To assess the impact of an intervention for teenage mothers with the involvement of maternal grandmothers on the prevalence of pacifier use in the first six months of life.
MethodsThis randomized clinical trial involved 323 teenage mothers, allocated to four groups: intervention with teenagers only, intervention with teenagers and their mothers, and respective controls. Six breastfeeding counseling sessions, including the recommendation to avoid the use of a pacifier, were delivered at the maternity ward and subsequently at the teenagers’ homes, at seven, 15, 30, 60, and 120 days postpartum. Data on infant feeding and pacifier use were collected monthly by interviewers blinded to group allocation. The impact of the intervention was measured by comparing survival curves for pacifier use in the first six months of life and mean time to pacifier introduction.
ResultsThe intervention had a significant impact on reducing pacifier use only in the group in which grandmothers were involved. In this group, the intervention delayed by 64 days the introduction of a pacifier (21–85 days), compared to 25 days in the group without the participation of grandmothers (65–90 days).
ConclusionsThe intervention reduced pacifier use in the first six months of life and delayed its introduction until beyond the first month when grandmothers were involved. The intervention did not have a significant impact when only teenage mothers were involved.
Avaliar o impacto de uma intervenção para mães adolescentes com a participação de avós maternas na prevalência de uso de chupeta nos primeiros 6 meses de vida.
MétodosEste ensaio clínico randomizado envolveu 323 mães adolescentes, alocadas para quatro grupos: intervenção com somente adolescentes, intervenção com adolescentes e suas mães e respectivos controles. Seis sessões de aconselhamento para amamentação, incluindo a recomendação de evitar o uso de chupeta, foram realizadas na maternidade e posteriormente nas casas das adolescentes ao 7°, 15°, 30°, 60° e 120° dias. Os dados sobre alimentação infantil e uso de chupeta foram coletados mensalmente por entrevistadores cegos a respeito da alocação dos grupos. O impacto da intervenção foi medido comparando as curvas de sobrevida para uso de chupeta nos primeiros 6 meses de vida eotempo médio de introdução de chupetas.
ResultadosA intervenção apresentou um impacto significativo sobre a redução do uso de chupeta somente no grupo em que as mães estiveram envolvidas. Nesse grupo, a intervenção mostrou atraso de 64 dias na introdução de chupeta (21 a 85 dias), em comparação a 25 dias no grupo sem a participação das avós (65 a 90 dias).
ConclusõesA intervenção reduziu o uso de chupeta nos primeiros 6 meses de vida e atrasou sua introdução além do primeiro mês com a participação das avós. A intervenção não teve impacto significativo somente com o envolvimento das mães adolescentes.
The use of pacifiers in infants has been associated with different adverse effects, e.g., possible reduction in breastfeeding duration1,2 and increased risk of developing otitis media,3,4 gastroenteritis,5 masticatory dysfunction,6 and distoclusion.7 Nevertheless, the use of pacifiers is a well consolidated habit in Brazil. A nation-wide survey8 conducted in 2008 reported that 43% of children younger than 1 year used pacifiers, with major regional differences: for instance, the prevalence found in the South (54%) was double that of the North (26%). Among the determinant factors of pacifier use in infants, studies have pointed to young maternal age9,10 and advice from grandmothers recommending its use.11
A recent systematic review found positive association between pacifier use and shorter duration of exclusive breastfeeding (EBF) in observational studies, although there are still gaps in the understanding the mechanisms involved in this association.12 While some researchers have suggested that pacifier could interfere with the breastfeeding technique, others have pointed out that pacifier use is a marker of breastfeeding difficulties.13,14
Data from two Brazilian surveys confirmed the pacifier use as strongly associated with the interruption of EBF and that the reduction of this oral habit contributed significantly to the increase in the rates of EBF between 1999 and 2008. While EBF prevalence among infants from all state capitals increased from 25.1% to 40.3%, pacifier use prevalence decreased from 58.5% to 41.6% in this nine-year period.14
Few experimental studies confirm that well designed intervention can be effective at reducing the pacifier use.13 However, none of these studies were performed with teenage mothers and/or maternal grandmothers. Having this in mind, the present study included the recommendation not to offer pacifier to children in a pro-breastfeeding intervention aimed at teenage mothers and maternal grandmothers, while cohabiting.15–17 The present study describes the effects of this intervention on the prevalence of pacifier use in the first six months of life.
MethodsDesignA randomized clinical trial was conducted involving teenage mothers, their children, and the infants’ maternal grandmothers, while cohabiting.
Reporting of this trial follows the CONSORT (Consolidated Standards of Reporting Trials) reporting guideline.18 The study was approved by the Research Ethics Committee of Hospital de Clínicas de Porto Alegre, Brazil. The trial was registered at ClinicalTrials.gov under the number NCT00910377 and title “Adolescent mothers, grandmothers, breastfeeding, and complementary feeding.”
SettingParticipants were recruited at the rooming-in maternity ward of Hospital de Clínicas de Porto Alegre, a general university hospital, which mostly receives patients from a low socioeconomic level. As a Baby Friendly Hospital, no pacifiers or artificial nipples are offered to the newborns, and all mothers are oriented not to use them after leaving the hospital.
SampleTeenage mothers aged <20 years, residing in the city of Porto Alegre, Brazil, who had given birth to healthy infants weighing ≥2500g and had initiated breastfeeding were considered eligible for the study. Mothers of twins and those who could not stay in the rooming-in setting due to health problems of the mother or child were not included in the study; neither were mothers living with their mothers-in-law (paternal grandmothers).
Eligible mothers were identified and then divided into two groups, namely, teenagers who cohabited, and those who did not cohabit, with their mothers. Subsequently, using block random allocation in groups of two (block size=2), mothers were allocated to either the intervention or the control group. Two spheres of similar texture and size, one bearing the word “Yes” (assignment to intervention group) and the other bearing the word “No” (assignment to control group) were drawn from a dark bag; participants were allocated to the study groups accordingly.
This resulted in a total of four groups: intervention with teenagers not cohabiting with their mothers, intervention with teenagers cohabiting with their mothers, and the respective controls (no intervention). In the group of mothers who did not cohabit with the infants’ maternal grandmothers, only the teenagers received the intervention. In the group were mother and maternal grandmother cohabited, both were exposed to the intervention.
The clinical trial was planned to evaluate rates of EBF and breastfeeding in the first year of life, but for the present article the outcome was the prevalence of pacifier use. The sample size was calculated to detect a difference of 25 percentage points in the prevalence of breastfeeding between the groups exposed and unexposed to the intervention, prevalence of the EBF in the first month in the control group 56%,19 with alpha error set at 5% and beta error at 20%. To compensate for possible losses and to enable multivariate analysis, the number of subjects that was actually included in each group was 50% greater than the minimum, totaling approximately 72 participants in each group.
MeasurementThe main goal of the intervention was to increase breastfeeding duration. A total of six counseling sessions on breastfeeding and infant feeding according to the principles of the World Health Organization20 were delivered to the mothers included in the two experimental groups. In addition to discussing several aspects related to breastfeeding, the mothers were advised not to offer pacifiers to their children. The arguments used to justify this recommendation included the association between pacifier use and early weaning,1,2 a higher incidence of diseases such as otitis3,4 and diarrhea,5 and an increased risk for orofacial disorders.6,7 Flip charts were used as support material to illustrate the contents covered by the intervention.
The first session was held at the maternity ward, individually, at different times for mothers and grandmothers. At the end of the first session, illustrated booklets especially designed for this project and covering several of the topics addressed were given to both mothers and grandmothers. The five subsequent sessions were held at the mothers’ homes at seven, 15, 30, 60, and 120 days postpartum. All the six sessions, which lasted about one hour each, were conducted by the same professional. The intervention team comprised a pediatrician, two nurses, and a nutritionist, all with extensive expertise in breastfeeding, three of whom were International Board Certified Lactation Consultants. The subjects were divided equally among the four professionals, who underwent a training to standardize the intervention. Subsequently, uniformity was tested in a pilot study. In addition, at the time of analysis, no systematic differences were found among the professionals that conducted the intervention.
Data collectionData were collected at different time points. At the maternity ward, once the teenage mothers and the maternal grandmothers agreed to participate in the study and signed an informed consent form, they were interviewed individually for the collection of sociodemographic data and pregnancy/delivery characteristics. Data on infant feeding and the use of a pacifier during the first six months of life were obtained monthly via telephone contact or home visits, whenever telephone contact failed – which occurred in less than 10% of the interviews. The interviewers were blind to group allocation.
Data analysisAll analyses were performed using the Statistical Package for the Social Sciences (IBM SPSS Statistcs for Windows, version 22.0, NY, USA), version 23.0, according to the intention-to-treat principle. First, in order to test whether randomization was successful, the characteristics of individuals in the control and intervention groups were compared using the chi-squared test with Yates's correction. Time to pacifier introduction was expressed in days, and calculated as median and 95% confidence interval. Prevalence rates for pacifier use in the first six months of life were compared between the groups using Kaplan–Meier survival curves, with differences assessed using the log-rank test.
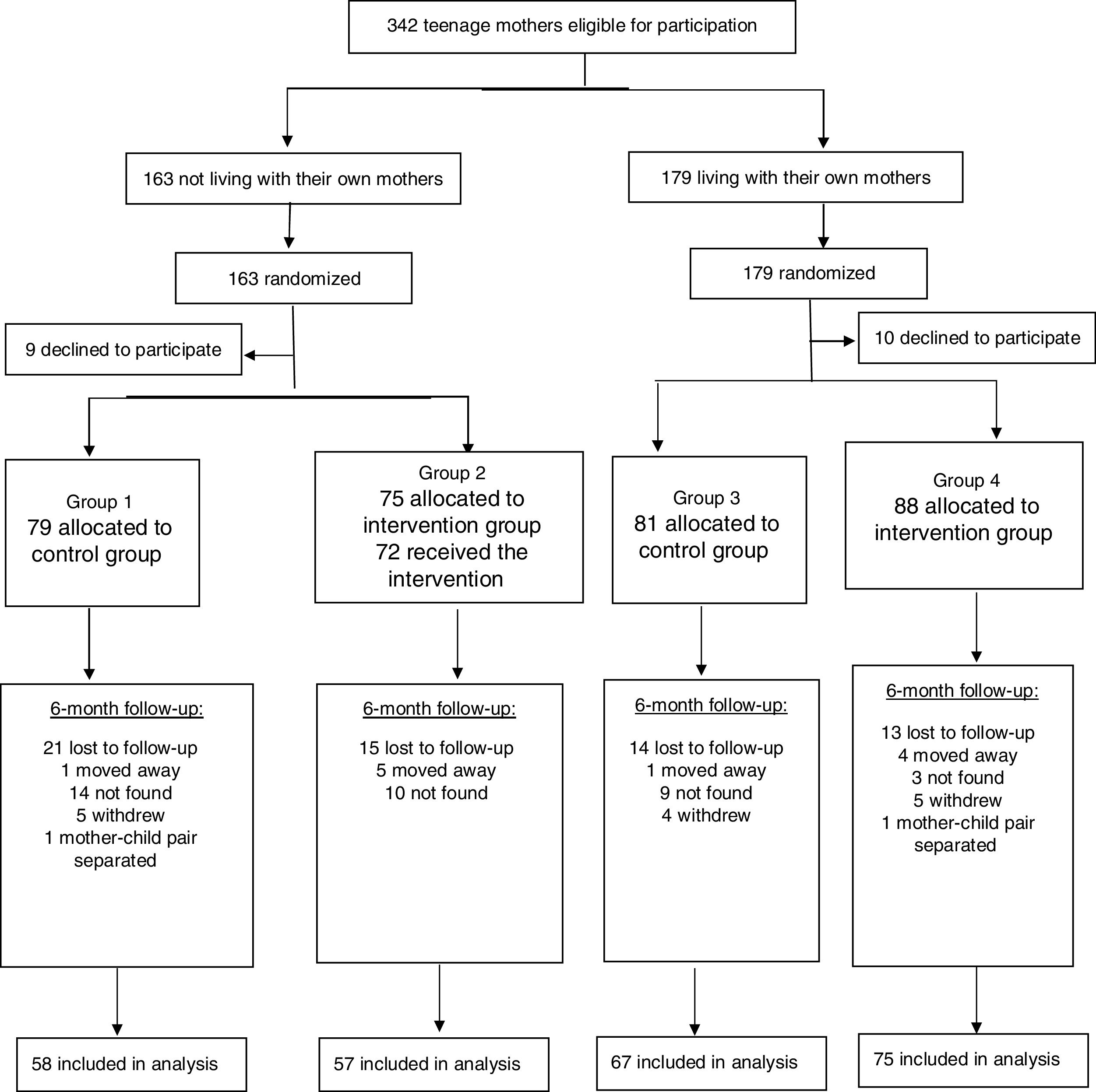
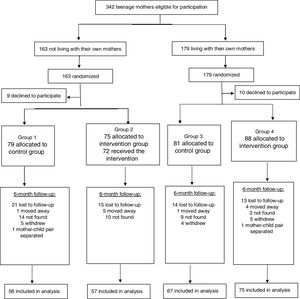
