The stable microbubble test on gastric aspirate and on amniotic fluid has been used for the diagnosis of respiratory distress syndrome in the newborn. However, no study has performed this test on oral aspirates from premature infants. The objective of this study was to evaluate the performance of the stable microbubble test on oral aspirates from preterm newborns to predict respiratory distress syndrome.
MethodThis study included infants with gestational age <34 weeks. Oral fluids were obtained immediately after birth and gastric fluids were collected within the first 30 minutes of life. The samples were frozen and tested within 72 hours.
ResultsThe sample was composed of paired aspirates from 64 newborns, who were divided into two groups: respiratory distress syndrome group (n=21) and control group (n=43). The median (interquartile range) of the stable microbubble count in the oral samples of infants with respiratory distress syndrome was significantly lower than that of infants who did not develop respiratory symptoms: respiratory distress syndrome group=12 (8–22) stable microbubbles/mm2; control group=100 (48–230)microbubbles/mm2 (p<0.001). The correlation between microbubble count in gastric and oral aspirates was 0.90 (95% confidence interval=0.85–0.95; p<0.001). Considering a cut-off point of 25microbubbles/mm2, the sensitivity and the specificity of the stable microbubble test were 81.4% and 85.7%, respectively.
ConclusionThe study suggests that the stable microbubble test performed on oral aspirate is a reliable alternative to that performed on gastric fluid for the prediction of respiratory distress syndrome in the newborn.
O teste das microbolhas estáveis no aspirado gástrico e no líquido amniótico foi usado no diagnóstico da síndrome do desconforto respiratório do recém-nascido. Contudo, nenhum estudo fez esse teste nos aspirados bucais de neonatos prematuros. O objetivo deste estudo foi avaliar o desempenho do teste das microbolhas estáveis em aspirados bucais de recém-nascidos prematuros para prever síndrome do desconforto respiratório.
MétodoEste estudo incluiu neonatos com idade gestacional < 34 semanas. Os fluidos orais foram obtidos imediatamente após o nascimento e os fluidos gástricos foram coletados nos primeiros 30 minutos de vida. As amostras foram congeladas e testadas em 72 horas.
ResultadosA amostra foi composta de aspirados pareados de 64 recém-nascidos, divididos em dois grupos: grupo de síndrome do desconforto respiratório (n = 21) e grupo de controle (n = 43). A mediana (intervalo interquartil) da contagem das microbolhas estáveis nas amostras de fluido oral dos neonatos com síndrome do desconforto respiratório foi significativamente menor que a dos neonatos que não desenvolveram sintomas respiratórios: grupo de síndrome do desconforto respiratório = 12 (8–22) microbolhas estáveis/mm2; grupo de controle = 100 (48–230) microbolhas/mm2 (p < 0,001). A correlação entre a contagem das microbolhas nos aspirados gástricos e bucais foi 0,90 (intervalo de confiança de 95% = 0,85–0,95; p < 0,001). Considerando um ponto de corte de 25 microbolhas/mm2, a sensibilidade e a especificidade do teste das microbolhas estáveis foram 81,4% e 85,7%, respectivamente.
ConclusãoO estudo sugere que o teste das microbolhas estáveis feito no aspirado bucal é uma opção confiável ao fluido gástrico para a predição da síndrome do desconforto respiratório do recém-nascido.
Currently, there has been a renewed interest in rapid diagnostic tests to assess the surfactant system function, such as the stable microbubble test (SMT), which may be explained by the pursuit of refining indications for early exogenous surfactant therapy.1–3 The SMT was developed by Pattle et al. in 1979 and was subsequently evaluated by Chida et al. in 1993. Since then, it has been used to analyze several organic fluids, showing excellent sensitivity and specificity for the diagnosis of respiratory distress syndrome (RDS) in amniotic fluid, gastric aspirate, and tracheal aspirate.3–9
In a study conducted prior to recommendation for routine use of continuous positive airway pressure (CPAP) in the delivery room, it has been suggested that stable microbubble (SMB) count in gastric aspirates collected in the delivery room immediately after birth was useful to predict the need for exogenous pulmonary surfactant in preterm newborns not requiring mechanical ventilation.7 Recently, Bhatia et al. showed that extremely premature infants suffering from RDS and treated with CPAP who had a high microbubble count on their gastric aspirate (>8SMB/mm2) evolved well and did not require mechanical ventilation.10
The SMT on oral aspirates obtained at birth was recently used to detect surfactant dysfunction in full-term newborns with transient tachypnea,11 but it has never been used for the diagnosis of RDS in preterm infants. Oral fluid samples are readily and easily available and can be collected without using a gastric tube, which could jeopardize initial care for premature newborns. Hence, the aim of this study was to assess the performance of the SMT on oral aspirates from preterm newborns to predict RDS.
