factor for CVC-BSI.30,31,33,35 In Japan (S4), it was verified that the longer time of catheter use (OR, 1.35; 95% CI: 1.14–1.60 for each week, p=0.0005) independently contributed to overall complications.32
A recent study (S6) found a mean of 11 days for PICC use, lower than the 25-day mean for long-term catheters (p=0.001). The large study sample and the multicenter nature are advantageous; however, it was a retrospective study. It is difficult to compare this study with institutions from developing countries, which have very different characteristics from those in the United States, where 66 (47%) of the 141 NICUs reported an incidence of CVC-BSI equal to zero.44 In Brazil, a prospective observational study carried out between 2011 and 2014 compared two periods with different empirical schemes for the treatment of late neonatal sepsis and found a BSI density of 17.29/1000 CVC-day.45 Even in the case of a study (S6) in which all participating institutions had already implemented the quality improvement initiative in PICC care, some factors remained unchanged (unit architecture and care environment) or were not evaluated (antibiotic therapy), suggesting that it is necessary to evaluate the maintenance package components and that other factors have the greatest impact on infection prevention. This study (S6) indicates that clinicians should focus their efforts on reducing CVC-BSI in infusion line maintenance care and timely removal of the central line when the CVC is no longer needed.34
The use of enteral contrast within 48h prior to sepsis, presence of CVC (S14), presence of two catheters (S10), weight≤750g, and intravenous lipid emulsion (S11) were statistically significant risk factors for CVC-BSI. However, due to the peculiarities of the studied population, the results may not be extrapolated to other institutions. However, these results confirm that apnea and hypotension may be useful clinical signs to guide the sepsis investigation and, additionally, the use of contrast deserves further investigation as a risk factor for CVC-BSI.42
In Brazil, prematurity and birth weight up to 1500g were factors associated with the suspicion of CVC-associated infection in two studies (S8, S9), but one used only clinical signs to determine the infection diagnosis36 and, in the other, the data were only analyzed according to absolute and relative frequencies, in addition to having a sample of only 18 newborns, which could lead to interpretation bias.37
There is lack of information about practices during patient care, such as use of parenteral nutrition, surgical procedures, type of implanted catheter, and other risk factors with potential for confusion. The literature indicates that skin fragility, the need for an infusion line for long periods, insertion made with limited use of sterile barriers, location of the infusion line placement, substitution by guidewire, contamination of the catheter hub, catheter use for more than seven days, and inexperienced and poorly qualified operators are among the significant risk factors for catheter-associated bloodstream infection in studies with neonatal populations.19,30,41
Studies have shown that neutropenia, prematurity, reduced numbers of nurses when compared to the number of patients, use of total parenteral nutrition, failures during catheter care (e.g., excessive catheter manipulation), and deficiencies in connection disinfection measures during manipulations have also been reported in studies with this type of population.33,46,47
It has been observed that low rates of catheter-associated infection, close to zero, are already a reality in health institutions in developed countries, since surveillance and infection control programs are used. When there is constant monitoring of the health team's adherence, through regular audits of the insertion procedure, during catheter maintenance and regarding hand hygiene, rates can be kept low at all times.48
The strength of the scientific evidence of the findings according to the GRADE28 system indicates that the absence of randomization in clinical trials shows a strong possibility of bias, with a lower level of evidence being initially attributed. Observational studies, such as those found in the study, are considered to be highly-biased. However, the GRADE system also evaluates the measure of effect, such as Relative Risk (RR) or Odds Ratio (OR), as a factor to increase the quality of evidence, since it is possible to obtain a higher effect size in observational studies, even in the presence of confounding factors.
In the present review, the risk factor with the greatest effect magnitude was the use of contrast in the CVC, with a nine-fold greater risk (S14) and birth weight≤750g, with a six-fold greater risk of infection (S11), whereas longer time of catheter use (S3 and S6), type of CVC (such as polyurethane and two-way catheters) (S12), and umbilical CVC for more than five days (S7) showed a two to three-fold higher risk of CVC-BSI. Additionally, CVC repair (S8) and use of a lipid solution (S11) have shown a two-fold increase in the risk for CVC-BSI.
The two studies (S1 and S9) with a higher level of evidence in favor, but that may be considered weak (grade “+1”) according to GRADE assessed gestational age and birth weight and use of parenteral nutrition as risk factors. However, they did not show the measures of effects for adequate analysis and quality assessment of the studies.
It should be pointed out that even in observational studies, with randomization, selection bias can be avoided, with better methodological quality and less risk of confusion, which is proposed for new studies.28
One of the limiting factors of this review is that few well-designed studies were found on CVC-BSI in Latin America. National data are still scarce on the evaluation of risk factors related to the clinical characteristics of the neonatal population and those related to the conditions of insertion and care in the maintenance of CVC and infusion lines, time of use, and reason for insertion and removal of central catheters. Furthermore, due to the ethical aspects involved, it is not feasible to carry out a clinical trial for CVC insertion and evaluation of its outcomes, which would allow the use of observational studies for a systematic review. Other studies should still address the training of the care team to prevent the complications associated with the presence of a CVC, especially bloodstream infections.
However, the authors consider that randomized studies of high methodological quality are necessary to assess the risks related to the use of CVC in neonates. Evidence-based interventions and adequate statistical evaluations of these complications may contribute to the reduction of this risk and aid the planning of the indication and time of permanence of CVCs.
ConclusionDespite the recommendations for reduction of CVC-associated bloodstream infections, they still show high incidence density rates in Brazil. Low birth weight, small gestational age, and time of use of the device are associated with a higher risk for these infections.
Considering the epidemiological and clinical importance of CVC-BSI, knowledge of the risk factors can contribute to the configuring of clinical protocols and establishing effective preventive measures in the care of patients with CVC.
This systematic review demonstrates that few studies on the risk factors for these infections have provided information on these infections in Brazil and, therefore, the need for more longitudinal studies and better prevention strategies to reduce infection is emphasized.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Rosado V, Camargos PA, Anchieta LM, Bouzada MC, Oliveira GM, Clemente WT, et al. Risk factors for central venous catheter-related infections in a neonatal population – systematic review. J Pediatr (Rio J). 2018;94:3–14.
Study carried out at Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil.