this study aimed to prepare a silkworm moth (Bombyx mori) antigenic extract and to perform skin prick tests with this extract in patients with allergic respiratory diseases; to evaluate serum specific immunoglobulin E (IgE) to Bombyx mori using ImmunoCAP® system and to report the frequency of positivity between the two methods and with clinical data.
Methodsthis was a cross‐sectional study with 99 children and adolescents diagnosed with asthma and/or allergic rhinitis, who had skin reactivity to at least one of the six aeroallergens tested. Clinical data were evaluated: skin prick tests with Bombyx mori in‐house extract, and total and specific IgE analysis using ImmunoCAP® were performed.
Resultsthe frequency of Bombyx mori specific IgE was found to be 52.5% and 60% using the skin prick test and ImmunoCAP®, respectively. An association between a positive skin test for Bombyx mori and the presence of allergic rhinitis, atopic dermatitis, and urticaria was observed, but the same was not true for asthma or allergic conjunctivitis. There was no relation with the severity of asthma or rhinitis symptoms.
Conclusionsa high frequency of sensitization to Bombyx mori was observed in a selected population of patients with respiratory allergic diseases in the city of Curitiba, state of Paraná, Brazil. The extract prepared from the wings of this moth species is effective in demonstrating this sensitivity.
Preparar extrato antigênico da mariposa do bicho‐da‐seda (Bombyx mori) e realizar testes cutâneos com esse extrato em pacientes com doenças respiratórias alérgicas, avaliar IgE sérica específica para Bombyx mori usando o sistema ImmunoCAP® e relatar a frequência de positividade entre os dois métodos e com dados clínicos.
MétodosEstudo transversal com 99 crianças e adolescentes com diagnóstico de asma e/ou rinite alérgica, que apresentaram reação cutânea a pelo menos um dos seis aeroalérgenos testados. Os dados clínicos foram avaliados; testes cutâneos com extrato de Bombyx mori e análise de IgE total e específica por ImmunoCAP® foram realizados.
ResultadosA frequência de IgE específica para Bombyx mori foi de 52,5% e 60%, respectivamente, pelo teste cutâneo e ImmunoCAP®. Foi observada uma associação entre o teste cutâneo positivo para Bombyx mori e a presença de rinite alérgica, dermatite atópica e urticária, mas o mesmo não ocorreu para a asma ou conjuntivite alérgica. Não houve relação com a gravidade dos sintomas de asma ou rinite.
ConclusõesAlta frequência de sensibilização à Bombyx mori foi encontrada em uma população selecionada de pacientes com doenças alérgicas respiratórias na cidade de Curitiba, estado do Paraná, Brasil. O extrato preparado a partir das asas dessa espécie de mariposa é eficaz em demonstrar essa sensibilidade.
Sensitization to inhalant allergens is a risk factor for the development of allergic diseases such as asthma and rhinitis. Knowledge about sensitizing allergens and their degree of exposure in different environments is essential for the diagnosis and treatment of allergic respiratory diseases. Dermatophagoides pteronyssinus and Blomia tropicalis mites are the main sensitizers for patients diagnosed with asthma and allergic rhinitis.1,2
The participation of insects in allergic respiratory reactions has been discussed for decades.3 The most extensively studied insect has been the cockroach, whose domestic infestation is a cause of asthma and is considered to be a public health issue.4 There have been descriptions of asthma and rhinitis triggered by species of flies and mosquitoes such as the mayfly and the caddis fly.5,6 A study conducted with asthmatic patients in the city of São Paulo, Brazil, evidenced positive skin prick test (SPT) with mosquito extract in 32.5% of cases, and positive SPT with moth extract in 65% of cases.7
There have been several reports of individuals who, during the process of silk production, developed respiratory allergic diseases. While caring for silkworm cocoons, workers are exposed directly to their inhalant antigens, present from the selection to the hatching of cocoons, when there is contact with dust from the moths’ wings.8 These allergens can trigger asthma,9 rhinitis, and conjunctivitis symptoms.10
The silkworm moth has cross‐reactivity with other species of moths and butterflies; it has been shown that patients with respiratory allergic diseases can develop symptoms from environmental exposure to their allergens.11,12 Concentrations of moth antigens verified by radioimmunoassay in samples of dust in the external environment (not home) for a period of three years were high and at levels comparable to those of pollen.
Skin tests with moth extract in allergic patients had 45% reactivity in this population.13 In 1997, allergy skin tests in atopic children in the city of Curitiba, state of Paraná, Brazil, detected 38.4% of positivity to moth extract (1:20 Heterocera weight/volume), the second most frequent after the Dermatophagoides pteronyssinus mite (97.5%). It was suggested that the high rate of sensitization to moth required a better evaluation of its clinical relevance.14
The aim of this study was to determine the sensitivity to Bombyx mori by SPT using silkworm moth wing antigens and specific serum immunoglobulin E (IgE) in children diagnosed with asthma and/or allergic rhinitis.
MethodsThis was a cross‐sectional study with non‐probabilistic sampling of 99 children and adolescents of both genders with a diagnosis of asthma and/or allergic rhinitis treated at the outpatient allergy clinic of Allergy and Pediatric Immunology Service, Hospital de Clínicas, Universidade Federal do Paraná, with positive SPT for at least one of the following antigens: Dermatophagoides pteronyssinus, Blomia tropicalis, Blattella germanica, Lolium multiflorum, dog epithelium, or cat epithelium.
The diagnosis and classification of respiratory allergic diseases (asthma and rhinitis) followed the recommendations of the Global Initiative for Asthma (GINA)15 and Allergic Rhinitis and its Impact on Asthma (ARIA),16 respectively.
The allergen extract of Bombyx mori was prepared from the wings of this species using the following method: the material from the insect was macerated with a pestle in a ceramic mortar and the content was degreased with ethyl ether. For antigen extraction, the approximate amount of 2g of insect particles was placed in 20mL of cold sterile buffer solution (PBS), mixed for six minutes, and allowed to stand for 48hours at 8°C. The mixture was then centrifuged for 15minutes at 12,000rpm, and the supernatant was filtered using sterile polyethersulfone filters (Millipore Express® PLUS Membrane Filters, USA) containing pores of 0.2 micrometers in diameters.
After bacteriological sterility tests, the filtrate was diluted in 50% glycerin, 1:20 (weight/volume). This final concentration was chosen to allow for comparison with the results of a similar study conducted in 1997, in which allergy skin tests were performed with allergen extract standardized at 1:20 from Heterocera sp. moth (Hollister‐Stier Laboratories®, USA) in children diagnosed with asthma and allergic rhinitis.14 This concentration of 1:20 is commonly used by manufacturers of glycerinated allergenic extracts for SPT. The allergen extract was stored in bottles with a dropper and kept refrigerated at 4°C.17
All subjects underwent the SPT with the extract prepared from Bombyx mori according to the following technique: the volar surface of the right forearm was cleaned with 70% ethyl alcohol; then, a drop of the extract was applied on the skin and the location marked with skin marker pen. The positive control was performed with histamine at a concentration of 10mg/mL (IPI/ASAC, Brazil) and the negative control with 50% saline/glycerol (IPI/ASAC, Brazil) with a 3‐cm distance between them. The puncture technique was used with a 27‐mm sterile needle applied superficially on the skin surface at an angle of 20°. After 15minutes, the reading of the reaction was performed with the aid of a millimeter‐scale ruler. The test was considered positive when the mean of the two perpendicular diameters of the papule was ≥ 3mm.18
Serum samples were separated into aliquots in Eppendorf tubes at ‐80°C until measurement of total IgE and specific IgE to allergens from Bombyx mori, Dermatophagoides pteronyssinus, Dermatophagoides farinae, Blomia tropicalis, Blattella germanica, dog epithelium, and cat epithelium through the ImmunoCAP® method. Each allergen was covalently coupled to a solid phase and reacted with specific IgE antibodies contained in the serum sample of patients.
In the case of Bombyx mori, the antigen coupled to the solid phase is derived from the wings of this kind of moth, and the allergen components are not available for this insect. Then, nonspecific antibodies were removed by washing and anti‐IgE antibodies were added, bound to the β‐galactosidase enzyme. After incubation, the antibodies (anti‐IgE‐enzyme) that were not bound were removed by a new washing process. The enzyme substrate contained in the development solution was added to the reaction medium. The reaction was then discontinued by adding the stop solution, and the fluorescence was measured, which is proportional to the amount of specific IgE in the sample. The concentration of total IgE was expressed in kU/L and for the specific IgE, kUA/L.19 Values > 0.7 kUA/L were considered positive.
The research project was approved by the Ethics Committee on Human Research of the HC‐UFPR; parents or guardians signed the informed consent and participants older than 12 years old signed the term of agreement.
For statistical analysis, the software program Statistica (Statsoft®, USA) was used. Measures of central tendency and dispersion were expressed as median, minimum and maximum values. The nonparametric Mann‐Whitney's test was used to estimate the difference of variables with asymmetric distribution. The estimated difference between categorical variables was performed by Fisher's exact test and the chi‐squared test. The univariate logistic regression model was used to estimate the probability of SPT positivity according to the levels of specific IgE to Bombyx mori. A minimum level of significance of 5% and a minimum test power of 85% were considered for all tests.
ResultsThe mean age of patients was 10.4 + 2.2 years with a range of 6‐15 years (95% CI: 9.9‐10.8) and 61 (61.6%) were males. Eighty‐seven patients had asthma (87.9%) and 95 had (95.9%) allergic rhinitis; 12 (12.1%) had only rhinitis, and four (4.0%) had only asthma.
Among patients with asthma, 31 (35.6%) were classified as having the intermittent or persistent mild form, 46 (52.9%) had persistent moderate, and ten (11.5%) had persistent severe asthma. Among patients with allergic rhinitis, 24 (25.3%) had the mild form (intermittent or persistent mild rhinitis) and 71 (74.7%) had persistent moderate/severe.
Eye symptoms (eye pruritus, tearing, conjunctival hyperemia, or congestion) were reported by 66.7% of patients. Among other allergic diseases, atopic dermatitis was diagnosed in nine (9.1%) patients and urticaria in six (6.1%).
A positive reaction at SPT to the Bombyx mori extract was observed in 52 patients (52.5%), the second in frequency after dust mites; Dermatophagoides pteronyssinus, 82 (82.8%), and Blomia tropicalis, 69 (69.7%). The other observed reactions were to cockroach (Blattella germanica), dog epithelium, rye grass (Lolium multiflorum) pollen, and cat epithelium with 17.2%, 16.2%, 15.1%, and 12.1% of positive SPTs, respectively.
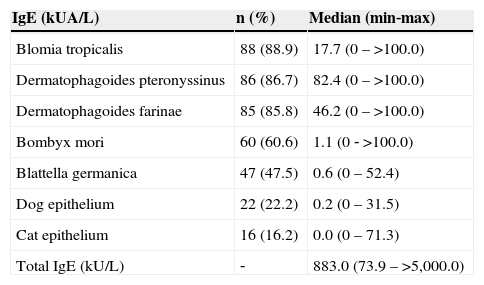
Table 1 shows the frequency of positive ImmunoCAP® tests using a cutoff of 0.7 kUA/L; the levels of specific IgE and total IgE were expressed as medians (minimum and maximum values).
Frequency of positivity to specific IgEs through Immunocap® (> 0.7 kUA/L) and serum levels of specific and total IgEs.
| IgE (kUA/L) | n (%) | Median (min‐max) |
|---|---|---|
| Blomia tropicalis | 88 (88.9) | 17.7 (0–>100.0) |
| Dermatophagoides pteronyssinus | 86 (86.7) | 82.4 (0–>100.0) |
| Dermatophagoides farinae | 85 (85.8) | 46.2 (0–>100.0) |
| Bombyx mori | 60 (60.6) | 1.1 (0 ‐ >100.0) |
| Blattella germanica | 47 (47.5) | 0.6 (0–52.4) |
| Dog epithelium | 22 (22.2) | 0.2 (0–31.5) |
| Cat epithelium | 16 (16.2) | 0.0 (0–71.3) |
| Total IgE (kU/L) | ‐ | 883.0 (73.9–>5,000.0) |
Univariate logistic regression analysis showed a good positive association between SPT and levels of specific IgE to Bombyx mori (p=0.006).
There was an association between SPT positivity for Bombyx mori and the presence of allergic rhinitis (p=0.04), atopic dermatitis (p=0.03), and urticaria (p=0.02). However, there was no association with the presence of asthma (p=0.36) and eye symptoms (p=0.14). Likewise, there was no difference in the severity of asthma (p=0.73) or allergic rhinitis symptoms (p=0.37).
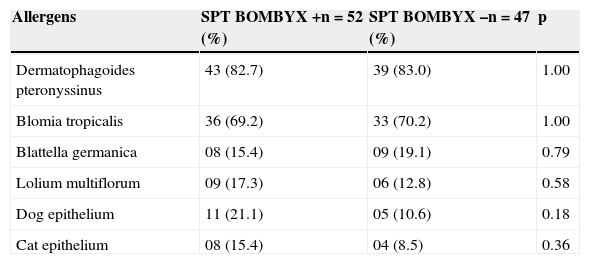
Regarding the frequency of positivity for Bombyx mori in relation to other SPTs, there was no significant association (Table 2).
Association between SPT positivity for Bombyx mori and other skin tests (n=99).
| Allergens | SPT BOMBYX +n=52 (%) | SPT BOMBYX –n=47 (%) | p |
|---|---|---|---|
| Dermatophagoides pteronyssinus | 43 (82.7) | 39 (83.0) | 1.00 |
| Blomia tropicalis | 36 (69.2) | 33 (70.2) | 1.00 |
| Blattella germanica | 08 (15.4) | 09 (19.1) | 0.79 |
| Lolium multiflorum | 09 (17.3) | 06 (12.8) | 0.58 |
| Dog epithelium | 11 (21.1) | 05 (10.6) | 0.18 |
| Cat epithelium | 08 (15.4) | 04 (8.5) | 0.36 |
Fisher's exact test.
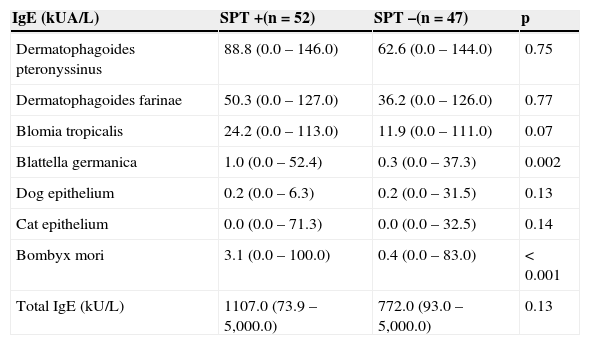
The analysis of serum levels of total and specific IgE antibodies in relation to the SPT for Bombyx mori tended to be higher in patients sensitized to the moth. However, only the serum levels of specific IgE to cockroach and to the moth were significantly elevated in these patients who were positive at the SPT for Bombyx mori (Table 3).
Serum levels of total and specific IgE according to SPT positivity for Bombyx mori (n=99).
| IgE (kUA/L) | SPT +(n=52) | SPT–(n=47) | p |
|---|---|---|---|
| Dermatophagoides pteronyssinus | 88.8 (0.0–146.0) | 62.6 (0.0–144.0) | 0.75 |
| Dermatophagoides farinae | 50.3 (0.0–127.0) | 36.2 (0.0–126.0) | 0.77 |
| Blomia tropicalis | 24.2 (0.0–113.0) | 11.9 (0.0–111.0) | 0.07 |
| Blattella germanica | 1.0 (0.0–52.4) | 0.3 (0.0–37.3) | 0.002 |
| Dog epithelium | 0.2 (0.0–6.3) | 0.2 (0.0–31.5) | 0.13 |
| Cat epithelium | 0.0 (0.0–71.3) | 0.0 (0.0–32.5) | 0.14 |
| Bombyx mori | 3.1 (0.0–100.0) | 0.4 (0.0–83.0) | < 0.001 |
| Total IgE (kU/L) | 1107.0 (73.9–5,000.0) | 772.0 (93.0–5,000.0) | 0.13 |
Mann‐Whitney's test
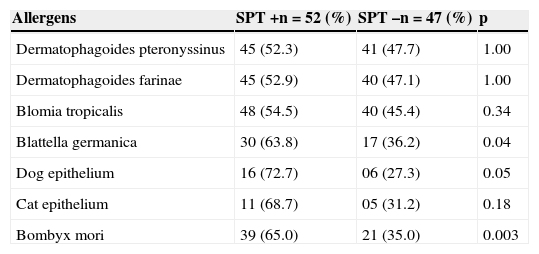
There was a significantly higher frequency of positivity of specific IgEs to dog epithelium, Blattella germanica, and Bombyx mori (Table 4).
Frequency of positivity for specific IgEs (>0.7 kUA/L) according to SPT positivity for Bombyx mori (n=99).
| Allergens | SPT+n=52 (%) | SPT–n=47 (%) | p |
|---|---|---|---|
| Dermatophagoides pteronyssinus | 45 (52.3) | 41 (47.7) | 1.00 |
| Dermatophagoides farinae | 45 (52.9) | 40 (47.1) | 1.00 |
| Blomia tropicalis | 48 (54.5) | 40 (45.4) | 0.34 |
| Blattella germanica | 30 (63.8) | 17 (36.2) | 0.04 |
| Dog epithelium | 16 (72.7) | 06 (27.3) | 0.05 |
| Cat epithelium | 11 (68.7) | 05 (31.2) | 0.18 |
| Bombyx mori | 39 (65.0) | 21 (35.0) | 0.003 |
Fisher's exact test.
The prevalence of respiratory allergic diseases has increased worldwide in the last decades.20,21 In Brazil, the same trend of increase in asthma and rhinitis symptoms has been observed.22 Knowledge about sensitizing aeroallergens is essential for diagnosis, treatment, and prevention.2
Dust mites are the main allergenic sources in patients with asthma and allergic rhinitis.23,24 The importance of insect inhalant antigens has been discussed for decades.3,7 In this study, the moth was the second most frequent aeroallergen regarding positivity verified by SPT and specific IgE serum levels, suggesting that this insect should be considered a sensitizing agent for patients with asthma and allergic rhinitis.
The study participants were not silk industry workers, and therefore had no occupational exposure to Bombyx mori. Kino and Oshima evaluated asthmatic patients at random and found sensitivity to moth and butterfly by SPT and radioallergosorbent test (RAST) in the sera of over one third of cases. The authors concluded that the insects are easily attracted to the artificial light of households and may cause sensitization and respiratory allergy symptoms.[11]
A group of asthmatics with no history of occupational exposure (n=50) showed a frequency of positivity at the SPT for Bombyx mori of 68%,12 higher than that observed in the present patients (52.5%); both studies used antigenic extract prepared from the wings of moths. This difference may have occurred because the population was studied in Japan, in smaller numbers, consisted of adults, and had different life habits and exposure to different climate and environment.
This study showed an association between SPT positivity for Bombyx mori and the corresponding specific serum IgE by ImmunoCAP®, which demonstrates the effectiveness of the extract made to test sensitization to moth. Moreover, in patients with positive SPT, levels of specific IgE to Bombyx mori were higher.
When evaluating the frequency of positivity to specific IgE for moth according to the severity of allergic rhinitis, it was observed that patients with more severe disease had more positive serum specific IgEs for moth by the RAST method,25 differently from that found for the population of the present study, in which there was more positivity at the SPT for Bombyx mori in patients with rhinitis; however, there was no correlation with symptom severity. This difference may be explained by the different methods used to detect specific IgE and by the higher number of participants in the study conducted in Japan.
Although the assessed population of patients with atopic dermatitis and urticaria was small, more dermatological allergic diseases were diagnosed in those reactive to moth. This could be explored in the future, as there have been few reports of allergic and irritant reactions in the skin after contact with moths.26,27
It is known that there is cross‐reactivity between insect allergens. It was demonstrated by RAST‐inhibition assay that there is cross‐reactivity between similar species, such as butterfly and moth,12 but also between different species, such as moth and mosquito.28
The molecular allergy techniques allowed for the identification of the major allergen of Bombyx mori larvae (Bom m 1), which is constituted by arginine kinase protein and displays cross‐reactivity with arginine kinase from the cockroach.29 In the study, it was observed that patients reactive to Bombyx mori at the SPT showed a higher frequency of positivity and higher levels of serum specific IgEs for Blattella germanica, which could be explained by the cross‐reactivity between them. However, the same did not occur when comparing the positive skin reactions between moth and cockroach, but the immunochemical analysis of these allergens was not performed.
Moreover, inhibition tests with extracts from moth and mites showed differences between their antibodies, demonstrating that there is no cross‐reactivity between them.12 In the study, there was no association between the frequency of skin reactivity at the SPT to mite and moth antigens (Dermatophagoides pteronyssinus and Blomia tropicalis). Similarly, there was no association between positivity at the SPT for Bombyx mori and the presence of specific serum IgE to mites (Dermatophagoides pteronyssinus, Dermatophagoides farinae, and Blomia tropicalis), probably because there is no cross‐reactivity between them.
One study from China identified another allergen component of Bombyx mori (Bom m 7), also obtained from the larvae of this insect, but consisting of tropomyosin protein.30 It is considered a pan‐allergen, able to show broad cross‐reactivity with components of other insects such as Der p 10 (from the Dermatophagoides pteronyssinus mite) and Bla g 7 (from the Blattella germanica cockroach).31,32 Therefore, to verify true allergic sensitization or cross‐reactivity, future studies should be performed, based on molecular allergy diagnosis, but using allergenic components of the Bombyx mori moth and not of its larvae as a cause of respiratory allergic symptoms.33,34
This study was the first on sensitization to the silkworm moth performed in Brazil, and showed the importance of Bombyx mori as a sensitizing allergen in children and adolescents diagnosed with allergic respiratory diseases (asthma and/or rhinitis). A high frequency of sensitization to Bombyx mori was found in patients evaluated by SPT with an extract prepared from the wings of moths; these results were confirmed by ImmunoCAP®, a well‐established method for detection of specific serum IgE.
The identification of this aeroallergen (moth), together with the other groups that compose the profile of allergic sensitization in this population, should make their treatment more efficient, as it will allow for adjustments in environmental control procedures, as well as provide new specific immunotherapy options in the future.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Araujo LM, Rosário Filho NA, Riedi CA. Respiratory allergy to moth: the importance of sensitization to Bombyx mori in children with asthma and rhinitis. J Pediatr (Rio J). 2014;90:176–81.
Study conducted at Allergy and Pediatric Immunology Service, Hospital de Clínicas, Universidade Federal do Paraná.