ResultsFig. 1 shows the flowchart of participants from the recruitment of teenage mothers to the last assessment, when children were 6 months old. Of the 342 mothers considered eligible for the study, 323 were included in the sample and 257 (80%) remained in the study until the child was 6 months old. The number of participants lost to follow-up was similar in the intervention and control groups (19% and 22%, respectively). The characteristics of the participants included in the study were as follows: maternal age (mean±standard deviation): 17.5±1.4 years (<15 years: n=13 – 4%; 15–17 years: n=126 – 39%; 18–19 years: n=184 – 57%); maternal skin color – white: 62.8%; average family income (median): 2.5 minimum wages (equivalent to US$195.00/month, according to exchange rate at the time); maternal schooling level ≥8 years: 52.9%; primiparity: 85.5%; vaginal delivery: 74.5%; male child: 50.3%; cohabitation with husband/partner: 62.2%.
There were no significant differences between the intervention and control groups in terms of mother's age, education level, cohabitation with child's father, and parity; child's sex, type of delivery and birth weight; and grandmother's age, education level, work outside the home, and experience with breastfeeding (data not shown). This absence of differences evidences that randomization was successful.
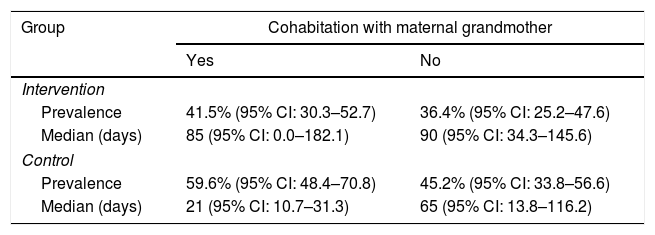
Table 1 shows the frequency of pacifier use in the first month of life and median time to pacifier introduction according to group and cohabitation with maternal grandmother. The intervention delayed by 64 days the introduction of a pacifier in the group of teenage mothers who cohabited with their mothers (both received the intervention), and by 25 days in those who did not cohabit (only the teenagers received the intervention).