MethodsThis study was conducted in the Department of Neonatology of Hospital São Lucas at Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Porto Alegre, Brazil, from January 2014 to September 2015. We used a convenience sample consisting of patients with a gestational age <34 weeks, whose birth occurred during periods when one of the researchers was present in the delivery room, and from whom it was possible to collect oral and gastric aspirates. Gestational age was determined by obstetric ultrasound performed before 20 weeks of gestation or, if unavailable, by last menstruation date and was subsequently confirmed by the New Ballard Score.12 Newborns with the following conditions that may compromise respiratory function were excluded from the study: congenital malformations incompatible with life, genetic syndromes, severe congenital heart defects, congenital diaphragmatic hernia, and presence of amniotic fluid with meconium. This study was approved by the Research Ethics Committee of PUCRS, decision report n. 372.742 of August 23, 2013. This committee waived the need for written consent since the study used biological material obtained from routine procedures, which was usually discarded but could still be used for research.13
Oral fluids were collected by the obstetrician during routine airway clearance immediately after birth, using a rubber suction bulb. After each oral aspiration, the collected material was immediately stored in a sterile generic collection container made of crystal polystyrene with an easy-to-open and firmly fitting lid. Any volume higher than 0.2mL was considered an adequate sample. Gastric aspirates were obtained and assessed by the neonatologist within 30min after all preterm births at <34 weeks of gestation as part of routine evaluation. Gastric aspirates that remained from initial testing and oral aspirates were frozen at −20°C for up to 96h.
Gastric and oral samples were simultaneously analyzed by the SMT within 48 and 96h after freezing, according to the method described by Pattle et al.4 with slight modifications.2,8,14 Firstly, samples were thawed at room air until visually melting and were gently homogenized. Next, nearly 40μL of fluids to be tested were suctioned into a Pasteur pipette (Brand GmbH & Co., Wertheimer, Germany) with a length of 11cm and 1mm in diameter and a manual rubber cap. With the pipette held vertically and placed with the tip almost touching the counting chamber (Neubauer Improve Bright-Line, Loptik Labor, Germany), the aliquot was quickly suctioned in and expelled out for nearly 6s (20 times) in the pipette, in order to promote aeration of samples. Immediately after that, the chamber was inverted and placed under a binocular microscope, forming a hanging drop. After 4min, the count area was examined with a 100× magnification, and the number of SMB (bubbles <15μm in diameter) per mm2 was counted. Non-spherical and black bubbles were not included in the count. The results of the count were expressed in stable microbubbles per mm2 (SMB/mm2). All tests were conducted by one of the authors (MASR), blinded to sample provenance.
Newborns were divided into two groups: (1) RDS group: infants diagnosed with RDS based on clinical criteria (expiratory grunting, tachypnea, costal and external retractions, requirement of FiO2>0.40) associated with a compatible radiological pattern, such as diffuse reticulogranular pattern and presence of air bronchograms; (2) Control group: newborns who did not develop respiratory symptoms.
The decision of using surfactant therapy and implementing ventilatory support was made exclusively by the neonatology team during newborn care, with no interference from the authors of this study. Data on maternal characteristics, perinatal variables, patient demographic characteristics, maximum FiO2, duration of oxygen supplementation, ventilatory support, use of exogenous surfactant, and outcome were recorded.
Continuous variables were expressed as absolute and relative frequencies. Continuous variables were expressed as mean and standard deviation and as median and interquartile range when found not to be normally distributed. Means were compared using Student's t test when variables were symmetrically distributed and with the Mann–Whitney test when they were asymmetrically distributed. A reference value of 15SMB/mm2 was established to determine pulmonary surfactant deficiency, based on a previous study with gastric aspirates.3 Pearson's correlation and linear regression analysis between microbubble counts in oral and gastric fluids were performed using logarithmic transformation. The performance of SMT on oral and gastric fluid samples to predict the development of RDS was determined by calculating the receiver operating characteristic (ROC) area under the curve and by assessing the sensitivity and specificity of the SMT on both samples for the diagnosis of RDS in newborns. The significance level was set at 5% (p<0.05). Statistical analysis was based on data processed and analyzed using the Excel 2010 software and IBM-SPSS Statistics for Windows, version 19.
ResultsThe initial sample comprised 72 patients, 8 of which were excluded: oral fluid was not collected from 3 newborns, and samples of 5 newborns were insufficient or inadequate for testing. Thus, 21 patients with RDS and 43 preterm infants without respiratory symptoms were included in the study. Mean birth weight was 1.465±517g; median gestational age was 31 weeks (interquartile range [IQR]=29–32 weeks); and there was an equal distribution between the sexes. In the overall sample, median count was 55SMB/mm2 (IQR=20–162SMB/mm2) in gastric aspirates and 60SMB/mm2 (IQR=16–150SMB/mm2) (p=0.54) in oral aspirates.
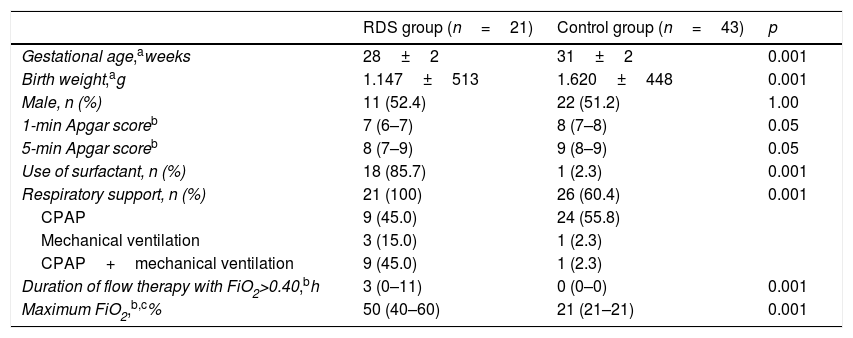
The demographic characteristics of the sample are shown in Table 1. Forty-two (65.6%) women were given two doses of antenatal corticosteroids, whereas 15 (24.5%), were given one dose. Maternal diseases included 25 (39.1%) cases of pregnancy-specific hypertensive disease, 5 (7.8%) cases of diabetes mellitus, and 2 (3.1%) cases of chorioamnionitis. A total of 49 (76.6%) children were delivered by cesarean section. Surfactant was administered to 18 (85.7%) newborns in the RDS group and to 1 (2.3%) in the control group (p<0.001), and median age of administration was 60min (IQR=50–180min). There were 5 (7.1%) cases of bronchopulmonary dysplasia and 4 (6.3%) deaths, all of which in the RDS group, 3 from extreme immaturity and 1 from Gram-negative bacillus septicemia. All newborns in the RDS group required respiratory support with CPAP and/or mechanical ventilation after admission to the neonatal intensive unit, whereas 26 (60.4%) patients in the control group were initially treated with CPAP as routine care (p<0.001). Two patients required mechanical ventilation in the control group, one from early Gram-negative bacillus septicemia and one from symptomatic congenital syphilis with normal chest radiograph.
Data on demographic characteristics, oxygen therapy, and ventilatory support for comparison between study groups.
| RDS group (n=21) | Control group (n=43) | p | |
|---|---|---|---|
| Gestational age,aweeks | 28±2 | 31±2 | 0.001 |
| Birth weight,ag | 1.147±513 | 1.620±448 | 0.001 |
| Male, n (%) | 11 (52.4) | 22 (51.2) | 1.00 |
| 1-min Apgar scoreb | 7 (6–7) | 8 (7–8) | 0.05 |
| 5-min Apgar scoreb | 8 (7–9) | 9 (8–9) | 0.05 |
| Use of surfactant, n (%) | 18 (85.7) | 1 (2.3) | 0.001 |
| Respiratory support, n (%) | 21 (100) | 26 (60.4) | 0.001 |
| CPAP | 9 (45.0) | 24 (55.8) | |
| Mechanical ventilation | 3 (15.0) | 1 (2.3) | |
| CPAP+mechanical ventilation | 9 (45.0) | 1 (2.3) | |
| Duration of flow therapy with FiO2>0.40,bh | 3 (0–11) | 0 (0–0) | 0.001 |
| Maximum FiO2,b,c% | 50 (40–60) | 21 (21–21) | 0.001 |
H, hours; RDS, respiratory distress syndrome.
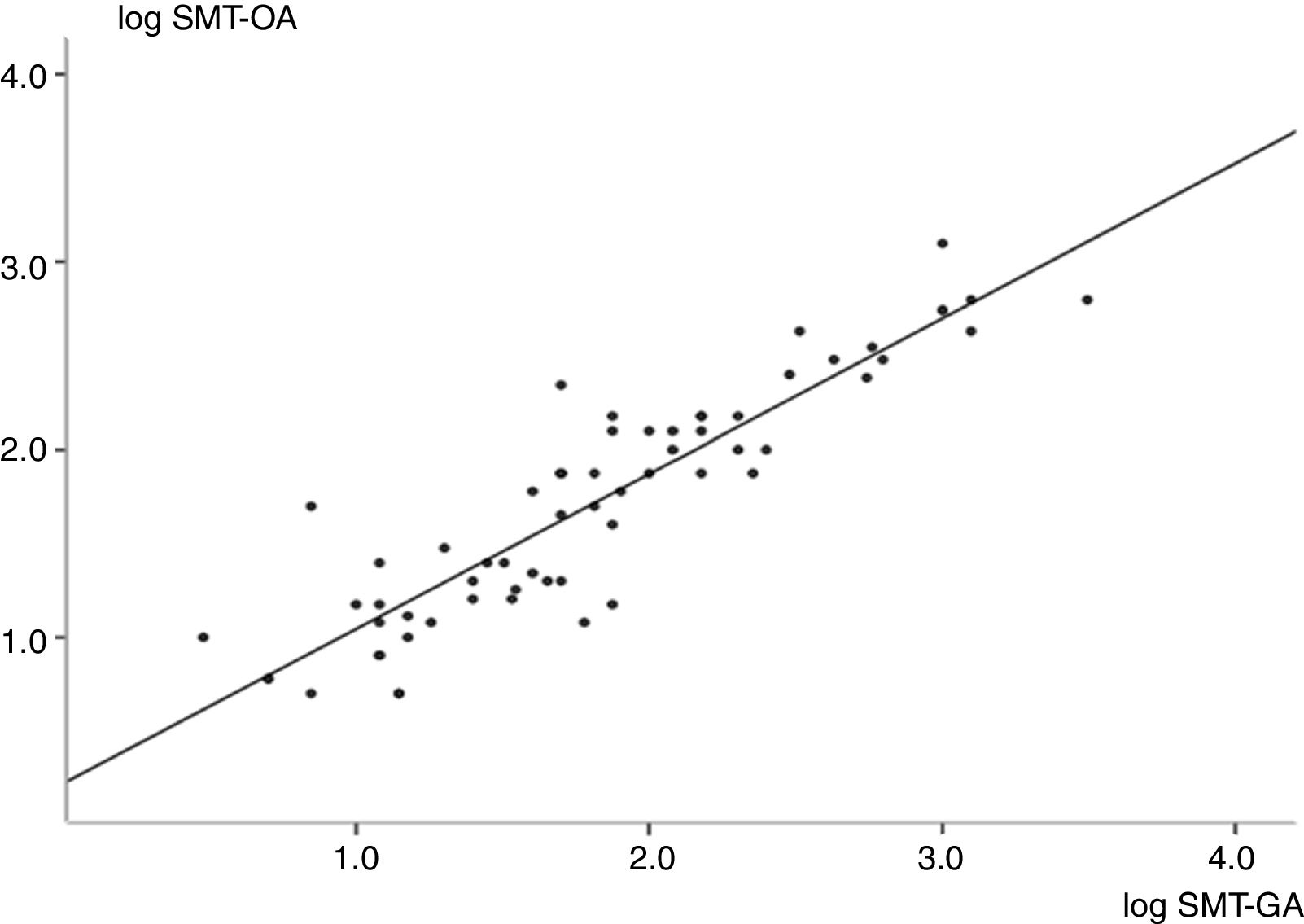
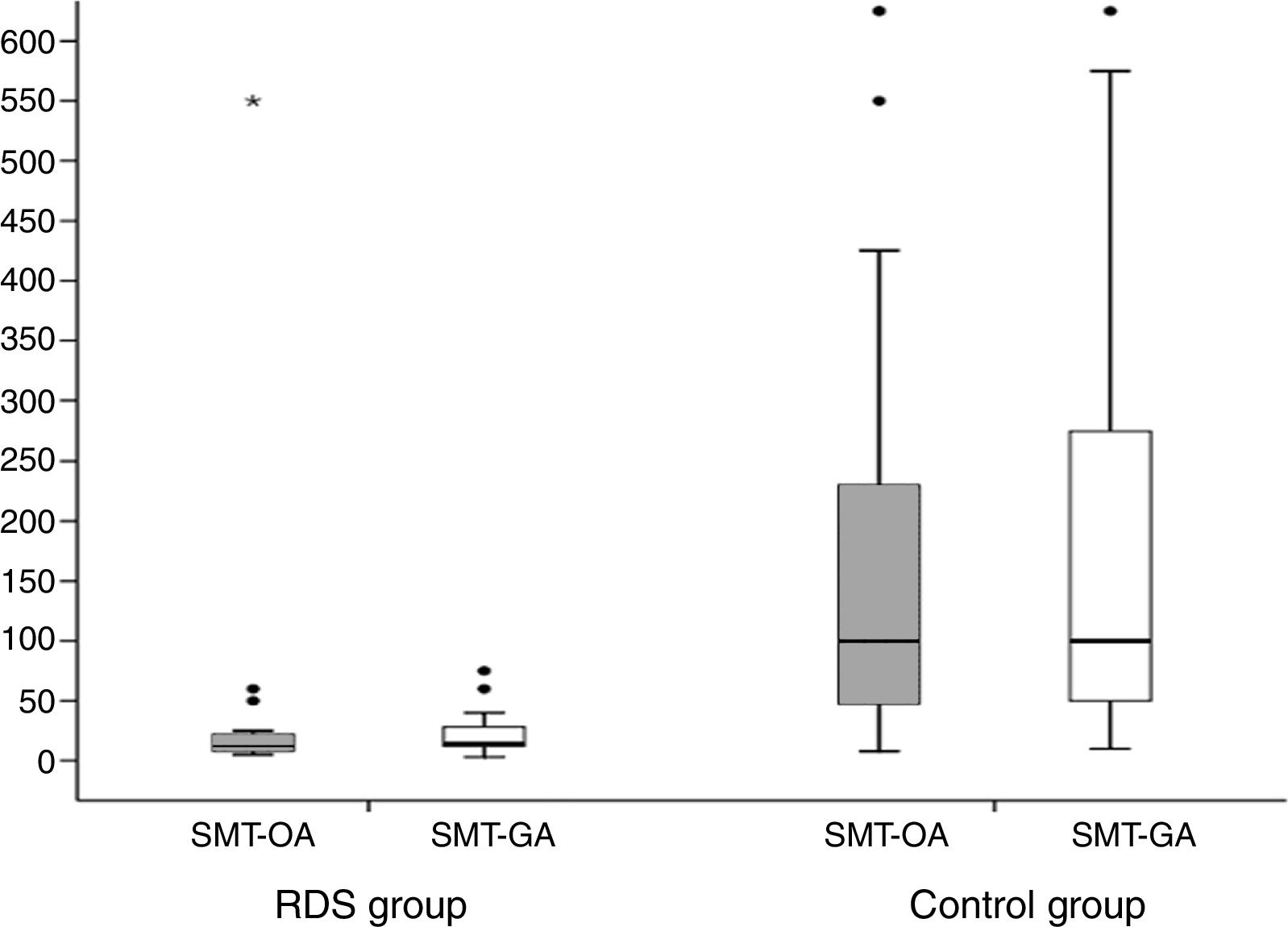
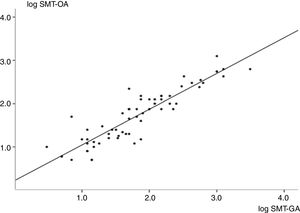
As shown in Fig. 1, a strong positive correlation was found between SMB in oral and gastric fluid samples. Fig. 2 shows the number of SMB per mm2 in oral and gastric aspirates for the two groups. Only one infant in the RDS group had a count of above 50SMB/mm2.
Linear correlation graph showing logarithmic transformation of microbubble counts in oral and gastric fluids (R=0.90; 95% confidence interval=0.85–0.95; p<0.001). Regression analysis found y=0.90x+0.22 (R2=0.81), where ‘y’ is microbubble count in oral aspirates, and ‘x’ is microbubble count in gastric aspirates. SMT-OA, stable microbubble test in oral aspirates; SMT-GA, stable microbubble test in gastric aspirates.
