to evaluate the frequency and factors associated with vascular complications after pediatric liver transplantation.
Methodrisk factors were evaluated in 99 patients under 18 years of age with chronic liver disease who underwent deceased donor liver transplantation (DDLT) between March of 1995 and November of 2009 at the Hospital de Clínicas de Porto Alegre, Brazil. The variables analyzed included donor and recipient age, gender, and weight; indication for transplant; PELD/MELD scores; technical aspects; postoperative vascular complications; and survival.
Resultsvascular complications occurred in 19 patients (19%). Arterial events were most common, occurred earlier in the postoperative period, and were associated with high graft loss and mortality rates. In the multivariate analysis, the following factors were identified: portal vein diameter ≤ 3mm, donor‐to‐recipient body weight ratio (DRWR), prolonged ischemic time, and use of arterial grafts.
Conclusionthe choice of treatment depends on the timing of diagnosis; however, in this study, surgical revision or correction produced worse outcomes than percutaneous angioplasty. The reduction of risk factors and early detection of vascular complications are key elements to a successful transplantation.
Avaliar a frequência e os fatores associados a complicações vasculares após transplante hepático pediátrico.
MétodoOs fatores de risco foram avaliados em 99 pacientes com mais de 18 anos de idade com doença hepática crônica submetidos a transplante hepático cadavérico (THC) entre março de 1995 e novembro de 2009 no Hospital de Clínicas de Porto Alegre, Brasil. As variáveis analisadas incluíram: idade, sexo e peso dos doadores e receptores; indicação de transplante; escores PELD/MELD; aspectos técnicos; complicações vasculares pós‐operatórias; e sobrevida.
ResultadosOcorreram complicações vasculares em 19 pacientes (19%). Os eventos arteriais foram mais comuns, tendo ocorrido precocemente no pós‐operatório, e foram associados a altas taxas de perda do enxerto e mortalidade. Em uma análise multivariada, foram identificados os seguintes fatores: diâmetro da veia porta ≤ 3mm, proporção de peso do doador/receptor (DRWR), tempo de isquemia prolongado e uso de enxertos arteriais.
ConclusãoA escolha do tratamento depende do momento do diagnóstico; contudo, nessa série, a cirurgia de revisão, ou correção cirúrgica, produziu resultados piores que a angioplastia percutânea. A redução dos fatores de risco e a detecção precoce de complicações vasculares são fundamentais para um transplante bem‐sucedido.
Liver transplantation is an accepted treatment option for children with chronic liver disease, with actuarial survival rates of up to 80% in five and 75% in ten years.1 Early causes of graft failure and mortality are mostly related to vascular complications, especially hepatic artery thrombosis (HAT) and portal vein thrombosis (PVT).2 There are a number of recognized risk factors for the development of these complications in the pediatric population, such as discrepancy between donor and recipient arterial and portal diameter, surgical skills, lower recipient weight,3 and small portal vein diameter.4 There are no consistent data regarding these risk factors in the pediatric population of Brazil.
The aim of this study was to assess the frequency of vascular complications in pediatric patients undergoing deceased donor liver transplantation (DDLT) at the Hospital de Clínicas de Porto Alegre, Brazil, and to identify the factors associated with these complications and mortality.
MethodsThe charts of 99 first liver transplant recipients under 18 years of age who underwent DDLT at the Hospital de Clínicas de Porto Alegre between March of 1995 and November of 2009 were retrospectively reviewed. The study was approved by the institution's ethics committee.
During this period, 128 liver transplants were performed on 121 children and adolescents (range: 4 months to 18 years). Of these, 29 were excluded from the sample: 13 who underwent emergency liver transplantation due to fulminant hepatitis, six who received living donor grafts, and three because the preservation solution wasn’t the University of Wisconsin solution (UW). The exclusion criteria were defined in order to avoid comparison bias based on immunological or technical factors influencing vascular complications. The patients were split into two groups for comparison: with vascular complications (n=19) and without vascular complications (n=88). These data were used for univariate and multivariate analysis in order to identify the associated factors.
Recipients were assessed for the following variables: age, gender, weight, transplant indication, PELD/MELD scores, type of allograft, type of anastomosis, vascular complications, management of these complications, and survival. Since data regarding graft weight was not available for all patients, the donor weight/recipient weight ratio (DRWR) was assessed.5
The diagnosis of vascular complications was established by a minimum of two imaging modalities and/or surgical confirmation. All transplants were performed by the same surgical team, and the piggyback technique with vena cava preservation was the standard procedure. Vascular anastomosis was performed under 3.5 × loupe magnifications. A PDS™ (Ethicon) (7‐0 polydioxanone monofilament) thread was used for arterial and portal sutures, and a non‐absorbable 5‐0 polypropylene monofilament was used on the hepatic veins. Running stitches were used for vessels larger than 3mm in diameter, and simple interrupted stitches for smaller vessels.
Postoperatively, blood flow in the hepatic artery, portal vein, and suprahepatic vena cava was assessed by Doppler ultrasonography (DUS) of the abdomen once a day during the first postoperative week; every other day on the second week; and once a week subsequently, for a total of 30 days. Outpatient DUS follow‐up was provided on the third and sixth postoperative months, and one year after transplantation. DUS was subsequently performed once a year or when patients developed clinical and/or biochemical abnormalities that justified testing. When DUS suggested a vascular complication, repeat ultrasound, angiography, computerized tomography (CT) scan with intravenous contrast, and/or surgery was performed to confirm or discard the diagnosis. Vascular complications were classified as arterial (hepatic artery) or venous (affecting the portal vein or suprahepatic vena cava). Early complications were defined as those occurring within 30 days of transplantation, whereas late complications were those that occurred after this period.
Statistical analysisQuantitative data were expressed as median, interquartile range, and minimum and maximum values, and categorical data were expressed as counts and percentages.
Univariate analysis was performed on Kaplan‐Meier curves compared by the log‐rank test, yielding hazard ratios, 95% confidence intervals, and their significance. A multivariate Cox model including significant variables (p<0.20) was constructed and used to adjust for confounders. A backward selection procedure was performed, and all variables with p ≥ 0.10 were removed. Data were analyzed and processed using the Statistical Package for Social Sciences (SPSS), version 17.0.
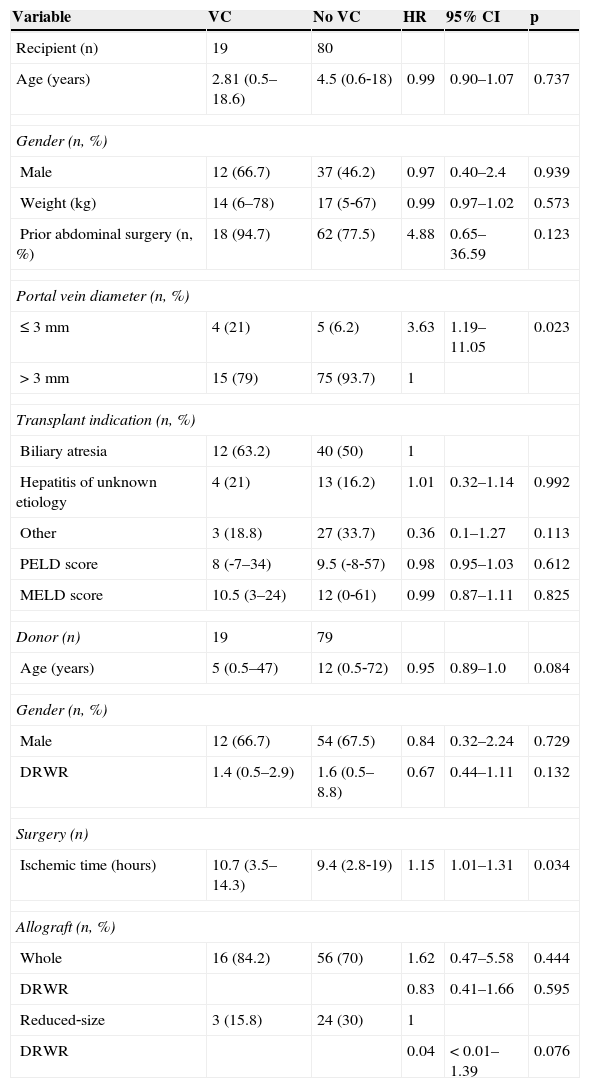
ResultsThe main indication for transplant was biliary atresia (52.5%), followed by hepatitis of unknown etiology (17.2%) and autoimmune hepatitis (7%). Median recipient weight was 17.0kg (range: 5.0–78.0kg), and the median PELD score was 9.0 (range: 8.0–57.0). A total of 99 patients were included in the study; 72 (72.7%) received an entire liver and 27 (27.3%) received a reduced or split graft. Vascular complications occurred in 19 (19.2%) patients. Median age, weight, and DRWR in the complication group were 2.1 years (range: 0.5–18.6 years), 14.0kg (range: 6.0–78.0kg), and 1.4 (range: 0.44–2.88), respectively (Table 1).
Sample characteristics and univariate analysis.
| Variable | VC | No VC | HR | 95% CI | p |
|---|---|---|---|---|---|
| Recipient (n) | 19 | 80 | |||
| Age (years) | 2.81 (0.5–18.6) | 4.5 (0.6‐18) | 0.99 | 0.90–1.07 | 0.737 |
| Gender (n, %) | |||||
| Male | 12 (66.7) | 37 (46.2) | 0.97 | 0.40–2.4 | 0.939 |
| Weight (kg) | 14 (6–78) | 17 (5‐67) | 0.99 | 0.97–1.02 | 0.573 |
| Prior abdominal surgery (n, %) | 18 (94.7) | 62 (77.5) | 4.88 | 0.65–36.59 | 0.123 |
| Portal vein diameter (n, %) | |||||
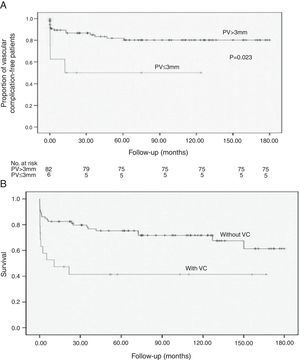
| ≤ 3 mm | 4 (21) | 5 (6.2) | 3.63 | 1.19–11.05 | 0.023 |
| > 3 mm | 15 (79) | 75 (93.7) | 1 | ||
| Transplant indication (n, %) | |||||
| Biliary atresia | 12 (63.2) | 40 (50) | 1 | ||
| Hepatitis of unknown etiology | 4 (21) | 13 (16.2) | 1.01 | 0.32–1.14 | 0.992 |
| Other | 3 (18.8) | 27 (33.7) | 0.36 | 0.1–1.27 | 0.113 |
| PELD score | 8 (‐7–34) | 9.5 (‐8‐57) | 0.98 | 0.95–1.03 | 0.612 |
| MELD score | 10.5 (3–24) | 12 (0‐61) | 0.99 | 0.87–1.11 | 0.825 |
| Donor (n) | 19 | 79 | |||
| Age (years) | 5 (0.5–47) | 12 (0.5‐72) | 0.95 | 0.89–1.0 | 0.084 |
| Gender (n, %) | |||||
| Male | 12 (66.7) | 54 (67.5) | 0.84 | 0.32–2.24 | 0.729 |
| DRWR | 1.4 (0.5–2.9) | 1.6 (0.5–8.8) | 0.67 | 0.44–1.11 | 0.132 |
| Surgery (n) | |||||
| Ischemic time (hours) | 10.7 (3.5–14.3) | 9.4 (2.8‐19) | 1.15 | 1.01–1.31 | 0.034 |
| Allograft (n, %) | |||||
| Whole | 16 (84.2) | 56 (70) | 1.62 | 0.47–5.58 | 0.444 |
| DRWR | 0.83 | 0.41–1.66 | 0.595 | ||
| Reduced‐size | 3 (15.8) | 24 (30) | 1 | ||
| DRWR | 0.04 | < 0.01–1.39 | 0.076 | ||
Data expressed as “median (interquartile range) [range]” or as “number (%).”
CI, confidence interval; DRWR, donor to recipient weight ratio; HR, hazard ratio; MELD, model for end‐stage liver disease; p, p‐value; PELD, pediatric model for end‐stage liver disease; VC, vascular complication.
Of the 19 patients with vascular complications, 16 received whole‐liver transplants and three received reduced‐size grafts (two left lobes and one right lobe and segment).4 No patients with vascular complications received split grafts. Vascular malformations were found in five patients (26.3%), and reduced portal vein diameter (≤ 3mm) in four (21%). Venous grafts were not used in these cases because the graft wasn’t always available or because the intraoperative evaluation concluded that the blood flow was good. Two patients (10.5%) needed grafts for arterial revascularization; in both cases, an autologous infrarenal aortic graft was used. Only one patient had never undergone abdominal surgery prior to transplantation.
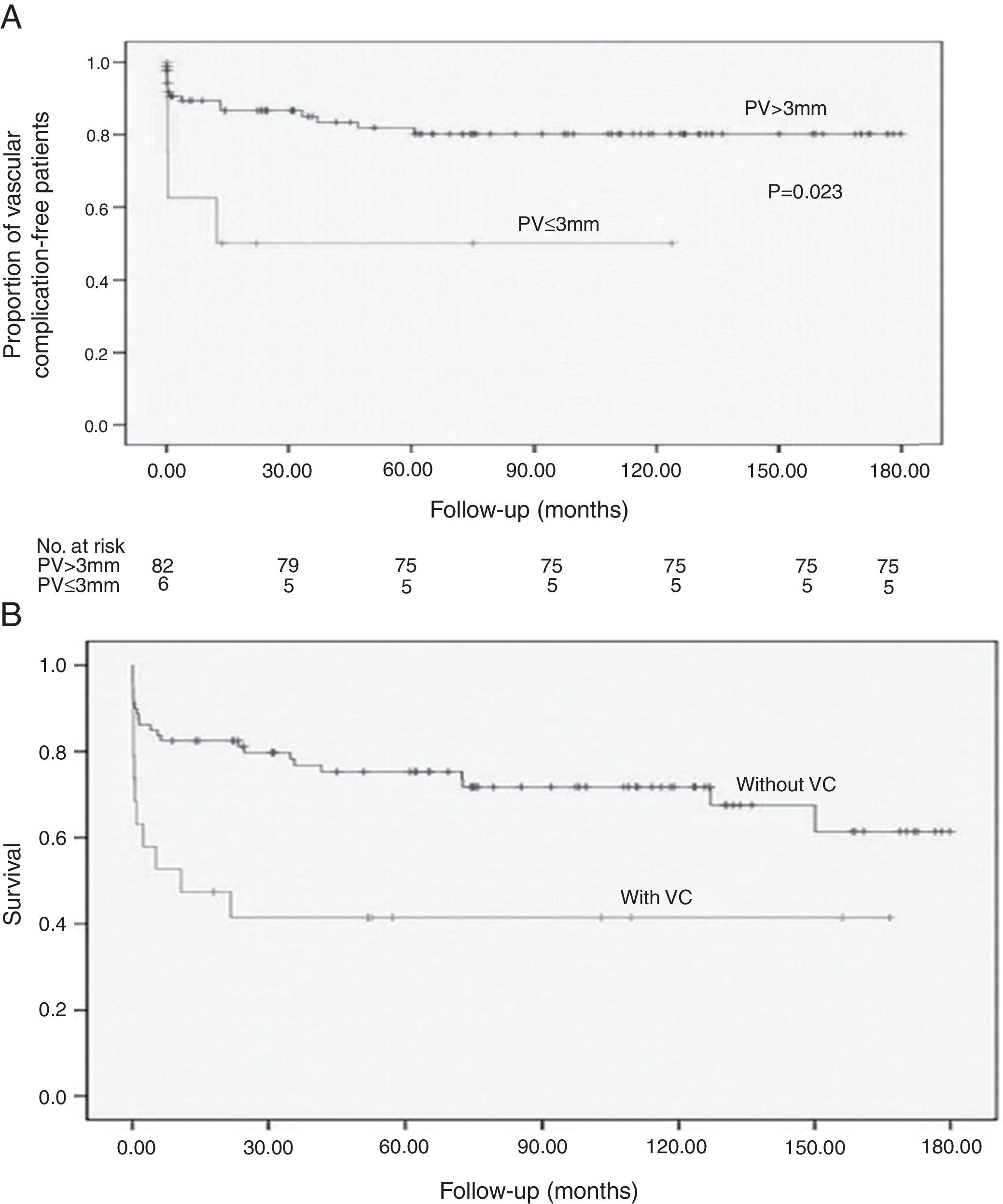
The most common complication was HAT (7%). Five patients (5%) developed PVT, three (3%) had hepatic artery stenosis, and two (2%) had portal vein stenosis. One patient had a mycotic aneurysm of the hepatic artery, and one developed stenosis of the suprahepatic‐caval anastomosis. Early vascular complications were most frequent, occurring in 11 patients (57.9%), with a mortality of 81.8%. Vascular complication‐free survival is shown in Fig. 1A.
In all patients, a diagnosis of vascular complication was suggested by DUS. The definitive diagnosis was established by repeated DUS in three patients (15.8%), angiography in eight (42.1%), CT scan with intravenous contrast in four (21%), and reoperation in seven (36.8%).
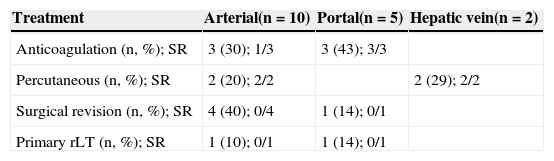
Clinical management with intravenous heparin (Liquemine®) and oral acetylsalicylic acid were administered in six patients, with a mortality rate of 33.3%. Percutaneous treatment was indicated in four patients: three underwent angioplasty and two received stents. The primary percutaneous management was with balloon dilatation; the stent was inserted when the balloon dilatation alone was insufficient. In one case, the stent was indicated because of arterial kinking. All percutaneous interventions were successful, and all four patients were alive at the end of follow‐up. Seven patients were submitted to surgery, five of whom (71.4%) underwent thrombectomy with reanastomosis. All died, including two who were awaiting transplantation. In the remaining two patients (28.6%), re‐transplantation (rLT) was the initial surgical treatment, but neither survived.
The outcomes are listed in Table 2. One patient in whom rLT was indicated died before any treatment could be administered. Overall mortality in patients with vascular complication was 57.9%, and seven deaths (36.8%) were directly correlated to the vascular complication. Other causes of death were sepsis, renal failure, pneumonia, pneumothorax, tuberculosis, intracranial bleeding, and chronic rejection.
Management of vascular complications.
| Treatment | Arterial(n=10) | Portal(n=5) | Hepatic vein(n=2) |
|---|---|---|---|
| Anticoagulation (n, %); SR | 3 (30); 1/3 | 3 (43); 3/3 | |
| Percutaneous (n, %); SR | 2 (20); 2/2 | 2 (29); 2/2 | |
| Surgical revision (n, %); SR | 4 (40); 0/4 | 1 (14); 0/1 | |
| Primary rLT (n, %); SR | 1 (10); 0/1 | 1 (14); 0/1 |
rLT, retransplantation; SR, success rate.
In the univariate analysis, portal vein diameter ≤ 3mm and prolonged ischemic time were significant risk factors for vascular complications (p < 0.05). A prior history of abdominal surgery also had significance as a risk factor (p < 0.20, HR 4.88). The results of univariate analysis are shown in Table 1.
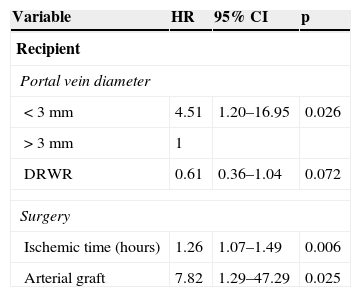
Stratification by graft type demonstrated that increased DRWR was a protection factor against vascular complications in patients receiving reduced grafts. After adjusting for confounders with Cox multivariate analysis, portal diameter ≤ 3mm (p=0.026; HR 4.51; Fig. 1B), DRWR (p=0.072; HR 0.61), prolonged ischemic time (p=0.06; HR 1.26), and the use of arterial grafts (p=0.025; HR 7.82) remained as highly significant risk factors for vascular complications (Table 3).
Factors associated with vascular complications in patients undergoing orthotopic liver transplantation. Cox multivariate regression analysis.
| Variable | HR | 95% CI | p |
|---|---|---|---|
| Recipient | |||
| Portal vein diameter | |||
| < 3 mm | 4.51 | 1.20–16.95 | 0.026 |
| > 3 mm | 1 | ||
| DRWR | 0.61 | 0.36–1.04 | 0.072 |
| Surgery | |||
| Ischemic time (hours) | 1.26 | 1.07–1.49 | 0.006 |
| Arterial graft | 7.82 | 1.29–47.29 | 0.025 |
CI, confidence interval; DRWR, donor to recipient weight ratio; HR, adjusted hazard ratio; p=p-value.
The survival of pediatric liver transplant recipients who developed vascular complications was significantly lower (Fig. 1B).
DiscussionLiver transplantation in the pediatric setting is technically challenging due to the reduced size of the vasculature and biliary tree. Discrepancies in portal vein and hepatic arterial diameter between the donor and recipient are expected.1 The incidence of vascular complications reported in the literature varies widely among centers, but is always higher than in adult samples.6,7
Arterial complications are most frequent, occurring after 3% to 9% of all transplants;8,9 the present study corroborates this finding. Early HAT is the most common arterial complication. In a systematic review, Bekker et al. reported the incidence of early HAT in pediatric patients as 8.3%, versus 2.9% in adult transplant recipients (p < 0.001).10 Duffy et al. reported HAT rates of 8% versus 3.9% in pediatric and adult patients, respectively, in a sample of 4,234 transplants performed on 3,558 patients at the University of California, Los Angeles, in the United States.7 Uchida et al. reported an incidence of HAT of 6.7% after LDLT in the pediatric population, with a ten‐year survival of 78%.3 The use of microvascular techniques in arterial reconstruction has diminished this complication, especially in children.
Patients with HAT are at a higher risk of allograft loss (53%), morbidity, and mortality (33%).10,12,13 All events are more severe in early complications.6,9,14 The fact that only one of the patients with HAT survived may be associated with late diagnosis and the lack of available grafts in time for rLT.
Venous complications are less frequent than arterial complications, and the most common is PVT.11 These findings were not different in the present patients. Unlike arterial complications, venous events occur later. Ueda et al. reported a 9% rate of portal vein complications in a review of 521 pediatric patients who underwent LDLT. Of the 47 patients in the portal event group, 38 were diagnosed with a complication 3 months after transplant.15 Moon et al. reported an 11.2% incidence of portal complications in another sample of patients undergoing LDLT (n=96). Once again, most complications occurred 3 months after transplantation.16 Kawano et al. also reported an incidence of 9% of late portal vein stenosis following LDLT; all patients were treated by interventional radiology.17 Still regarding living donation, another study reported an incidence of 15% of portal complications in the pediatric group, associating with a discrepancy in portal vein diameter.11,18
Portal vein hypoplasia is one of the main risk factors for vascular complications after pediatric liver transplantation, particularly in children with biliary atresia.12 Suzuki et al. found a portal vein diameter of less than 3.5mm to be the single most sensitive and specific predictor of portal stenosis.4 In a study of 71 pediatric transplant recipients, Broniszczak et al. reported a 16.9% rate of vascular thrombosis, with PVT occurring only in patients with portal hypoplasia.19 In the present study, four of 19 patients with vascular complications (21%) had portal hypoplasia. Biliary atresia was the primary liver disease in all cases. Unlike the study by Broniszczak, only one of the present patients with portal hypoplasia developed PVT after transplantation. Nevertheless, an intraoperative finding of portal diameter ≤ 3mm was a statistically significant predictor of vascular complications in the postoperative period (p=0.026), although the number of patients limited the accuracy of the present findings, which should be interpreted with caution. This findings need to be confirmed in other studies. Venous grafts were not used in these cases because portal flow was present and considered adequate after Fogarty balloon portal vein dilation.
In their study of HAT, Stewart et al. found an ischemic time of 12hours or more to be a significant risk factor for vascular complications (p < 0.001).20 In the present sample, four out of six patients with prolonged ischemic time developed HAT. Lower body weight and higher graft to recipient weight ratio (GRWR) were also inflicted as risk factors for HAT in the pediatric population.3 GRWR could not be assessed because graft weight wasn’t available in most cases. This data has only been routinely collected in the last 5 years. Lack of appropriate data is a limitation of retrospective studies.
In order to try to evaluate the impact of graft size on vascular complications, DRWR was assessed. Oh et al. also assessed DRWR, and noted that patients who receive allografts from small donors have significantly higher HAT rates (p=0.002).5 In the present study, a high DRWR was a protective factor after stratification by graft type (whole vs. partial). In patients with reduced‐size grafts, the higher the DRWR, the lower the potential for vascular complications. This phenomenon is believed to be due to the larger vessel diameter.
The use of arterial grafts for vascular reconstruction has also been debated as a risk factor. In the present study, multivariate analysis demonstrated that patients requiring arterial grafts had higher rates of vascular complications (p=0.025). A meta‐analysis conducted by Bekker et al. found four studies assessing the use of arterial grafts as a risk factor for vascular complications. In three of these studies, two of which employed multivariate analysis, arterial grafts were indeed found to be a risk factor.5,10,21 A recent publication by Backes et al. reported their experience with arterial grafts in 58 recipients, 38 during primary liver transplantation and 20 rLT. The incidence of early HAT was 6.8% in primary liver transplantation recipients, and none in the rLT. Iliac artery graft with infrarenal aortic anastomosis was the technique of choice; however, the study wasn’t fit for the evaluation of risk factors.22
At least three modalities are available for the treatment of vascular complications: revascularization, rLT, or clinical management. The choice depends on the timing of diagnosis. rLT provides the best outcomes and is the treatment of choice in most groups; however, it is severely limited by the scarcity of donors.10,23 In early vascular complications, attempts at emergency revascularization through percutaneous intervention (angioplasty) or surgical re‐exploration is the first step in management, particularly in HAT.24,25 In the event of irreversible cell damage, rOLT is the only option.26 Revascularization success rates are approximately 50%.10 Thrombectomy is not indicated in late‐onset HAT, which is usually complicated by ischemia and biliary tract injury, and is thus best treated by rLT.27 Patients with late‐onset PVT and portal hypertension but no liver function compromise may benefit from splenorenal shunting. If intra‐hepatic portal veins are permeable, a meso‐Rex shunt may be performed rLT is mandatory in early‐onset PVT with graft dysfunction.28
In the present study, high mortality rates were observed in patients undergoing revision of anastomosis with thrombectomy; patients managed with percutaneous intervention (angioplasty) had better outcomes. Few patients underwent rLT, but several died while awaiting the procedure, as reported elsewhere.29 In the present sample, the time to rLT limited the results, since the scarcity of donors delayed the rescue procedure. Outcomes are encouraging in asymptomatic patients treated with early revascularization after incidental diagnosis of vascular complications on DUS.24,25 Graft survival rates after revascularization are substantially higher in this group than in symptomatic patients (81.8% vs. 40%).24
ConclusionDespite technical progress in pediatric liver transplantation, vascular complications are still a significant determinant of allograft loss, increasing postoperative morbidity and mortality.
Arterial complications are more common, occur early in the postoperative period, and are associated with high rates of graft loss and patient mortality. Conversely, venous complications are less frequent, occur late in the postoperative period, and have no significant effect on graft loss or mortality rates.
Strategies for identification and mitigation of risk factors, prevention of technical complications, and protocols for early detection of vascular complications may reduce the need for rLT, thus producing a long‐term positive effect on treatment of patients with end‐stage liver disease. Development of these strategies is a challenge yet to be overcome.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Orlandini M, Feier FH, Jaeger B, Kieling C, Vieira SG, Zanotelli ML. Frequency of and factors associated with vascular complications after pediatric liver transplantation. J Pediatr (Rio J). 2014;90:169–75.
Study conducted at the Pediatric Liver Transplantation Group, Hospital de Clínicas de Porto Alegre, Brazil.