Prevalence of pacifier use in the first month of life and time to pacifier introduction according to group and cohabitation with maternal grandmother.
| Group | Cohabitation with maternal grandmother | |
|---|---|---|
| Yes | No | |
| Intervention | ||
| Prevalence | 41.5% (95% CI: 30.3–52.7) | 36.4% (95% CI: 25.2–47.6) |
| Median (days) | 85 (95% CI: 0.0–182.1) | 90 (95% CI: 34.3–145.6) |
| Control | ||
| Prevalence | 59.6% (95% CI: 48.4–70.8) | 45.2% (95% CI: 33.8–56.6) |
| Median (days) | 21 (95% CI: 10.7–31.3) | 65 (95% CI: 13.8–116.2) |
95% CI, 95% confidence interval.
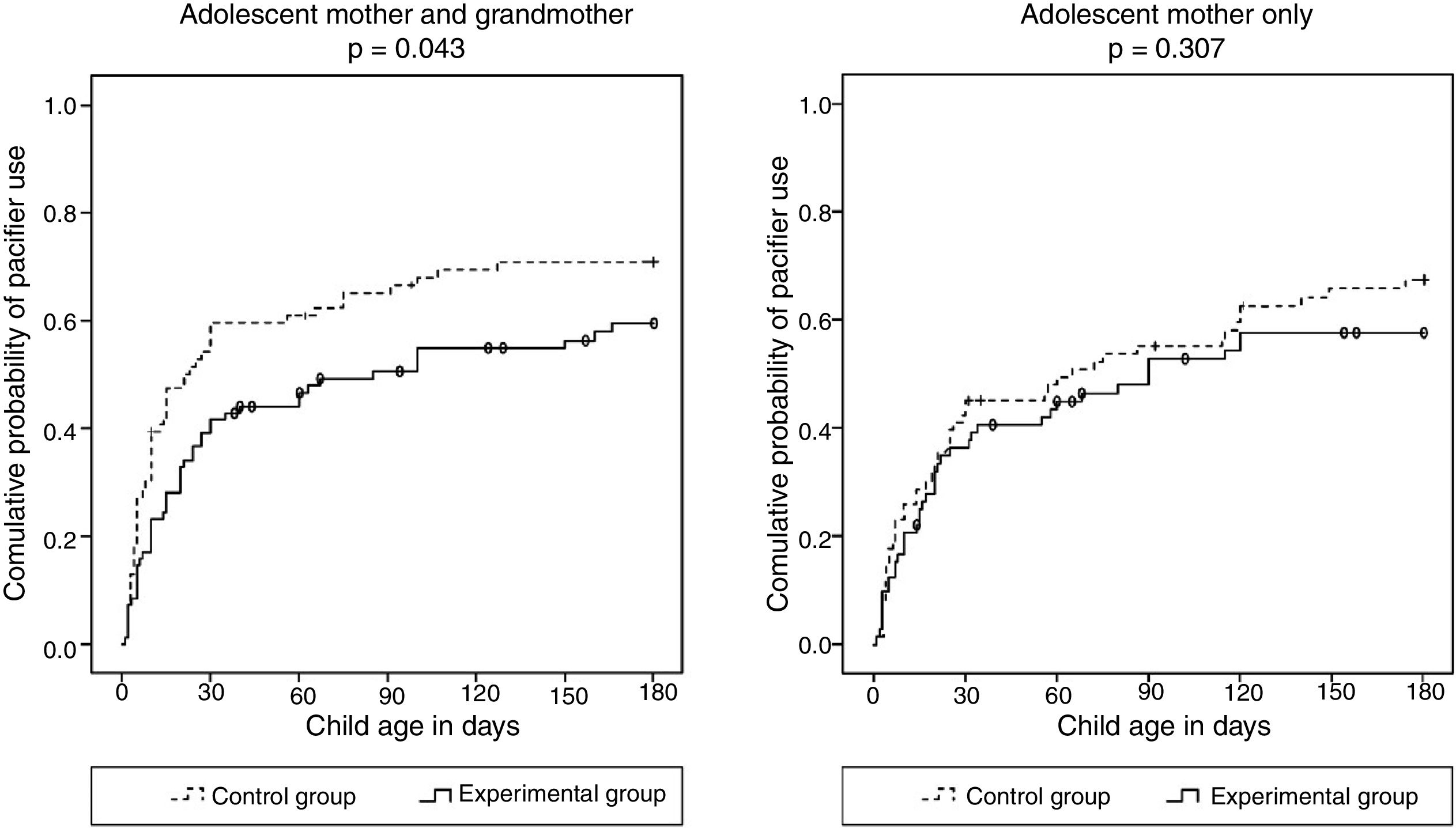
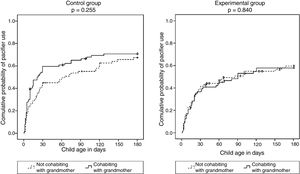
Survival curves showed that the intervention had a significant impact on reducing pacifier use, but only in the group where the infants’ mothers cohabited with the maternal grandmothers (Fig. 2).