Box plot comparing stable microbubble (SMB) count/mm2 in oral and gastric fluids for each group. SMT-OA: RDS group: median=12SMB/mm2 (interquartile range [IQR]=8–22SMB/mm2), Control group: median=100SMB/mm2 (IQR=48–230SMB/mm2) (p<0.001). SMT-GA=RDS group=14SMB/mm2 (IQR=12–28SMB/mm2), Control group=100SMB/mm2 (IQR=50–275SMB/mm2) (p<0.001). RDS, respiratory distress syndrome; SMT-OA, stable microbubble test in oral aspirates; SMT-GA, stable microbubble test in gastric aspirates.
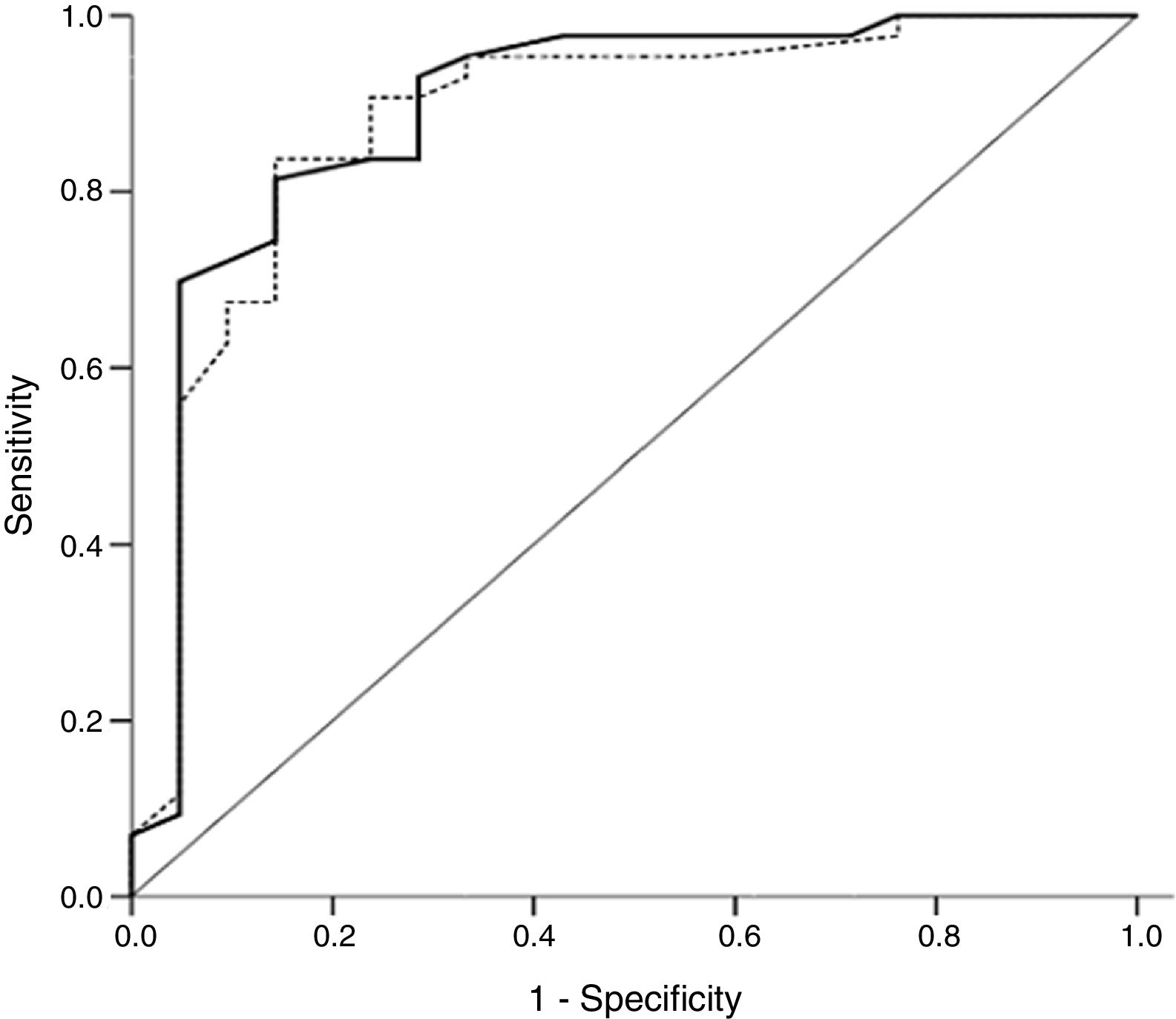
A ROC curve was built to determine the best cutoff point of SMB count to predict adequate surfactant function in our samples. The area under the curve was 0.89 (95% CI: 0.81–0.97; p<0.001) in oral aspirates and 0.88 (95% CI: 0.80–0.96; p<0.001) in gastric aspirates (Fig. 3). Considering a cutoff point at which sensitivity and specificity are similar (25SMB/mm2) in oral aspirates to predict the diagnosis of surfactant deficiency in newborns, sensitivity was 81.4% (95% CI=67.4–90.3) and specificity was 85.7% (95% CI=65.4–95.0). In gastric aspirates, a count of <15SMB/mm2, as used in previous studies, showed a sensitivity of 95.4% (95% CI=84.5–98.7%) and a specificity of 61.9% (95% CI=41.9–79.3%). In turn, a count of <25SMB/mm2 showed a sensitivity of 90.7% (95% CI=78.4–96.3%) and a specificity of 71.4% (95% CI=50.0–86.2%). The combined use of both tests did not lead to significant changes in sensitivity and specificity and would not change the decision on the number of neonates requiring exogenous surfactant therapy.
Receiver operating characteristic curve for microbubble count in oral fluid samples (full line) and gastric fluid samples (dotted line) for the diagnosis of respiratory distress syndrome. Area under the curve: SMT-OA=0.89 (95% confidence interval [CI]=0.81–0.97; p<0.001); SMT-GA=0.88 (95% CI=0.80–0.96; p<0.001). SMT-OA, stable microbubble test in oral aspirate; SMT-GA, stable microbubble test in gastric aspirate.
The present study showed that microbubble count in oral aspirates is similar to that found in gastric aspirates, and that SMT may be routinely performed on oral samples for the diagnosis of RDS. The use of SMT for the diagnosis of RDS has already been demonstrated in the literature with several types of biological materials, including gastric fluid.3,8,9 The advantages of those tests are its ease of implementation, since it can be