Primary objectives were to analyze the prevalence of obstructive sleep apnea in (1) boys and girls, and (2) severe asthma versus moderate and mild cases. The authors hypothesized that girls and severe asthma would have a higher prevalence of obstructive sleep apnea.
MethodsCross-sectional evaluation of asthmatic children attending a tertiary Pediatric Pulmonology clinic. The authors performed a history, physical examination, pulmonary function test, and home sleep apnea test.
ResultsThe authors studied 80 consecutive patients, 7–18 years old, mean age of 11.6 years (standard deviation 2.7), 51.3% female, and 18.5% obese. Pulmonary function tests were obtained from 80 volunteers, 45% with obstruction pattern. Home sleep apnea tests were available from 76 volunteers, with a mean obstructive respiratory index of 1.8 events/h. Obstructive sleep apnea was found in 49 volunteers (61.2%). The authors did not find associations between obstructive sleep apnea and sex or asthma severity.
ConclusionsObstructive sleep apnea was frequent among these asthmatic children. Sex and asthma severity were not risk factors. Considering the interrelationship of both diseases, it is worth keeping in mind the possibility of obstructive sleep apnea among children and teenagers with asthma.
Asthma and obstructive sleep apnea (OSA) are inflammatory diseases of the respiratory tract that can affect all age groups.1,2 Asthma prevalence is high in Brazil, affecting around 20% of the population.3
OSA affects 1–4% of the pediatric population according to the 3rd International Classification of Sleep Disorders.4 Asthma and OSA share many risk factors such as allergic rhinitis (AR), upper airway and systemic inflammation, gastroesophageal reflux disease (GERD), and obesity.5 Moreover, both are influenced by circadian rhythm, age, and sex.6 There is a paucity of studies regarding asthma and OSA in children, but there is evidence that OSA increases the length of stay in hospitalizations due to asthma.7
Age and sex have a significant role in asthma and OSA presentation. Asthma is more prevalent in adult females than males, but during childhood, the association is more complex: until 6 years old, boys are more affected than girls.8 This is explained by the smaller airway and dysanaptic growth of lung parenchyma (boys develop the parenchyma slower than girls).9 No difference in asthma prevalence is found between 7 and 9 years – airway and parenchyma are equal in boys and girls and hormonal changes have not yet begun. Afterward, girls are more affected – the airway turns larger in boys and sexual hormones modify inflammation since estrogen and progesterone have inflammatory effects according to different receptors and testosterone has anti-inflammatory effects.8 During childhood, the prevalence of OSA in children without comorbidity is equal in boys and girls, but during adulthood, men are more affected and this sex-related difference in OSA prevalence begins during puberty.4 However, a questionnaire-based study found OSA more prevalent among asthmatic girls than boys, though this finding was a post-hoc analysis.10
Even though the American Academy of Sleep Medicine recommends the polysomnographic evaluation for the diagnosis of OSA in children be performed at the sleep center (type I), some authors have found it feasible to use home polygraphy type III (known as home sleep apnea testing – HSAT, monitoring flow, respiratory effort, and oximetry) as an optional tool.11
The objectives of this study are: (1) analyze the prevalence of obstructive sleep apnea in: (1) boys and girls, and (2) severe asthma versus moderate and mild cases. Secondary objectives are: (1) analyze the effect of the pubertal stage on OSA.
Considering that the asthma presentation is associated with age, sex, and OSA, and questionnaire-based studies described that OSA was more prevalent in girls than boys,10 and with asthma severity,6 the authors hypothesized that among children with asthma, OSA measured by an objective method would be more prevalent in girls than boys, and among those with severe asthma comparing to mild to moderate cases.
Materials and methodsThis cross-sectional study enrolled 80 consecutive patients, recruited from a tertiary Pediatric Pulmonology clinic between December 2016 and July 2018. The sample size was calculated considering OSA more frequent in girls (OR, 2.55)10 and in severe asthma (16 OSA in 29 severe asthmatic patients vs 16 OSA in 79 mild/moderate asthma)6 both considering alfa 0.05 and beta 0.80. The authors calculated 68 children, and expecting 20% of follow-up losses, the authors decided to recruit 80 volunteers.
Inclusion criteria were 7–18 years old and medical diagnosis of asthma according to the Brazilian Consensus:12 FEV1 <80% predicted or ≥12% variation post-beta-agonist, or 3 or more: (1) more than one episode of wheezing per month, (2) cough or wheezing, by morning or at night, after laughter, crying or exercise, (3) cough without viral infections, (4) allergic rhinitis or conjunctivitis, (5) family history of atopy or asthma, (6) improvement of symptoms with bronchodilator or inhaled corticosteroids. The authors had children already under treatment in the clinic and some that were new patients.
Exclusion criteria were craniofacial and/or thoracic malformations, genetic syndromes, bronchopulmonary dysplasia, bronchiolitis obliterans, neuromuscular diseases, sickle cell disease, cystic fibrosis, or those unable to understand or perform protocol's tests. Parents’ informed consent and patients’ assent were required before enrolling in the study. The project was approved by the Ethics Committee of Universidade Federal de São Paulo (#1.794.705).
Interview, physical examination, pulmonary function tests (PFT), and HSAT were performed. The authors applied unstandardized (asthma history) and standardized questionnaires (Brazilian criteria for socioeconomic classification).13 The authors also collected data about current asthma medication and asthma exacerbations in the previous 12 months.
Asthma symptoms were defined as not controlled in the long term if there were systemic corticosteroid use, emergency room visit, or hospital admission due to asthma exacerbation in the previous 12 months. Short-term control was based on the Global Initiative for Asthma (GINA) questionnaire that evaluate the control in the past 4 weeks,14 and inquiries about daytime and nighttime symptoms, use of short-acting beta-agonist, and limitations due to asthma.
All volunteers self-evaluated their pubertal stage using Tanner figures for boys and girls.15 Those with any pubertal development 2 or more were classified as pubescent. Body-mass index was calculated using CDC definition and classified as z-score. Rhinitis was defined as intermittent or persistent (mild, moderate/severe) according to ARIA definition.16
PFT was acquired on Koko® (Longmont, USA), with Polgar reference.17 All exams were performed by the same medical doctor (CFS), during the afternoon, with 1 h maximum intervals. Per protocol, every volunteer should perform the test before and after 20 min taking 400 mcg of salbutamol through a metered-dose inhaler and a spacer. Volunteers with asthma exacerbations were rescheduled for two weeks later.
HSAT was performed using a three-channel cardiorespiratory monitor (Philips Stardust®), regardless of sleep symptoms, on the entire sample. Even though the gold standard for sleep studies in children is PSG type I this is not easily accessible in every part of the studied country. Another major concern is its costs. Since there are some studies showing that PSG type III is feasible and accurate in children,11 we opted to use this method.
The exams were revised by two polysomnographic technicians with extensive experience with pediatric sleep studies, using AASM rules. Afterward, they were revised by a sleep physician (A.K.S.) who did not attend the volunteers. Technicians and physicians were blinded to the study hypothesis. HSAT was performed the night after the PFT, so HSAT also was rescheduled in case of asthma exacerbations.
OSA was defined as those with habitual snoring and one or more obstructive respiratory events (OREI) per hour of recording time. OREI ≥1 was defined as mild OSA, ≥5 as moderate, and ≥10 as severe OSA. The authors used the pediatric criteria in the entire sample. The authors considered acceptable studies with at least 2 h of register of each channel without artifact.18 When an acceptable study was not obtained on the first night, the authors invited the volunteer to repeat the exam. If the volunteer did not agree, the authors kept the data for those with at least 60 min of valid data.