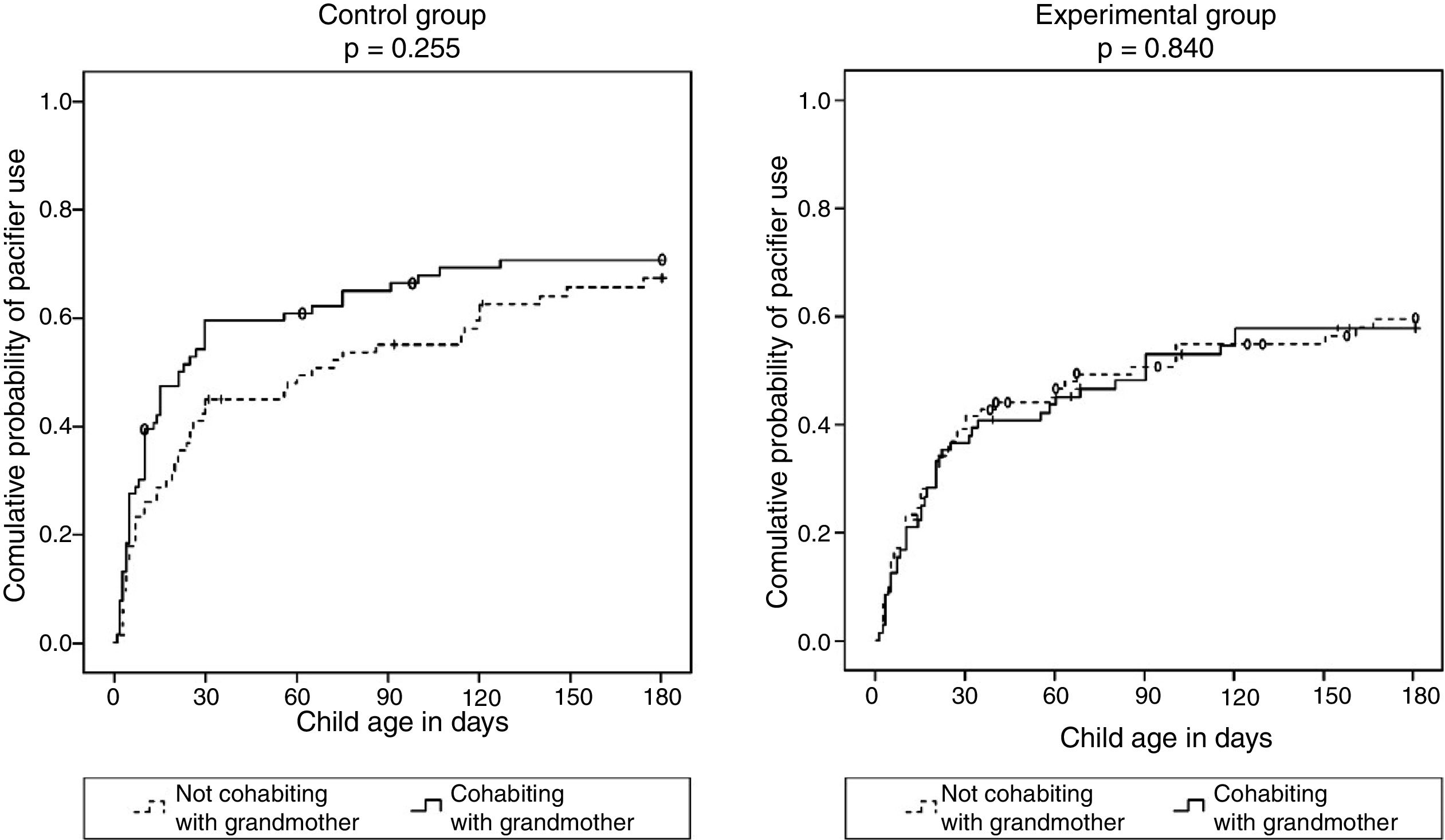
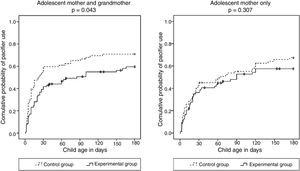
In order to test the influence of maternal grandmothers on the outcome of interest, survival curves for pacifier use were compared separately in the intervention and control groups (Fig. 3). This comparison revealed that, in the control group, the probability of using a pacifier in the first six months of life was greater when the infants’ mothers cohabited with the maternal grandmothers, however with a non-significant difference. In the experimental group, in turn, the curves for mothers cohabiting and not cohabiting with the maternal grandmother were virtually superimposed.
DiscussionFew studies so far have assessed the impact of interventions on reducing pacifier use in the first six months of life of infants. In fact, to the authors’ knowledge, no similar study has investigated pacifier use after the involvement of both teenage mothers and maternal grandmothers in an intervention. Therefore, the present investigation has the strength of being the first to describe the impact of a pro-breastfeeding intervention on reducing pacifier use in children of teenage mothers, with the involvement of maternal grandmothers, when cohabitating.
The intervention was successful in reducing pacifier use in the first six months of life when the maternal grandmothers cohabited with the teenagers, i.e., when they were exposed to the intervention. Apparently, involving grandmothers in the intervention eliminated any negative influence that they might have had on the subject; this was demonstrated by the absence of differences in the likelihood of pacifier use after the intervention, regardless of whether the grandmother did or did not cohabit with the teenage mother (Fig. 3). Mauch et al.11 in Australia, had already reported that grandmothers may be relevant sources of advice supporting the use of pacifiers. In that study, almost one-third