To evaluate the quality of the human milk expressed at home and at a human milk bank.
MethodsThis a retrospective, analytical, and observational study, performed by assessing titratable acidity records and the microbiological culture of 100 human milk samples expressed at home and at a human milk bank, in 2014. For the statistical analysis, generalized estimating equations (GEE) and the chi-squared test were used.
ResultsWhen comparing the two sample groups, no significant difference was found, with 98% and 94% of the samples being approved among those collected at the milk bank and at home, respectively. No main interaction effect between local and titratable acidity records (p=0.285) was observed, and there was no statistically significant difference between the expected and observed values for the association between the collection place and the microbiological culture results (p=0.307).
ConclusionsThe quality of human milk expressed at home and at the milk bank are in agreement with the recommended standards, confirming that the expression of human milk at home is as safe as expression at the human milk bank, provided that the established hygiene, conservation, storage, and transport standards are followed.
Avaliar a qualidade do leite humano ordenhado em domicílio e no Banco de Leite Humano.
MétodosEstudo retrospectivo, realizado por meio da avaliação dos registros da acidez titulável e dos resultados de cultura microbiológica de 100 amostras de leite humano ordenhado em domicílio e em um Banco de Leite Humano, no ano de 2014. Para análises estatísticas foram utilizadas as Equações de Estimação Generalizadas (Generalized Estimating Equations - GEE) e o teste Qui-quadrado.
ResultadosNa comparação dos dois grupos de amostras, não foi detectada diferença significativa, sendo que 98% e 94% das amostras foram aprovadas entre as coletadas no Banco de Leite e em domicílio, respectivamente. Não foi observado efeito principal de interação entre local e grau de acidez titulável (p=0,285) e não se constatou diferença estatisticamente significante entre os valores observados e esperados para associação entre local de coleta e o resultado da cultura microbiológica (p=0,307).
ConclusõesA qualidade do leite humano ordenhado em domicílio e no Banco de Leite Humano estão de acordo com o padrão preconizado, comprovando que a ordenha de leite humano em domicílio é tão segura quanto a ordenha no Banco de Leite Humano, desde que sejam seguidas as normas de higiene, conservação, armazenamento e transporte estabelecidas.
Situations such as prematurity, newborn hospitalization in a neonatal unit, maternal diseases, or low milk production may lead to difficulties in establishing and maintaining breastfeeding (BF).1 Therefore, the use of donated human milk (HM) has become an efficient alternative for providing nutrition2 to newborns in special conditions and a way of maintaining milk production by donor nursing mothers.3
In this sense, human milk banks (HMBs) are specialized services, responsible for actions of BF promotion, protection, and support, as well as the performance of activities of HM collection, processing, quality control, and distribution.3
The process used to define the quality of expressed human milk (EHM) is the result of adequate hygienic-sanitary conditions, from expression to administration, and the joint evaluation of several parameters, including nutritional, immunological, chemical, and microbiological characteristics, thus providing confirmation of the final product's safety.3,4
In Brazil, as well as in other countries, HM milking is allowed in the donor's home, provided that the established hygiene, conservation, storage, and transport standards are met.3,5–7 This way, greater participation of donor nursing mothers is ensured and, consequently, a greater production of EHM.
In view of the importance of home milking as a strategy for collecting EHM and the lack of studies on the safety of this type of milking, it was considered important to evaluate the quality of the EHM at home and in the HMB.
MethodsA retrospective study was carried out by analyzing the records of the processing of the EHM samples in the year 2014 evaluated by the HMB of Hospital de Clínicas de Uberlândia, Universidade Federal de Uberlândia (HCU-UFU), Minas Gerais, Brazil. This study was approved by the Ethics Committee on Research with Human Beings of the institution where it was carried out, Opinion No. 1,289,959, of October 21, 2015.
To define the sample size, the calculation was performed using the G*Power8 program (Statistical power analyses, G*Power 3.1, Germany), and the minimum total sample size was defined as 84 samples. It was decided to collect the data from 100 samples of EHM, representing 50 samples in each group (group 1: milking at home; group 2: milking at the HMB).
Simple random sampling was used for the selection of the analyzed EHM samples. Initially, the records of the nursing mothers registered in 2014 were identified and separated by place of milking, and two selections were carried out by drawing lots. In the first, fifty mothers were randomly selected in each group, and each mother had, on average, six samples of EHM; in the second, one of the EHM samples of each previously selected mother was selected by drawing lots.