performed at bedside, its agility to deliver results (around 5min), and its low cost.1 The SMT on gastric aspirates has been introduced in our service as part of routine care and as a method to assist in the early indication of surfactant to symptomatic, very low birth weight neonates treated with CPAP.1 Estorgato et al. recently published a study aiming to assess the performance of the SMT in oral aspirates for detecting deficiencies in surfactant function among neonates with transient tachypnea, but the use of oral fluid samples for diagnosing RDS in preterm infants has not been assessed yet.11
The diagnosis of RDS was based on clinical and radiological parameters. Occasionally, RDS may be confused with other lung diseases. However, no laboratory test has sufficient sensitivity and specificity to be used as the gold standard diagnostic test. Our study found that preterm infants who developed RDS had a significantly lower microbubble count compared to neonates without signs of respiratory distress, in both oral and gastric aspirates. These findings corroborate those observed in previous studies involving gastric and tracheal fluids.3,8,15–17
The present study also found a strong positive correlation between microbubble count in gastric and oral aspirates, indicating that oral SMT may be an alternative to evaluate pulmonary maturity in preterm newborns at birth. This finding was expected because oral fluids would be similar to gastric content at birth.
Stable microbubble count in oral aspirate was found to be very accurate for the diagnosis of RDS, with a sensitivity of 81.4% and a specificity of 85.7% for a cutoff point of 25SMB/mm2. These performance values were similar to those found in gastric fluid for a cutoff point of 15SMB/mm2 (95.4% and 61.9%, respectively) and in other studies assessing gastric aspirates (97% and 83%, respectively).3 In turn, the values found here were slightly lower than those found in tracheal aspirates (sensitivity of 89–96% and specificity of 97–100%).8,9 Thus, our findings suggest that the SMT in oral aspirates may be a useful test to diagnose RDS.
The present study has some limitations. The SMT could not be performed in 10% of oral fluid samples, due to lack of (or insufficient) material, showing one of the possible difficulties in using this test as a diagnostic tool. In these cases, the SMT could be performed in gastric aspirates.