of the mothers were advised by their own mothers or mothers-in-law to offer a pacifier to their infants. The present authors believe that many of the grandmothers included in this study were unaware of the problems associated with pacifier use, and that the intervention was an opportunity for them to learn about the risks associated with this practice.
Pacifier introduction was delayed from the first month to the third month in the group in which grandmothers received the intervention. This finding is especially relevant, as the negative effect of pacifier use on breastfeeding duration is believed to be minimal, if existing at all, when the device is introduced after the infant's first month of life.21
It is important to observe that the intervention did not have any impact on reducing pacifier use when only the teenage mother was involved. Nevertheless, as demonstrated elsewhere, the intervention with teenagers resulted in increase in the duration of EBF16 and a lower risk for weaning in the first year of life.17 These findings suggest that teenage mothers are sensitive to behavioral changes aimed at promoting the health of their children, when successfully targeted by interventions. In this sense, it is possible that the negative result found in this sample of teenagers not cohabiting with their mothers may be due to the fact that pacifier use was not the focus of the intervention.
Three clinical trials in the literature were found addressing the use of pacifiers: one carried out in Canada22 one in Brazil,23 and a community-based cluster-randomized trial conducted in Denmark.24 Similarly to this trial, the interventions tested in those studies focused primarily on breastfeeding promotion, but all of them had a significant impact on reducing pacifier use. Nevertheless, differently from this study, those three trials included women of all ages and did not involve grandmothers in the intervention.