Of the selected EHM samples, the results of the titratable acidity evaluation were initially collected, which identify the level of acidity expressed in degrees Dornic (°D) of raw EHM.1,3 The HM has an original acidity with values ranging between 1 and 4°D, but under conditions that favor the proliferation of microorganisms of the primary and secondary microbiota, lactic acid is produced and, consequently, there is an increase in the EHM acidity.3 Acidity ≥8°D disqualifies the EHM for consumption.1,3,9 The Dornic acidity measurement is, therefore, a way to classify and select the donated HM before its pasteurization, using a simple and inexpensive test.4
The EHM samples that attained titratable acidic value <8°D were pasteurized and submitted to the microbiological quality control, based on the classical procedure for the presence (positive) or absence (negative) of total coliforms.3 The use of this procedure is recommended because it is simple, economically feasible, and safe, minimizing the possibility of false-negative results.10 Thus, the results of the microbiological culture of the EHM samples that attained values of titratable acidity <8°D were collected. The samples that showed titratable acidity results ≥8°D were replaced by the next sample from the same nursing mother with titratable acidity <8°D, or a new drawing of lots was performed to select a new sample.
All nursing mothers selected as donors received verbal and written instructions on how to milk and store the EHM, according to the recommendations of the National Sanitary Surveillance Agency (ANVISA) and the Brazilian Network of HMB (Rede BRH-BR).1,3
The HMB HCU-UFU has a qualified team capable of conducting home visits, when the guidelines are reinforced and the hygienic-sanitary conditions of the environment are verified. The guidelines are: choose a quiet, clean, pet-free place close by; tie up the hair and put on a cap; remove rings, wristwatch, and bracelets; place a mask or cloth diaper over nose and mouth; always keep nails short and clean; sanitize hands and forearms to the elbow with running water and soap; clean the breasts only with filtered water before starting the milking; discard the first few streams of milk (0.5–1.0mL); use a glass container with sterilized plastic cap (supplied by HMB) or boiled for 15min (counting from the start of the boiling); store the bottles in a clean and closed place (cabinet or container); do not touch the inside of the bottle or cap; express the milk directly into the bottle, placing it under the areola; at the end of the milking, close the bottle and immediately freeze it in the upright position; on the next milking, in case of complementation of the previously collected volume, use another flask or glass container boiled for 15min to collect the milk and put it inside the one that is already frozen; do not fill the entire bottle, leaving the milk volume approximately 2–3cm below the edge; identify the container (full name of the nursing mother and date and time of milking); keep containers with milk in the freezer up to a maximum of ten days; store the milk separately from other foods; where no exclusive storage is available, the human milk must be placed inside another impermeable container (plastic bag or container); in case of milking using a suction pump, the latter should be sanitized after milking with plenty of running water and liquid soap, and before each milking, place the pump in hypochlorite solution (1 tablespoon) with filtered water (1l) during 60min (this solution should be changed every eight hours), and then rinse under running water; the frozen milk must be transported to the HMB inside a thermal box with ice.1,3
The HM expressed at home is collected by the HMB HCU-UFU team; it is transported using their own car and trained employees for safe and adequate transportation.1
For the statistical analysis, generalized estimating equations (GEE) were used with an unstructured working correlation matrix to compare the estimated marginal means (EMM) of two values of titratable acidity (repeated measures) of EHM from two sites (fixed factors: residence and HMB); the random factors considered were the donor and the donated sample itself; since the distribution of the response variable is different from the normal distribution, it was decided to use the model with gamma response with log binding.11 To compare the observed frequencies of microbiological culture results in both groups, the chi-squared test of independence was used with Cramer's V correction.12 For all analyses, the level of significance was set at 5%.
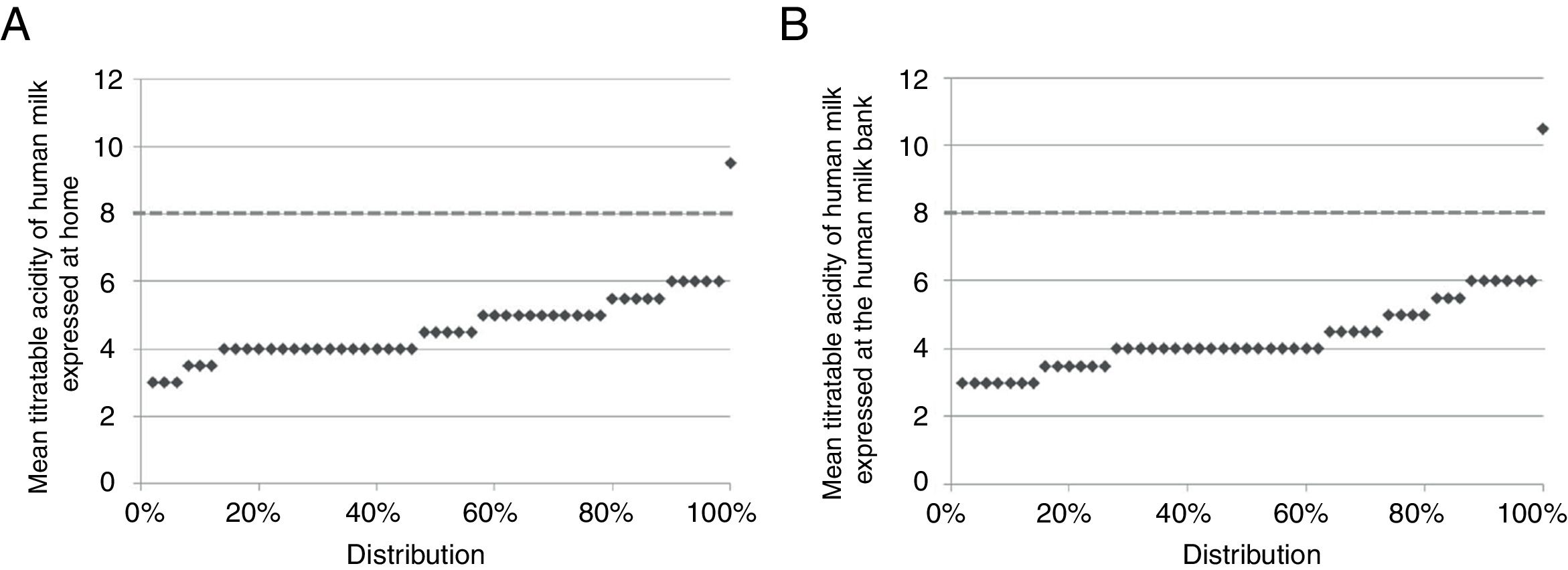
ResultsTitratable acidityThe mean values of titratable acidity of HM samples expressed at home varied between 3°D and 9.5°D, and between 3°D and 10.5°D in the samples collected in the HMB, with only 2% (n=1) in each group showing a titration grade ≥8°D, considered inadequate for consumption (Fig. 1).1,3,9
No main effect was observed for the collection site, with EMM=4.64; 95% CI=±0.29 at home and EMM=4.39; 95% CI=±0.33 at the HMB (Wald χ2=1.14, df=1, p=0.285).
Microbiological culturesOf the analyzed HM samples collected at home, 6% (n=3) had a positive microbiological culture for total coliforms; and of those analyzed samples expressed at the HMB, 2% (n=1) had positive cultures.
No difference was identified between the observed and expected values for the association between the collection site and the microbiological culture result (χ2=1.04, df=1, p=0.307, φc=0.102).
DiscussionTitratable acidity values <8°D were identified in both collection sites in 98% of the analyzed EHM samples. This value is close to that found in other studies, ranging from 88 to 99.2%4,9,13 without differentiating milking sites; 96%1,14 in samples of HM expressed exclusively at home; and 78%15 in samples collected only at the HMB. The variation found in the results suggests the importance of hygienic-sanitary control in all processing steps, regardless of the collection site.3 The entire process of home collection under the responsibility of the HMB of this study was evaluated in a previous study, which confirmed the quality control of the process.1
The microbiological culture was positive in 6% of the EHM samples at home and in 2% of the samples collected at the HMB. In other studies, the evaluation of microbiological culture indicated positive results in 5%16 and 5.6%17 of the samples of EHM in HMB; 7%18 positive in HM samples expressed at home, without using strict hygiene criteria during milking and storage; and 2%1 positive when the hygienic-sanitary criteria were established for home milking. Considering that the presence of the coliform group indicates non-compliance with the recommended hygienic-sanitary procedures, HM may be a vehicle for pathogenic microorganisms and, therefore, pasteurization and microbiological control are important to ensure safe use of EHM.3
No statistically significant differences were found in the titratable acidity and microbiological culture evaluations of the EHM between the two collection sites; therefore, there was no interference of the collection site in the quality of the EHM, which may be a result of compliance with hygienic-sanitary recommendations established for HMB.3 Thus, the importance of milking in compliance with these recommendations is emphasized as an indicator of milk quality control.
Milking at the donor's home is allowed and encouraged,3,5–7 and it is a safe and effective way to obtain HM in larger volumes, maintaining sufficient HM stocks in HMB to meet the demands.1 However, it is necessary to provide adequate guidance to the milk donors on milking and storage of milk and to ensure safe transportation.1,3,5 This study demonstrated the quality and safety of home milking when compared to collection at a HMB, according to the quality and safety criteria of the EHM, through the titratable acidity and microbiological culture evaluation.
The study suggests that home milking is safe for obtaining HM, providing that the hygienic-sanitary standards are observed. It should be emphasized that the study was carried out in a HMB accredited to the BLH-BR Network (Brazilian Network of Human Milk Banks), which encourages home milking, guarantees donor guidance, conducts systematic evaluation and home visits, and has its own car and trained personnel for the safe transport of EHM.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the nutritionist Marília and the team of the Human Milk Bank of Hospital de Clínicas da Universidade Federal de Uberlândia for their support of this study.
Please cite this article as: Borges MS, Oliveira AM, Hattori WT, Abdallah VO. Quality of human milk expressed in a human milk bank and at home. J Pediatr (Rio J). 2018;94:399–403.
Study carried out at Universidade Federal de Uberlândia (UFU), Faculdade de Medicina, Programa de Pós-graduação em Ciências da Saúde, Uberlândia, MG, Brazil.