The great advantage of the SMT in oral fluid samples is the rapid availability of material for testing, since oral aspirates are usually collected immediately after an infant's birth by the obstetrician, thus representing the easiest and more universal tool for early assessment of fetal maturity. Likewise, the collection of oral fluid samples has some advantages over that of gastric and tracheal fluids, in the sense that it does not require uncomfortable invasive procedures that may jeopardize initial care for premature infants, such as placement of a gastric tube and/or tracheal intubation.
Our study showed that the SMT may indicate the early surfactant therapy in some neonates who developed RDS, even in those treated with CPAP as routine care, because initial clinical signs of surfactant deficiency are still subtle or doubtful, and no radiological sign is specific for the diagnosis of this condition. The SMT may be used in combination with other clinical criteria for treatment decision-making. In our service, early exogenous surfactant therapy is indicated for preterm neonates treated with CPAP if they had a microbubble count <15SMB/mm2 in gastric aspirates and clinical signs of respiratory failure. Otherwise, the use of surfactants is postponed until the onset of symptoms of respiratory distress in association with requirement of FiO2 ≥0.40.
The results of the present study suggest that the SMT on oral aspirates with a cutoff point of approximately 25SMB/mm2 has a high sensitivity to diagnose surfactant dysfunction in preterm newborns, representing a reliable alternative to gastric aspirate. Additionally, this alternative method enables material to be collected in any obstetric center and contributes to early identification (i.e. within the first hour of life) of preterm infants with surfactant deficiency and risk of developing RDS, possibly improving indications for treatment with exogenous surfactant.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Ribeiro MA, Fiori HH, Luz JH, Garcia PC, Fiori RM. Rapid diagnosis of respiratory distress syndrome by oral aspirate in premature newborns. J Pediatr (Rio J). 2019;95:489–94.
Study linked to Faculdade de Medicina, Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, RS, Brazil.



![Box plot comparing stable microbubble (SMB) count/mm2 in oral and gastric fluids for each group. SMT-OA: RDS group: median=12SMB/mm2 (interquartile range [IQR]=8–22SMB/mm2), Control group: median=100SMB/mm2 (IQR=48–230SMB/mm2) (p<0.001). SMT-GA=RDS group=14SMB/mm2 (IQR=12–28SMB/mm2), Control group=100SMB/mm2 (IQR=50–275SMB/mm2) (p<0.001). RDS, respiratory distress syndrome; SMT-OA, stable microbubble test in oral aspirates; SMT-GA, stable microbubble test in gastric aspirates. Box plot comparing stable microbubble (SMB) count/mm2 in oral and gastric fluids for each group. SMT-OA: RDS group: median=12SMB/mm2 (interquartile range [IQR]=8–22SMB/mm2), Control group: median=100SMB/mm2 (IQR=48–230SMB/mm2) (p<0.001). SMT-GA=RDS group=14SMB/mm2 (IQR=12–28SMB/mm2), Control group=100SMB/mm2 (IQR=50–275SMB/mm2) (p<0.001). RDS, respiratory distress syndrome; SMT-OA, stable microbubble test in oral aspirates; SMT-GA, stable microbubble test in gastric aspirates.](https://static.elsevier.es/multimedia/00217557/0000009500000004/v2_201908280659/S0021755717310653/v2_201908280659/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w90K3EErzaXq47TPDDaeTjoE=)
![Receiver operating characteristic curve for microbubble count in oral fluid samples (full line) and gastric fluid samples (dotted line) for the diagnosis of respiratory distress syndrome. Area under the curve: SMT-OA=0.89 (95% confidence interval [CI]=0.81–0.97; p<0.001); SMT-GA=0.88 (95% CI=0.80–0.96; p<0.001). SMT-OA, stable microbubble test in oral aspirate; SMT-GA, stable microbubble test in gastric aspirate. Receiver operating characteristic curve for microbubble count in oral fluid samples (full line) and gastric fluid samples (dotted line) for the diagnosis of respiratory distress syndrome. Area under the curve: SMT-OA=0.89 (95% confidence interval [CI]=0.81–0.97; p<0.001); SMT-GA=0.88 (95% CI=0.80–0.96; p<0.001). SMT-OA, stable microbubble test in oral aspirate; SMT-GA, stable microbubble test in gastric aspirate.](https://static.elsevier.es/multimedia/00217557/0000009500000004/v2_201908280659/S0021755717310653/v2_201908280659/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w90K3EErzaXq47TPDDaeTjoE=)