As the present study shows, prevalence rates for pacifier use in our setting remain high. In the latest national survey, conducted in 2008,8 Porto Alegre was the Brazilian state capital with the highest prevalence of pacifier use in the first year of life (59.5%). That survey reported a reduction in the use of pacifiers from 57.7% in 1999 to 42.6% in 2008 in Brazil as a whole, and from 69.2% to 59.5% in Porto Alegre. These reductions are probably the result of nation-wide campaigns held in the first of week of August every year, as well as of the implementation of multiple actions as part of the Brazilian National Breastfeeding Program, which include, in addition to breastfeeding promotion, protection, and support, the recommendation not to use a pacifier.
In 1999–2000, the prevalence of pacifier use in the first month of life found in a population with the same profile as the present study (young mothers seen at the same hospital) was 67.6%.25 Comparing that result with the one obtained in the control group of the present study, it was found that, after six to nine years, the prevalence of pacifier use in the first month of life decreased by 15.1% (from 67.6% to 52.5%), which is less than the reduction observed with the intervention involving grandmothers (reduction of 18.1%).
This study corroborates, albeit indirectly, the findings of the study by Buccini, who attributed one-third of the decline in interruption of EBF in Brazil between 1999 and 2008 to the temporal variation of pacifier use.26 The intervention tested in the present study, in addition to reducing the prevalence of pacifier use, significantly increased the duration of EBF: by 67 days for the group which included grandmothers and 46 days for the group which did not include grandmothers.16 Furthermore, the intervention was successful in increasing the prevalence of breastfeeding in the first year of life: the chance of maintaining breastfeeding in the first year of life increased by 49% in the group of adolescent mothers who did not live with grandmothers and by 26% in the group of mothers who cohabited with grandmothers.17
This study has some limitations, e.g., the fact that the intervention did not focus primarily on reducing pacifier use. It is speculated that, had this been the focus, the intervention would probably have been more effective, especially in the group of teenagers not cohabiting with their mothers. Another limitation was the high rate of participants lost to follow-up: around 20%. This is an inherent limitation in studies that require follow-up, especially when involving young adults living in urban peripheries in developing countries.27 However, because this was a randomized clinical trial and the number of losses was similar in both the intervention and control groups, the authors believe that this limitation was not an important source of bias.
It is important to emphasize that the present findings reinforce the association between reduced pacifier use and longer breastfeeding duration: the same intervention reduced pacifier use and increased breastfeeding duration at the same time.16,17 There are at least four routes to explain this association, which can also occur simultaneously: pacifier use by itself can reduce breastfeeding duration28; the introduction of the pacifier occurs due to difficulties in breastfeeding29; baby personality and mother-baby interaction11; and, the profile of mothers and their families determining the option of breastfeeding and avoiding pacifiers.30 However, this study does not help to explain the mechanisms involved in this association, namely, how not using a pacifier can benefit breastfeeding and vice versa.
The prevalence of pacifier use in the first six months of life is still high in this setting, suggesting the need for strategies aimed at reducing this habit. In this sense, the intervention described, consisting of several breastfeeding counseling sessions and including the recommendation to avoid the use of pacifiers, aimed at teenage mothers and maternal grandmothers when cohabiting, and held over the first five months of life of the infants, proved to be useful in reducing pacifier use in the first six months of life and delaying its introduction until after the first month. This reduction was probably due to the fact that potential negative influences from the maternal grandmothers regarding the use of pacifiers may have been neutralized by the intervention. Finally, the absence of impact when only the teenage mothers were exposed to the intervention reinforces the need for novel strategies specifically designed for this age group.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Giugliani ER, Nunes LM, Issler RM, Santo LC, Oliveira LD. Involvement of maternal grandmother and teenage mother in intervention to reduce pacifier use: a randomized clinical trial. J Pediatr (Rio J). 2019;65:166–72.
Study conducted at Programa de Pós-Graduação em Saúde da Criança e do Adolescente, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil.