To determine the main indications and assess the most common adverse events with the administration of hypnotic propofol in most pediatric clinical scenarios.
SourcesA systematic review of PubMed, SciELO, Cochrane, and EMBASE was performed, using filters such as a maximum of five years post-publication, and/or references or articles of importance, with emphasis on clinical trials using propofol. All articles of major relevance were blind-reviewed by both authors according to the PRISMA statement, looking for possible bias and limitations or the quality of the articles.
Summary of the findingsThrough the search criterion applied, 417 articles were found, and their abstracts evaluated. A total of 69 papers were thoroughly studied. Articles about propofol use in children are increasing, including in neonates, with the majority being cohort studies and clinical trials in two main scenarios: upper digestive endoscopy and magnetic resonance imaging. A huge list of adverse events has been published, but most articles considered them of low risk.
ConclusionsPropofol is a hypnotic drug with a safe profile of efficacy and adverse events. Indeed, when administered by non-anesthesiologists, quick access to emergency care must be provided, especially in airway events. The use of propofol in other scenarios must be better studied, aiming to reduce the limitations of its administration by general pediatricians.
Determinar as principais indicações e examinar os eventos adversos mais comuns com uso do hipnótico propofol na maioria dos cenários clínicos pediátricos.
FontesRealizada revisão sistemática da literatura nas bases de dados PubMed, Scielo, Cochrane e EMBASE, aplicando-se filtros como máximo de cinco anos de publicação e/ou referências ou publicações relevantes em outras hipóteses com enfoque em ensaios clínicos envolvendo o propofol. Todos os artigos de maior relevância foram avaliados cegamente pelos dois autores, de acordo com o PRISMA Statement, observando os riscos de vieses e qualidades ou limitações dos estudos.
Resumo dos achadosAtravés dos mecanismos de pesquisa, 417 artigos foram encontrados e separados logo após, de acordo com os critérios de inclusão. Um total de 69 artigos foram estudados. Destacam-se a produção científica crescente sobre o propofol em crianças, incluindo recém-nascidos, sendo a grande maioria dos trabalhos coortes retrospectivos ou prospectivos, bem como ensaios clínicos com o propofol nos principais cenários: endoscopia digestiva alta e ressonância magnética. Ampla gama de eventos adversos foi citada, mas a maioria dos trabalhos não as consideraram significativas.
ConclusõesO propofol apresenta um seguro perfil de eficácia e segurança. Quando administrado por médicos não-anestesistas, deve-se redobrar o cuidado para ação rápida em emergências, especialmente de vias aéreas. A aplicação do fármaco em outros contextos deve ser estudada em maior profundidade, afim de dirimir a dificuldade do uso por pediatras.
Propofol (2-6-diisopropylphenol) is one of the most widely used anesthetic drugs for numerous procedures. Its anesthetic properties, first described in 1973, primarily involve the increase in the inhibitory tone as mediated by gamma-aminobutyric acid (GABA) in the GABA-A receptors, causing an increase in the chloride influx into postsynaptic neurons, thus inhibiting the transmission of electrical impulses and varying levels of sedation.1 In adult patients, the use of propofol is routine and based on solid evidence regarding its safety and efficacy.2 Since the early 1990s, a large number of publications have evaluated the use of propofol at different doses in children of all ages, with a wide range of outcomes, including potential severe adverse events or large cohorts of patients without major adverse events, generating distrust and difficulty in the use of the drug, especially among non-intensivist pediatricians.
Its rapid action onset and termination, justified by its high fat solubility and free passage through the blood-brain barrier, make propofol an intriguing drug. However, its large volume of distribution and its potential to remain in adipose tissue may prolong the action time and adverse events, as well as in patients with liver and/or kidney dysfunction.3 In pediatrics, the difference in propofol clearance is extremely important in prescription limitation. In preterm and full-term newborns, the clearance is only 10%–38% of the drug in its active form, when compared to values in adults. This fact also greatly restricts the prescription of propofol, particularly in newborns (NBs).4
Approved for use in children by the US Food and Drug Administration (FDA) in 1989, the propofol infusion in Brazil country is almost exclusively limited to maintenance of general intravenous or mixed anesthesia and its continuous use in intensive care units is rare, due to potential severe adverse events such as hypotension, myocardial depression, anaphylaxis, and propofol infusion syndrome (PIS), whose signs and symptoms can be extremely severe.5 Therefore, the number of studies confirming the safety of propofol in children, especially in newborns, is small, considering the difficulty in conducting clinical trials in this population.
Thus, the aim of this review is to evaluate the main indications for propofol prescription in all pediatric age groups and to evaluate the hypothesis that the appropriate use of propofol in children is safe, as well as to verify, as the main outcome, the main adverse events (AEs) related to several scenarios for pediatric sedation, through a systematic review of the literature.
MethodsThe Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)6 checklist was used to obtain and analyze the evaluated articles. A systematic literature review was conducted inthe PubMed, SciELO (Virtual Health Library [VHL] descriptors), EMBASE, and Cochrane databases, with the following descriptors, respectively, in PubMed (Medical Subject Headings - MeSH): propofol, child, pediatrics, neonatology and drug-related side effects and adverse reactions; in VHL, propofol, pediatrics, neonatology, and side effects and drug-related adverse reactions; in Cochrane: propofol and children and adverse reactions. Moreover, considering that PIS is a severe adverse event, but poorly described in children, this study used a parallel search with the descriptors propofol and propofol infusion syndrome AND children, both listed identically in the two databases. To better understand the adverse events of propofol in newborns, the authors also conducted a parallel search with the following MeSH descriptors: Propofol and Neonatology.
Inclusion and exclusion criteriaThe review included articles describing the main indications of propofol and/or its adverse events in patients aged 0–20 years old, as well as important references from the reviewed articles. Relevant bibliographic references found in classical or systematic reviews; clinical trials and human-only studies were also considered. As exclusion criteria, the following were removed: editorials, letters to the editor, case reports, articles written in a language other than Portuguese, English, or Spanish, and articles that were submitted after the search but were not related to the scope of the work and/or could not be recovered in their entirety. At the time of the search, the following filters were applied in PubMed to obtain the most current and applicable data: publication date up to five years, clinical trials, clinical conferences, comparative studies, Congress, guideline, historical article, journal article, meta-analyses, multicenter study, observational study, practice guideline, all articles with or without United States government funding, review, humans, and systematic review. The last search was performed on March 25, 2019.