PFT was analyzed according to American Thoracic Society criteria.19 Asthma severity was classified based on GINA criteria.14
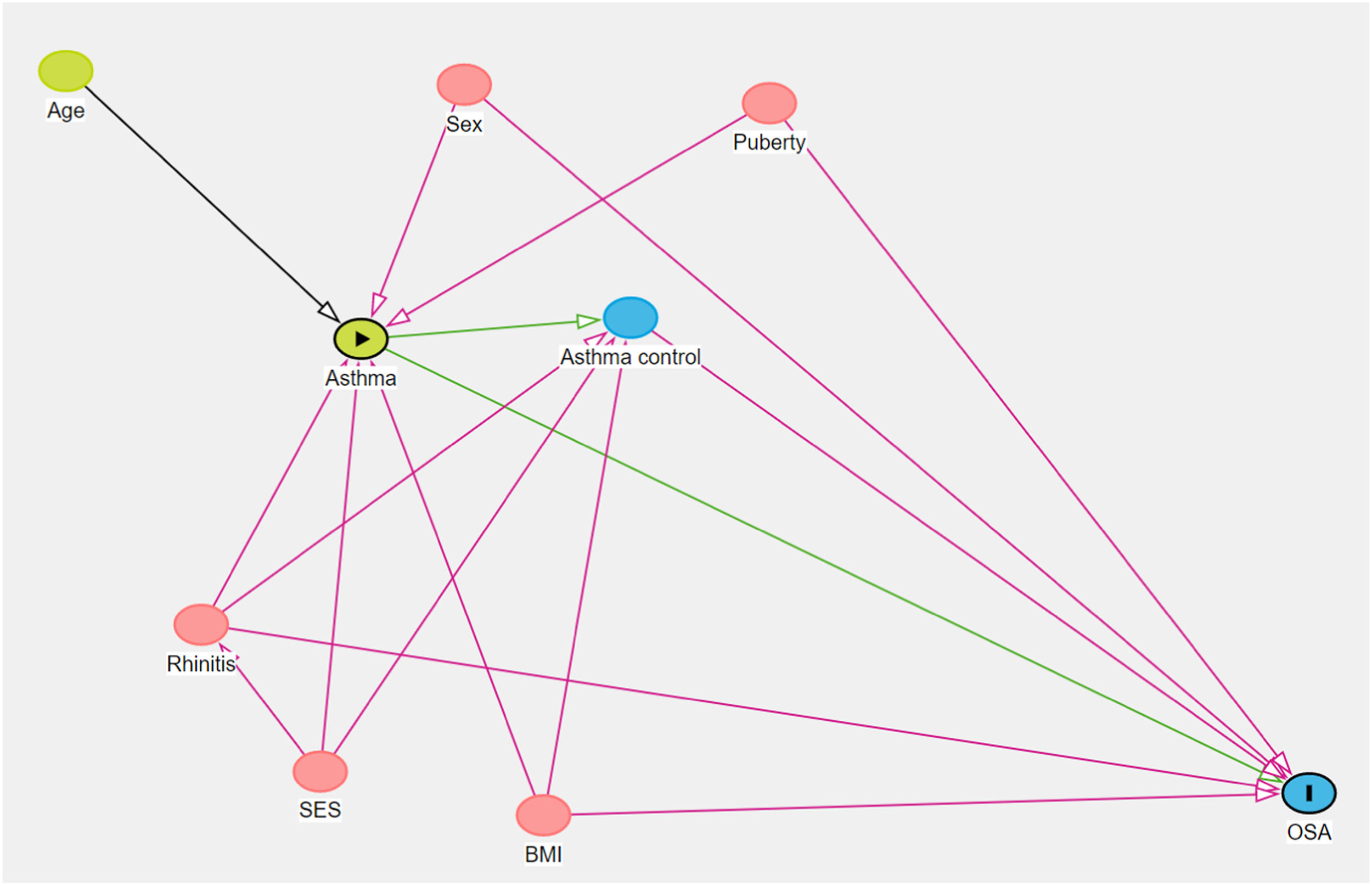
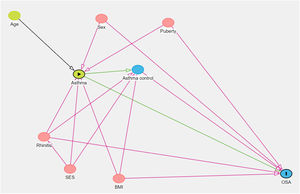
Analysis of possible confounders was made with a Directed Acyclic Graph (DAG); neither age, sex, BMI, pubertal stage, rhinitis, socioeconomic status, or asthma control were identified as cofounders (Figure 1). DAG considers the relationship among variables to determine if any of them is a confounder or not. The idea of “acyclic” is that you should not come back to the beginning of the path (exposure) following the arrows that link the variables.20 There are rules to consider if the path is open or closed when a variable is related to another. In this case, if the authors controlled for any of the above variables, we would introduce bias since the path would be opened.
Directed Acyclic Graph regarding the relationship among asthma and obstructive sleep apnea (OSA). Asthma is influenced by age, rhinitis, body mass index (BMI), socioeconomical status (SES), and puberty. Asthma influences asthma control which also receives influence from rhinitis and BMI. OSA is influenced by sex, rhinitis, BMI, puberty, asthma, and asthma control. Graphic made on Dagtty.net. Red arrows show variables that would introduce bias if controlled. ►, exposition, I, outcome.
Data were summarized as mean and standard deviation and frequencies were compared using chi-square tests. The authors considered significant p < 0.05.
ResultsTo achieve the sample size calculation, 148 volunteers were invited, 28 declined participation and 23 had exclusion criteria. Of those who accepted to participate, 17 did not attend the appointed meeting, and 80 completed the protocol.
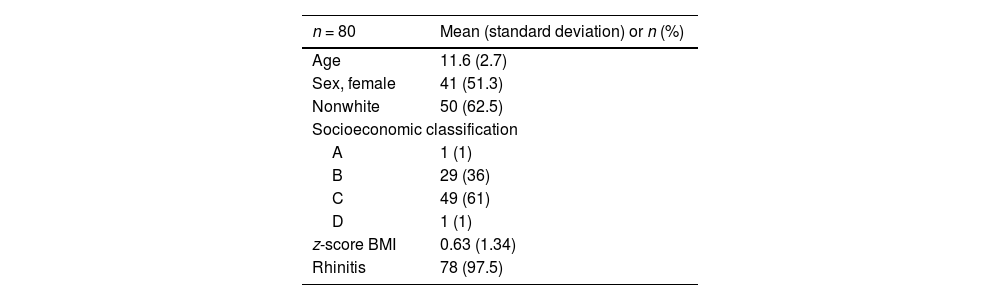
The mean age was 11.6 years, standard deviation 2.7, 41 were female (51.3%), 30 white (37.5%). Sociodemographic are summarized in Table 1. According to the body mass index z-score, nutritional status was: 2 (2.5%) underweight, 63 eutrophics (79%), and 15 obese (18.5%). Puberty was present in 17 volunteers (6 boys and 11 girls). Rhinitis was present in 78 volunteers; 12 (15%) had mild rhinitis and the remaining had moderate/severe rhinitis. Asthma classification based on GINA criteria was 3 (4%) mild, 8 (10%) moderate, and 69 (86%) severe. Asthma control in the past 4 weeks using the GINA questionnaire was found in 35 (43.7%) and in long term in 27 (33.7%).
Sociodemographic and clinical data.
Socioeconomic classification is based on Critério Brasil Classification.13 A is the higher income class. The average income per year in dollars for each class is A – $ 44,400, B – $ 6600, C – $ 6780, and D – $ 1300.
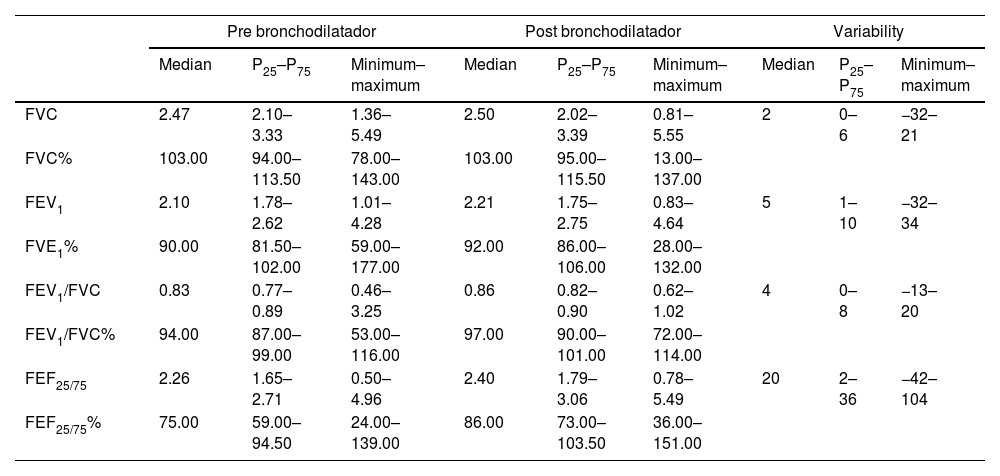
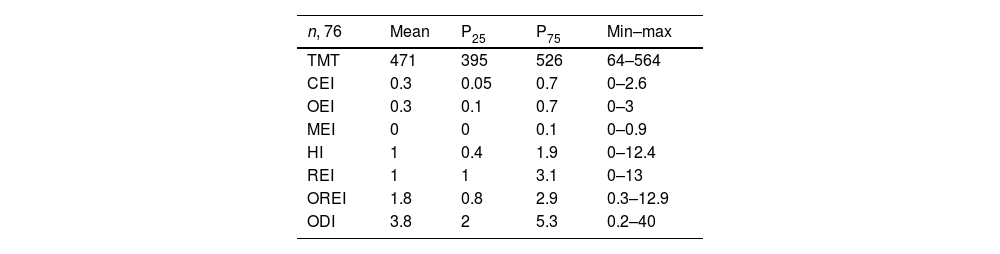
The authors obtained PFT classified as A or B according to ATS19 of 71 volunteers. Considering the 80 PFT obtained, a normal lung function pattern was found in 45 (56.2%) volunteers; 32 (40%) had an obstructive ventilatory defect (30 mild and two moderate); three had unspecific abnormalities. Data regarding the PFT are summarized in Table 2. Acceptable HSAT exams were obtained from 76 volunteers. Mean OREI was 1.8 events/h, mild OSA was found in 30 volunteers (37.5% of the sample), moderate in 16 (20%), and severe in 3 (3.8%). Habitual snoring was found in 9 volunteers. HSAT data are summarized in Table 3.
Pulmonary function tests.
FVC, forced vital capacity (in l); FEV1, forced expiratory volume in 1 second (in l); FEF25/75, forced expiratory flow between 25 and 75% of expiratory curve (in l/s); %, percentage of the predicted.
Home sleep apnea test data.
| n, 76 | Mean | P25 | P75 | Min–max |
|---|---|---|---|---|
| TMT | 471 | 395 | 526 | 64–564 |
| CEI | 0.3 | 0.05 | 0.7 | 0–2.6 |
| OEI | 0.3 | 0.1 | 0.7 | 0–3 |
| MEI | 0 | 0 | 0.1 | 0–0.9 |
| HI | 1 | 0.4 | 1.9 | 0–12.4 |
| REI | 1 | 1 | 3.1 | 0–13 |
| OREI | 1.8 | 0.8 | 2.9 | 0.3–12.9 |
| ODI | 3.8 | 2 | 5.3 | 0.2–40 |
TMT, total monitoring time (in minutes); CEI, central event index; OEI, obstructive event index; MEI, mixed event index; HI, hypopnea index; REI, respiratory event index; OREI, obstructive respiratory index; ODI, oxygen desaturation index.
With exception of TMT, which is expressed in minutes, all other variables are expressed as events per hour.
Among the volunteers with mild/moderate asthma, 7/10 had OSA (5 mild and 2 moderate) while among those with severe asthma 42/66 had OSA (25 mild, 14 moderate, and 3 severe). The authors did not find an association between OSA and sex (chi-square, 2.578, p, 0.108), asthma severity (Fisher's test, 0.091, p, 0.763), asthma control (short term: chi-square, 0.94, p, 0.759, long term: chi-square, 0.638, p, 0.424), or puberty status (chi-square test, 3.036, p, 0.081). The authors have found OSA in 75% of boys and 57.5% of girls (61.2% of the entire sample).
Severe OSA was found in three volunteers: they were all pubescent, with perennial rhinitis, severe asthma, not controlled in the short and long term, had nasal turbinate hypertrophy, tonsils III/IV, and HSAT with oxygen saturation nadir below 90%. Obesity, ogival palate, and gastroesophageal reflux were present in two of them.
DiscussionThis cross-sectional study of asthmatic children in a tertiary Pediatric Pulmonology clinic showed a high prevalence of OSA (61.2%) diagnosed by HSAT. However, neither sex, asthma severity, asthma control (short or long term), nor puberty status were predictors of OSA.
There is still a paucity of data regarding OSA among asthmatic children. A Chinese questionnaire-based study with almost 22 thousand asthmatic children, found 12% with sleep-disorder breathing.21 The authors have only found three studies in the pediatric population with both asthma and OSA objective measurements: He et al.22 studied 5–18 years-old children and found an OSA prevalence of 57%. A second study found a higher prevalence of OSA in children with asthma (6.6% vs 3.8% in non-asthmatic children).23 However, a third study did not find any difference in the prevalence of OSA among asthmatic versus non-asthmatic children.24 The different prevalences in OSA diagnosis among those studies and might be explained by different cut points to diagnose OSA, older children in the present study, and lower FEV1 values than in He22 and Sulit23 studies. Even though the ICSD-34 recommends a cut point of 1 for the obstructive apnea-hypopnea index to consider an OSA diagnosis in the pediatric population, different studies have chosen different cut points.25 Contrary to adults, most pediatric studies are based on statistical normality values and not on health outcomes.25
The authors did not find any statistically significant difference among boys and girls, puberty and pre-puberty, or asthma control (in the short and long term) among those with and without OSA. The present study's sample had a vast majority of children with severe asthma, reflecting the characteristic of the reference center. In accordance with the amount of severe asthma, the authors found 40% of PFT abnormal, which is not common in asthmatic children who tend to have a more preserved lung function than adults with severe asthma.14
The authors obtained acceptable HSAT exams in 95% of cases (81% on the first attempt). Previous studies had 81 to 100% of acceptable exams considering 1 repetition if needed.26-29 The prevalence of OSA in the present sample was higher than the prevalence in epidemiologic studies, mainly in children without asthma (62.5 vs 1%).4 The high prevalence of AR and severe asthma in our sample could explain our high OSA prevalence. However, the authors did not find associations among either AR or asthma severity in the present study's volunteers. The fact that the vast majority had moderate/severe AR and severe asthma could have reduced the variability necessary to find differences among these groups.
This study has some strengths: asthma and OSA were defined by questionnaires and objective measurements, and the authors obtained a high rate of acceptable sleep exams. However, the authors also faced some limitations such as a few volunteers with mild asthma and OSA evaluation with an alternative method instead of the gold standard type I polysomnography.
ConclusionIn summary, the authors found a high prevalence of OSA (61.2%) in a sample of mainly severe asthmatic children and adolescents. Nor sex, age, pubertal stage, asthma classification, or control were statistically significant as OSA predictors. High suspicion of the comorbidity must also be in mind as OSA since it may lead to detrimental metabolic and cognitive functions.
Grant supportAssociação Fundo de Incentivo à Pesquisa (AFIP), Methods of Epidemiological and Clinical Research (MECOR), Coordenação de Aperfeiçoamento do Ensino Superior (CAPES).
Associação Fundo de Incentivo à Pesquisa (AFIP), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Apoio à Pesquisa do Estado de São Paulo (FAPESP) and Methods of Epidemiological and Clinical Operations Research (MECOR).
The article has been registered in preprint format under the doi: 10.22541/au.165279606.69918679/v1.