Thus, the following results were obtained:
- •
Criterion A – propofol and children, after applying the filters, showed the following search details: ((“propofol”[MeSH Terms] OR “propofol”[All Fields]) AND (“child”[MeSH Terms] OR “child”[All Fields] OR “children”[All Fields])) AND (“2014/03/25”[PDat]: “2019/03/25”[PDat] AND “humans”[MeSH Terms]) – 346 articles
- •
Criterion B – propofol and drug-related side effects and adverse reactions – search details: ((“propofol”[MeSH Terms] OR “propofol”[All Fields]) AND (“drug-related side effects and adverse reactions”[MeSH Terms] OR (“drug-related”[All Fields] AND “side”[All Fields] AND “effects”[All Fields] AND “adverse”[All Fields] AND “reactions”[All Fields]) OR “drug-related side effects and adverse reactions”[All Fields] OR (“drug”[All Fields] AND “related”[All Fields] AND “side”[All Fields] AND “effects”[All Fields] AND “adverse”[All Fields] AND “reactions”[All Fields]) OR “drug related side effects and adverse reactions”[All Fields])) AND (“2014/03/25”[PDat]: “2019/03/25”[PDat] AND “humans”[MeSH Terms]) – 30 articles
- •
Criterion C – propofol and neonatology - ((“propofol”[MeSH Terms] OR “propofol”[All Fields]) AND (“neonatology”[MeSH Terms] OR “neonatology”[All Fields])) AND (“2014/03/27”[PDat]: “2019/03/25”[PDat]) – 15 articles
- •
Criterion D – propofol and children and propofol infusion syndrome - ((“propofol infusion syndrome”[MeSH Terms] OR (“propofol”[All Fields] AND “infusion”[All Fields] AND “syndrome”[All Fields]) OR “propofol infusion syndrome”[All Fields]) AND (“child”[MeSH Terms] OR “child”[All Fields] OR “children”[All Fields])) AND (“2014/03/27”[PDat]: “2019/03/25”[PDat])– 18 articles
- •
Criterion E (SciELO) – propofol infusion syndrome – 1 article; propofol infusion syndrome and children; as well as any of the above combinations with the VHL descriptors: no articles found.
- •
Criterion F (Cochrane) – propofol and children and Cochrane evidence – 4 articles
The PRISMA Statement was used as a checklist for required items and, within them, plausible items to be applied to the work. Item 4 of PRISMA individualizes the acronym PICOS (P – Patient, Problem or Population. I – Intervention. C – Comparison, control or comparator. O – Outcome(s)), which is individualized below for this review:
- •
Study participants: pediatric age group, from newborn infants to the end of adolescence (20 years), as defined by the World Health Organization;
- •
Intervention: propofol use in most pediatric settings of sedation, without aiming at a comparison regarding safety and efficacy with other hypnotic drugs;
- •
Comparisons with the other studies:Tables 1–3 show all the studies found. During the discussion, more relevant topics in different sedation scenarios were compared between one or more authors;
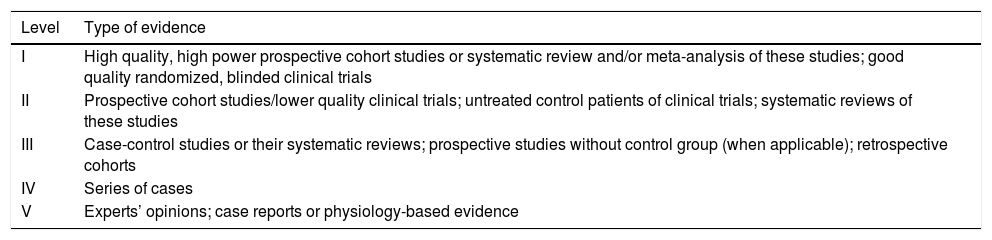
Table 1.Levels of evidence for prognostic studies.7
Level Type of evidence I High quality, high power prospective cohort studies or systematic review and/or meta-analysis of these studies; good quality randomized, blinded clinical trials II Prospective cohort studies/lower quality clinical trials; untreated control patients of clinical trials; systematic reviews of these studies III Case-control studies or their systematic reviews; prospective studies without control group (when applicable); retrospective cohorts IV Series of cases V Experts’ opinions; case reports or physiology-based evidence Table 3.Summary of articles involving the use of propofol in pediatrics.
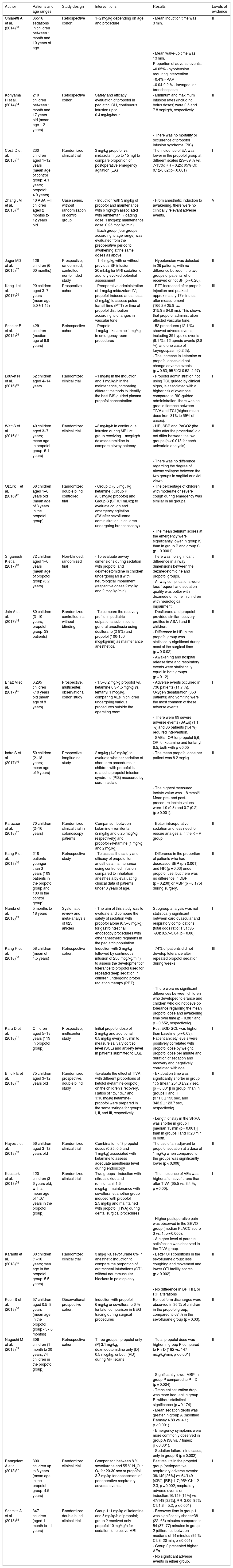
Author Patients and age ranges Study design Interventions Results Levels of evidence Chiaretti A et al. (2014)33 36516 sedations in children between 1 month and 10 years of age Retrospective cohort 1−2 mg/kg depending on age and procedure - Mean induction time was 3 min. II - Mean wake-up time was 13 min. Proportion of adverse events: −0.05% - hypotension requiring intervention −0.4% - PAP −0.04-0.2 % - laryngeal or bronchospasm Koriyama H et al. (2014)34 210 children between 1 month and 17 years old (mean age 1.2 years) Retrospective cohort Safety and efficacy evaluation of propofol in pediatric ICU, continuous infusion up to 0.4 mg/kg/hour - Minimum and maximum infusion rates (including bolus doses) were 0.5 and 7.8 mg/kg/h, respectively. II - There was no mortality or occurrence of propofol infusion syndrome (PIS) Costi D et al. (2015)35 230 children aged 1–12 years (mean age of control group: 4.1 years; propofol: 4.0 years) Randomized clinical trial 3 mg/kg propofol vs. midazolam (up to 15 mg) to compare proportion of postoperative emergency agitation (EA) The incidence of EA was lower in the propofol group at different scales (29–39 % vs. 7-15%; RR = 0.25; 95% CI: 0.12-0.62; p < 0.001) I Zhang JM et al. (2015)36 40 ASA I–II children aged 2 months to 12 years old Case series, without randomization or control group - Induction with 3 mg/kg of propofol and maintenance with 6 mg/kg/h associated with remifentanil (loading dose: 1 mcg/kg; maintenance dose: 0.25 mcg/kg/min) - From anesthetic induction to awakening, there were no clinically relevant adverse events. V - Each group (four groups according to age range) was evaluated from the preoperative period to awakening at the same doses as above. Jager MD et al. (2015)37 126 children (6–60 months) Prospective, randomized, controlled, non-blinded study - 1–6 mg/kg with or without previous SF infusion, 20 mL/kg for MRI sedation or auditory evoked potential assessment - Hypotension was detected in 26 patients, with no difference between the two groups of patients who received or not SF (p = 0.26). II Kang J et al. (2017)38 20 children aged 3–7 years (mean age 5.0 ± 1.45) Prospective cohort - Preoperative administration of 1 mg/kg midazolam IV; propofol-induced anesthesia (2 mg/kg) to assess pulse transit time (PTT) or time of propofol distribution according to changes in vascular tone - PTT increased after propofol injection and peaked approximately 17 minutes after measurement (166.2 ± 25.9 vs. 315.9 ± 64.9 ms). This shows that propofol administration affected vascular tone. III Scheier E et al. (2015)39 429 children (median age of 6.8 years) Retrospective cohort - Propofol 1 mg/kg + ketamine 1 mg/kg in emergency room procedures - 52 procedures (12.1 %) showed adverse events, including 39 hypoxic events (9.1 %), 12 apneic events (2.8 %), and one case of laryngospasm (0.2 %). II - The increase in ketamine or propofol doses did not change adverse events (p = 0.63; 95 %CI 0.52–2.97) Louvet N et al. (2016)40 62 children aged 4–14 years Randomized clinical trial −1 mg/kg in the induction, and 1 mg/kg/h in the maintenance, comparing different methods to identify the best BIS-guided plasma propofol concentration - Propofol administration not using TCI, guided by clinical signs, is associated with a higher risk of overdose compared to BIS-guided administration; there was no great difference between TIVA and TCI (higher mean dose from 31% to 59% of cases). I Watt S et al. (2016)41 40 children aged 3–7 years; mean age in propofol group: 5.1 years) Randomized clinical trial −3 mg/kg/h in continuous infusion during MRI vs. group receiving 1 mcg/kg/h dexmedetomidine to compare airway patency - HR, SBP and PaCO2 (the latter after the procedure) did not differ between the two groups (p < 0.013 for each univariate analysis). II - There was no difference regarding the degree of airway collapse between the two groups in sagittal or axial views. Ozturk T et al. (2016)42 68 children aged 1–8 years old (mean age of 3 years in the propofol group) Randomized, double blind controlled trial - Group C (0.5 mg / kg ketamine); Group P (0.5 mg/kg propofol) and Group S (SF 0.1 mL/kg) to evaluate cough and emergency agitation (EA)after sevoflurane administration in children undergoing bronchoscopy) - The percentage of children with moderate or severe cough during emergency was similar in all groups. II - The mean delirium scores at the emergency were significantly lower in group K than in group P and group S (p = 0.0001) Sriganesh K et al. (2017)43 72 children aged 1–6 years (mean age of propofol group (3.2 years) Non-blinded, randomized trial - To evaluate airway dimensions during sedation with propofol and dexmedetomidine in children undergoing MRI with neurological impairment (respective doses 2 mg/kg and 2 mcg/kg/min) There was no significant difference in airway dimensions between the dexmedetomidine and propofol groups. II - Airway complications were less frequent and sedation quality was better with dexmedetomidine in children with neurological impairment. Jain A et al. (2017)44 80 children (3–10 years; propofol group: 39 patients) Randomized controlled trial without blinding - To compare the recovery profile in pediatric outpatients submitted to general anesthesia using desflurane (2-8%) and propofol (100-150 mcg/kg/min) as maintenance anesthetics. - Desflurane and propofol provided similar recovery profiles in ASA I and II children. II - Difference in HR in the propofol group was statistically significant during most of the surgical time (p = 0-0.02). - Awakening and hospital release time and respiratory events were statistically equal in both groups (p = 0.12). Bhatt M et al. (2017)45 6,295 children <18 years old (mean age of 8 years) Prospective, multicenter, observational cohort study - 1.5–3.2 mg/kg propofol vs. ketamine 0.9-1.5 mg/kg vs. fentanyl 1 mcg/kg, comparing AEs in children undergoing various procedures outside the operating room - Adverse events occurred in 736 patients (11.7 %). Oxygen desaturation (353 patients) and vomiting were the most common of these adverse events. I - There were 69 severe adverse events (SAEs) (1.1 %) and 86 patients (1.4 %) required intervention. - SAEs - OR for propofol 5,6; OR for ketamine and fentanyl 6.5, both with p < 0.05 Indra S et al. (2017)46 50 children (2–18 years; mean age of 9 years) Prospective longitudinal study 2 mg/kg (1−9 mg/kg) to evaluate whether sedation of short-term procedures in children with propofol is related to propofol infusion syndrome (PIS) measured by serum lactate. - The mean propofol dose per patient was 8.2 mg/kg II - The highest measured lactate value was 1.8 mmol/L. Mean pre- and post-procedure lactate values were 1.0 (0.3) and 0.7 (0.2) (p < 0.001). Karacaer et al. (2018)47 70 children (2–16 years) Randomized clinical trial in colonoscopy patients Comparison between ketamine + remifentanil (2 mg/kg and 0.25 mcg/kg respectively) and propofol + ketamine (1 mg/kg and 2 mg/kg) - Better intraoperative sedation and less need for rescue analgesia in the K + P group II Kang P et al. (2018)48 218 patients younger than 3 years (109 patients in the propofol group and 109 in the control group) Retrospective study - To assess the safety and efficacy of propofol for anesthesia maintenance using controlled infusion compared to inhalation anesthesia by evaluating clinical data of patients under 3 years of age. - Difference in the proportion of patients who had decreased SBP (p < 0.001) and HR (p = 0.03) under propofol use, but there was no difference in DBP (p = 0.238) or MBP (p = 0.175) during surgery. II Narula et al. (2018)49 5 months to 18 years Systematic review and meta-analysis of 625 articles - The aim of this study was to evaluate and compare the safety of sedation with propofol alone (0.5–3 mg/kg) for gastrointestinal endoscopy procedures with other anesthetic regimens in the pediatric population. Subgroup analysis was not statistically significant between cardiovascular and respiratory complications. (total odds ratio: 1.31; 95 %CI: 0.57–3.04, p = 0.08) I Kang R et al. (2018)50 58 children (mean of 4.5 years) Retrospective cohort Induction with 2 mg/kg followed by continuous infusion of 250 mcg/kg/min) to assess the development of tolerance to propofol used for repeated deep sedation in children undergoing proton radiation therapy (PRT). −74% of patients did not develop tolerance after repeated propofol sedation during weeks III - There were no significant differences between children who developed tolerance and children who did not develop tolerance regarding the mean propofol dose and awakening time over time (p = 0.887 and p = 0.652, respectively). Kara D et al. (2018)51 Children aged 5–18 years (119 in propofol group) Prospective, multicenter study Initial propofol dose of 2 mg/kg and additional 0.5 mg/kg every 3−5 min to measure salivary cortisol level (SCL) and anxiety level in patients submitted to EGD Post-EGD SCL was higher than baseline (p = 0.03). Patient anxiety levels were positively correlated with propofol dose by weight, propofol dose per minute and duration of sedation and recovery and negatively correlated with age. I Biricik E et al. (2018)52 75 children aged 3–12 years old Randomized, prospective, double blind study -Evaluate the effect of TIVA with different proportions of ketofol (ketamine-propofol) on the children’s recovery. Ratios of 1:5, 1:6.7 and 1:10 mg/kg ketamine-propofol were prepared in the same syringe for groups I, II, and III, respectively. - Extubation time was significantly shorter in group 1: 5 (mean 254.3 ± 92.7 sec. [p = 0.001]) in group I than in groups II and III (371.3 ± 153 sec. and 343.2 ± 123.7 sec, respectively) II - Length of stay in the SRPA was shorter in group I [median 15 min (p = 0.001)] than in groups I and II: 20 min in both. Hayes J et al. (2018)53 56 children aged 3–12 years old Randomized clinical trial Combination of 3 propofol doses (0.25, 0.5 and 1 mg/kg) associated with ketamine to assess adequate anesthesia level during endoscopy The use of an adjuvant to propofol sedation at a dose of 1 mg/kg when compared to the groups was significantly lower (p < 0.008). II Kocaturk et al. (2018)54 120 children (3–6 years old, with a mean age of 4.67 years in the propofol group) Randomized clinical trial Two groups - induction with nitrous oxide and remifentanil 1.5 mcg/kg + maintenance with sevoflurane; another group induced with propofol 2.5 mg/kg and maintained with propofol (TIVA) during dental surgical procedures - The incidence of AEs was higher after sevoflurane than after TIVA (65.5 vs. 3.4 %, p = 0.00). I - Higher postoperative pain was observed in the SEVO group (median FLACC score 3 vs. 1, p = 0.000). - A higher level of parental satisfaction was observed in the TIVA group. Karanth et al. (2018)55 80 children (1–10 years; men age in the propofol group: 5.5 years) Randomized clinical trial 3 mg/g vs. sevoflurane 8% in anesthetic induction to compare the proportion of orotracheal intubations (OTI) without neuromuscular blockers in palatoplasty - Better OTI conditions in the sevoflurane group: less coughing and movement and lower OTI facility scores (p < 0.002) II - No difference in BP, HR, or RR alterations Koch S et al. (2018)56 57 children aged 0.5–8 years (mean age in the propofol group - 57.6 months) Observational prospective cohort Induction with propofol 6 mg/kg or sevoflurane 6 % for later comparison in EEG tracing during surgical procedures Epileptiform discharges were observed in 36 % of children in the propofol group, compared to 67 % in the sevoflurane group (p = 0.03). II Nagoshi M et al. (2018)59 306 children (1 month to 20 years; 74 children in the propofol group) Retrospective cohort Three groups - propofol only (P) 3.1 mg/kg; dexmedetomidine only (D) 0.5 mcg/kg; or both (PD) during MRI scans - Total propofol dose was higher in group P compared to P + D (182 vs. 147 mcg/kg/min; p < 0.001) II - Significantly lower MBP in group P compared to P + D (p = 0.004) - Transient saturation drop was more frequent in group B, without statistical significance (p = 0.174). - Mean sedation depth was greater in group A (modified Ramsay 4.89 vs. 4.1; p < 0.001) - Emergency symptoms were more commonly observed in group A (38 vs. 7 times; p < 0.001). - Sedation failure: nine cases, only in group B (p = 0.002) Ramgolam A et al. (2018)57 300 children up to 8 years (mean age in the propofol group: 4.5 years) Randomized clinical trial Comparison between 8 % sevoflurane and 55 % N2O in O2 for 20-30 sec or propofol 3-5 mg/kg for assessment of perioperative respiratory adverse events Best results in the propofol group (perioperative respiratory adverse events: 39/149 [26%] vs. 64/149 [43%], [RR]: 1.7; 95%CI: 1.2-2.3; p = 0.002; respiratory adverse events on induction:16/149 [11%] vs. 47/149 [32%], RR: 3.06; 95% CI: 1.8 – 5.2, p < 0.001) I Schmitz A et al. (2018)58 347 children (aged 1 month to 11 years) Randomized double blind clinical trial Group 1: 1 mg/kg of ketamine and 5 mg/kg/h of propofol; group 2 received only propofol 10 mg/kg/h for sedation for elective MRI - Recovery time in group 1 was significantly shorter:38 (22–65) minutes compared to 54 (37–77) minutes in group 2 (difference between medians of 14 minutes (95 % CI: 8−20 min; p < 0.001) II - Group 2 presented higher AEs - No significant adverse events in either group. ICU, intensive care unit; PIS, propofol infusion syndrome; PTT, pulse transition time; PAP, Positive airway pressure; BIS, bispectral index; ICT, infusion control time; TIVA, total intravenous anesthesia; HR, heart rate; SBP, systemic blood pressure; ASA, American Society of Anesthesiology; PRT, proton radiation therapy; EGD, esophagogastroduodenoscopy; SLC, salivary levels of cortisol; EEG, electroencephalogram; SEVO, sevoflurane; MRI, magnetic resonance imaging.
- •
Outcomes: efficacy (with emphasis on percentage of successful sedations with propofol), safety (with emphasis on the reporting of adverse events), and evaluation of the clinical situations in which propofol was used; and
- •
Study designs: placed individually in each table.
Aiming to define the evidence levels of each article, the present review adapted the classification according to the study by Burns et al.,7 more precisely what is shown in Table 1 of this review. The body of the article describes the definition of each degree and thus added an additional column in each table with the appropriate degree of evidence, therefore proposing recommendations in a more scientific manner.
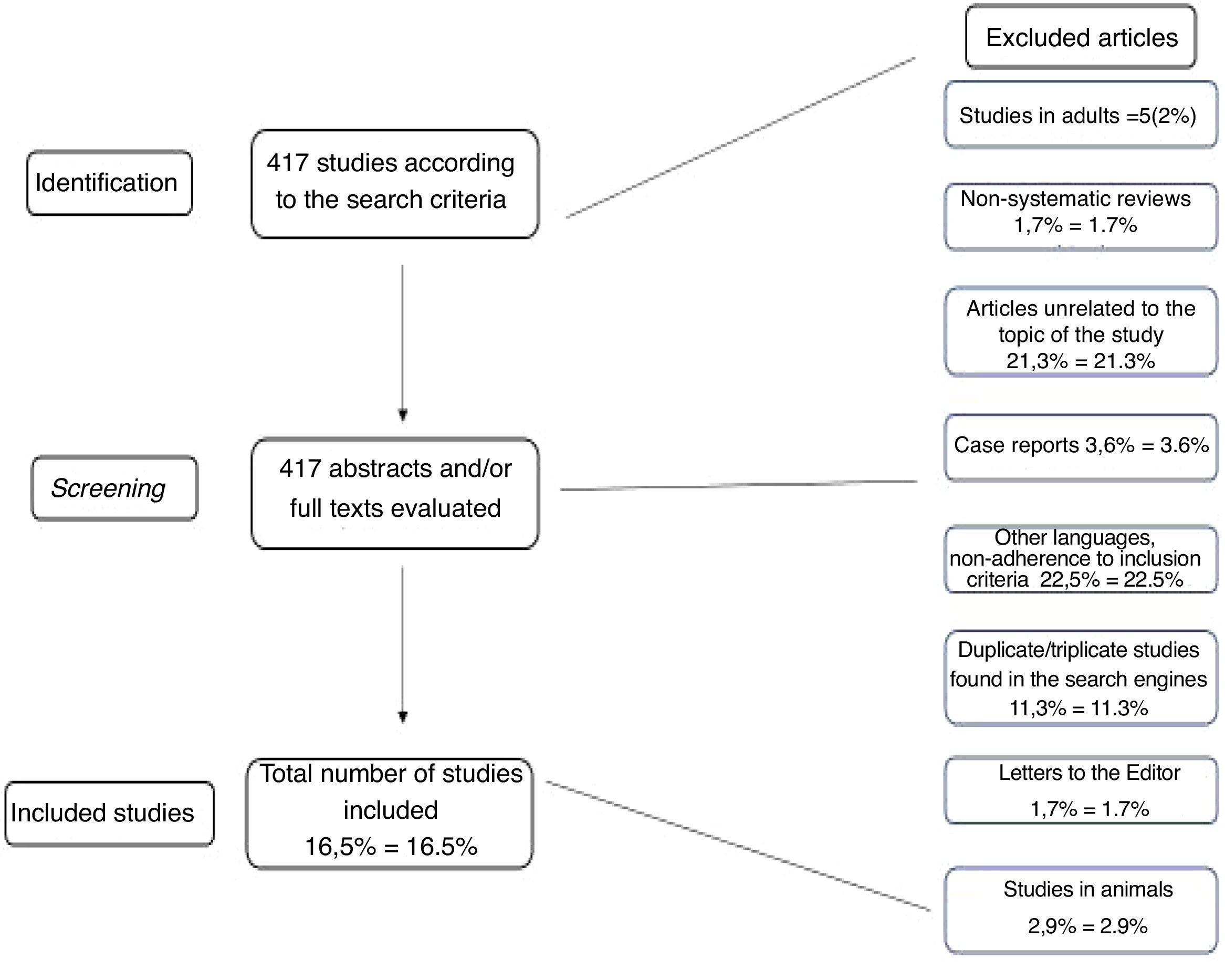
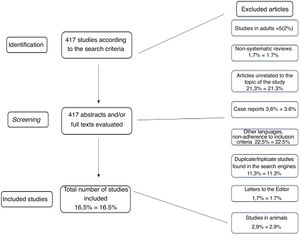
ResultsAccording to the search engines described above, 417 possible publications were identified. Each was separately analyzed by the two authors through their abstracts, and after applying the exclusion criteria explained above, 69 articles (16.5 % of the initial total) were assessed in their entirety and included in the tables or in other parts of the article. Many articles were found to be in duplicate and/or triplicate according to search engines, and that is why only articles that were directly related to the study objectives were included in the tables. This redundancy in the findings was almost total when the authors verified the articles cited by EMBASE.
The article selection process, as well as the reading of all abstracts and their inclusion in the results, was performed independently and separately between the two authors. Similarly, both authors jointly assessed the levels of evidence of the articles to which they apply, according to Table 1. The methodology for article selection is summarized in Fig. 1.
Most articles evaluated propofol use in procedures performed outside the operating room, especially upper digestive endoscopy and magnetic resonance imaging (MRI), or included propofol as clinical trial arms with other hypnotics and/or sedatives for safety and efficacy assessment.
Due to the large number of articles found, it was decided to divide the content of the research results into three large tables, each addressing different aspects of propofol use in pediatrics. The tables were divided into columns according to the PRISMA Statement, observing the types of studies, as well as their results, clinical and future research implications, and possible biases found in the studies.
DiscussionTo date, to the best of the authors’ knowledge, a systematic review addressing the use of propofol in several pediatric settings has not been found. Other reviews or meta-analyses addressed specific aspects, such as its use in digestive endoscopy or imaging exams, with a much smaller number of articles to be analyzed.
The number of articles published in recent years on the subject, which reflects the “universalization” of propofol use, as shown above, mainly outside the operating room and by non-anesthesiologists. The authors believe this is a huge gain due to the efficacy and safety of this hypnotic drug, demonstrated in the most varied situations according to the tables. After the discontinuation of hypnotics such as chloral hydrate and the low availability of dexmedetomidine (effective non-parenteral sedation options),8 the use of the parenteral route has increased, leading to the need for greater knowledge of the emergency management of potential adverse events associated with propofol, which appear less often with other hypnotic or sedative drugs.
Evidence based recommendationsAlthough the scope of this study is only of a systematic review with critical analysis of the selected texts, through the evaluation of the degrees of evidence of each article, conclusions are drawn below.
Use in neonatologySummary of recommendations
- •
There are no recommendations for the routine use of propofol for rapid surfactant instillation in the delivery room. The only study found did not have a control group (Level II).
- •
There is no recommended optimal dose of propofol in the delivery room for the same purpose as the abovementioned one, with high doses correlating with worse clinical outcomes. Similarly, very heterogeneous doses were also used in minor surgical procedures, limiting their prescription (Level II/III).
- •
Propofol in combination with ketamine is related to fewer intubations after minor surgery when compared to inhaled anesthetics (Level II).
- •
The routine use of propofol as a pre-electroencephalogram hypnotic drug is not recommended, and there is no definition regarding the adequate dose (Level I).
- •
The use of propofol in NBs should be regularly and carefully evaluated and the staff appropriately trained to manage adverse events, especially respiratory events, which may reach 50 % of sedations (Level I).
- •
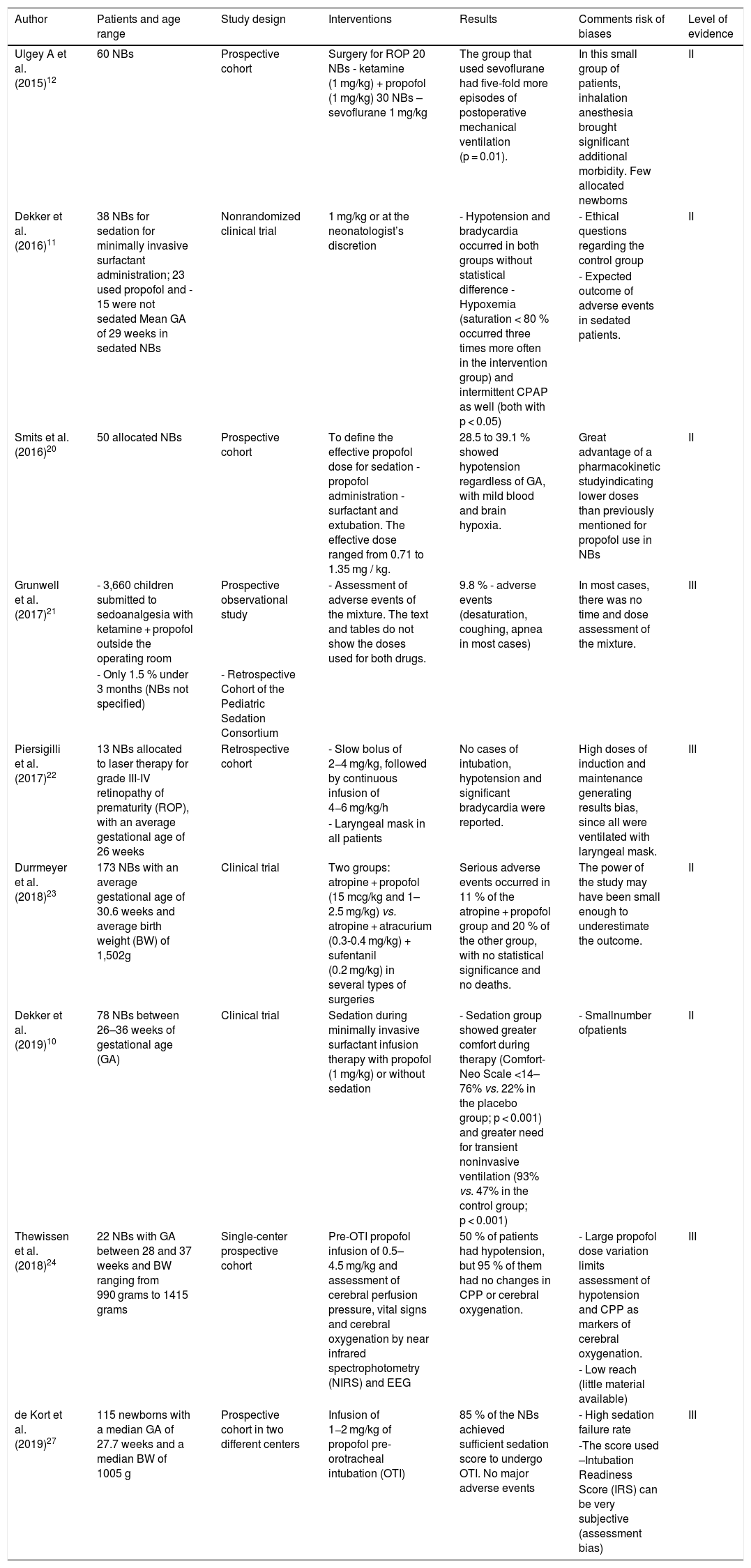
Historically, newborn sedation is a paradigm to be broken. Fortunately, the present moment is a time of transition in which the need for sedoanalgesia in newborns is more evident due to humanitarian reasons and to ensure the safety and effectiveness of several procedures. Regarding the eleven articles mentioned in the neonatology field, the indications, doses and AEs caused by propofol were very diverse. Doses of 1 mg/kg were more often found; however, doses up to 4.5 mg/kg have been reported (Table 2). The practice of propofol use for NBs outside the operating room is very rare, given the fear related to PIS and the low general experience of neonatologists with the drug; in fact, there have been reports of PIS with single-dose propofol use in newborns.9
Summary of articles involving the use of propofol in newborns.
| Author | Patients and age range | Study design | Interventions | Results | Comments risk of biases | Level of evidence |
|---|---|---|---|---|---|---|
| Ulgey A et al. (2015)12 | 60 NBs | Prospective cohort | Surgery for ROP 20 NBs - ketamine (1 mg/kg) + propofol (1 mg/kg) 30 NBs – sevoflurane 1 mg/kg | The group that used sevoflurane had five-fold more episodes of postoperative mechanical ventilation (p = 0.01). | In this small group of patients, inhalation anesthesia brought significant additional morbidity. Few allocated newborns | II |
| Dekker et al. (2016)11 | 38 NBs for sedation for minimally invasive surfactant administration; 23 used propofol and - 15 were not sedated Mean GA of 29 weeks in sedated NBs | Nonrandomized clinical trial | 1 mg/kg or at the neonatologist’s discretion | - Hypotension and bradycardia occurred in both groups without statistical difference -Hypoxemia (saturation < 80 % occurred three times more often in the intervention group) and intermittent CPAP as well (both with p < 0.05) | - Ethical questions regarding the control group | II |
| - Expected outcome of adverse events in sedated patients. | ||||||
| Smits et al. (2016)20 | 50 allocated NBs | Prospective cohort | To define the effective propofol dose for sedation - propofol administration - surfactant and extubation. The effective dose ranged from 0.71 to 1.35 mg / kg. | 28.5 to 39.1 % showed hypotension regardless of GA, with mild blood and brain hypoxia. | Great advantage of a pharmacokinetic studyindicating lower doses than previously mentioned for propofol use in NBs | II |
| Grunwell et al. (2017)21 | - 3,660 children submitted to sedoanalgesia with ketamine + propofol outside the operating room | Prospective observational study | - Assessment of adverse events of the mixture. The text and tables do not show the doses used for both drugs. | 9.8 % - adverse events (desaturation, coughing, apnea in most cases) | In most cases, there was no time and dose assessment of the mixture. | III |
| - Only 1.5 % under 3 months (NBs not specified) | - Retrospective Cohort of the Pediatric Sedation Consortium | |||||
| Piersigilli et al. (2017)22 | 13 NBs allocated to laser therapy for grade III-IV retinopathy of prematurity (ROP), with an average gestational age of 26 weeks | Retrospective cohort | - Slow bolus of 2−4 mg/kg, followed by continuous infusion of 4−6 mg/kg/h | No cases of intubation, hypotension and significant bradycardia were reported. | High doses of induction and maintenance generating results bias, since all were ventilated with laryngeal mask. | III |
| - Laryngeal mask in all patients | ||||||
| Durrmeyer et al. (2018)23 | 173 NBs with an average gestational age of 30.6 weeks and average birth weight (BW) of 1,502g | Clinical trial | Two groups: atropine + propofol (15 mcg/kg and 1–2.5 mg/kg) vs. atropine + atracurium (0.3-0.4 mg/kg) + sufentanil (0.2 mg/kg) in several types of surgeries | Serious adverse events occurred in 11 % of the atropine + propofol group and 20 % of the other group, with no statistical significance and no deaths. | The power of the study may have been small enough to underestimate the outcome. | II |
| Dekker et al. (2019)10 | 78 NBs between 26–36 weeks of gestational age (GA) | Clinical trial | Sedation during minimally invasive surfactant infusion therapy with propofol (1 mg/kg) or without sedation | - Sedation group showed greater comfort during therapy (Comfort-Neo Scale <14–76% vs. 22% in the placebo group; p < 0.001) and greater need for transient noninvasive ventilation (93% vs. 47% in the control group; p < 0.001) | - Smallnumber ofpatients | II |
| Thewissen et al. (2018)24 | 22 NBs with GA between 28 and 37 weeks and BW ranging from 990 grams to 1415 grams | Single-center prospective cohort | Pre-OTI propofol infusion of 0.5–4.5 mg/kg and assessment of cerebral perfusion pressure, vital signs and cerebral oxygenation by near infrared spectrophotometry (NIRS) and EEG | 50 % of patients had hypotension, but 95 % of them had no changes in CPP or cerebral oxygenation. | - Large propofol dose variation limits assessment of hypotension and CPP as markers of cerebral oxygenation. | III |
| - Low reach (little material available) | ||||||
| de Kort et al. (2019)27 | 115 newborns with a median GA of 27.7 weeks and a median BW of 1005 g | Prospective cohort in two different centers | Infusion of 1−2 mg/kg of propofol pre-orotracheal intubation (OTI) | 85 % of the NBs achieved sufficient sedation score to undergo OTI. No major adverse events | - High sedation failure rate | III |
| -The score used –Intubation Readiness Score (IRS) can be very subjective (assessment bias) |
ROP, retinopathy of prematurity; CPAP, continuous positive airway pressure; GA, gestational age; BW, birth weight; CPP, cerebral perfusion pressure; EEG, electroencephalogram.
Most studies had small sample size as a limitation, as well as the absence of a pre-established dose for use in these age groups; similarly, the studies focused on sedation for OTI, surgeries to correct retinopathy of prematurity, or minor procedures in the neonatal ICU. With the surfactant administration techniques for premature infants, some groups have questioned the need for sedoanalgesia in this group of patients. This was observed in an important article by Dekker et al.,10 in which one control group received no medication for surfactant administration via catheter, and neither group (the other group received propofol at a minimum dose of 1 mg/kg) showed any significant statistically significant morbidity. Years before, the same Dutch group led by Dekker et al.12 demonstrated that the rapid surfactant instillation technique was easier in children that had received propofol.11 Despite these results, the use of a control group in a procedure such as the above is highly questionable.
Other studies, however, and for the same purpose, reported numbers of propofol-related AEs that constitute a matter of concern. Hypotension (up to 50 %), transient hypoxemia (10–11 %), and unfavorable conditions for OTI stand out, when propofol was used as the sole hypnotic for OTI of premature infants at doses up to 2 mg/kg in the induction.11,12 Among the reviewed studies, there were no sentinel events resulting from the use of propofol.
Propofol and neurotoxicityFor a long time, a certain "resistance" to propofol use in newborns came from animal studies, in which brain toxicity, especially caused by benzodiazepines, was long-standing. Jia, one of the greatest authors on the subject, used propofol in different models. In a study of rat monocytes and macrophages submitted to propofol use in vitro, there was a reduction in the proportion of proinflammatory cytokines, such as interleukins 6 and 8, in addition to tumor necrosis factor.13 In a subsequent publication, Tuet al. demonstrated that cell oxidative stress, as well as cytokine-induced mitochondrial injury as the abovementioned ones, decreased with exposure to propofol.14 In this article, hippocampal neuron apoptosis was reduced in vitro with simultaneous exposure to dexmedetomidine, a fact previously described by other authors. In other specific groups, such as adults with hepatic encephalopathy in hemodiafiltration, as well as NBs with acute renal dysfunction and the maternal propofol bolus effect during emergency cesarean deliveries, there were no AEs.15–17 In a more recent article, Olutoye et al.18 stated that the actual incidence of neuronal damage with any type of sedoanalgesia in humans is still unknown. According to the Food and Drug Administration (FDA), the main agents involved in this topic are inhalation gases such as sevoflurane and isoflurane, and benzodiazepines with or without propofol.
The authors suggest other options such as opioids, alpha-2 agonists (clonidine or dexmedetomidine), and the minimization of exposure in pregnant women in the third trimester of pregnancy or in children under 3 years of age. Another article on the subject was published by Jiang et al.,19 in a review that addressed several mechanisms by which propofol can lead to neurotoxicity. The induction of factors that generate tissue hypoxia (long-chain RNA) and propofol-induced immunomodulation may even reduce the chemotherapy response of patients with the expression of some tumor suppression genes and/or proteins.
Table 2 shows the main articles about propofol use in neonates20–27 that are not detailed in the discussion, some of which have already been described here. Table 2 also shows the summary of articles involving the use of propofol in newborns.
Main clinical indications of propofol use in childrenSummary of recommendations
- •
Propofol at doses of 1−2 mg/kg, for procedures outside the operating room, is safe and well tolerated for most clinical situations (Level II).
- •
The team using propofol should have the necessary material and experience to deal with cardiopulmonary emergencies, after comparing propofol AE rates with those of other hypnotics and sedatives (Level I).
- •
Propofol has shown in several studies to be the most appropriate drug for post-sevoflurane emergency agitation (EA) control and should be strongly considered in these situations (Level II).
- •
According to a meta-analysis published by Cochrane in 2013,25 in more than 14,045 children, propofol has been confirmed to be indicated for the prevention of AE mentioned above.
- •
The use of continuous infusion of propofol outside the operating room is contraindicated, considering the potential severe AEs shown in studies cited in Table 3 (Level I).
- •
Dexmedetomidine seems to be safer and more protective of airway tone compared to propofol, especially for procedures requiring only sedation, such as MRI (Level II).
- •
Due to the highly heterogeneousdoses of propofol used with or without other concomitant inducing agents in emergency procedures, it is not possible to attest to the benefit of joint administration of propofol with midazolam or ketamine with more than 11 % of AEs with the mixtures (Level I).
- •
Propofol as an adjuvant agent in preoperative sedation seems to be safe at doses of 1−2 mg/kg, facilitating anesthetic induction and intraoperative anesthesia maintenance (Level II).
- •
There is no doubt that one of the greatest representatives of translational medicine is here: the vast majority of the studies presented in the abovementioned tables address the use of propofol as an adjuvant for endoscopy and bronchoscopy, as has occurred for years with adults. With a large number of studies, these patients have already been evaluated in meta-analyses, with well-determined results. The most recent review, dated July 2018,26 showed in six studies with 273 children receiving propofol that the odds ratio for cardiopulmonary AEs was higher in patients using propofol (1.87; 95 % CI: 1.09–3.20; p = 0.02). In 2012, a group from Amsterdam27 reviewed 182 articles looking for the best alternative to sedation for EGD (esophagogastroduodenoscopy), including eleven clinical trials, and concluded that propofol was the gold standard agent for EGD compared to all other agents.
The need to discuss the best sedative came from the inconclusive 2016 meta-analysis published by Cochrane,28 which aimed to evaluate midazolam as a sedative for procedures, concluding that it was not superior regarding efficacy and safety after the review of all articles. Additionally, as previously stated, the removal of chloral hydrate, an important hypnotic agent, has increased the need for sedation replacement with safety. Hong et al.29 published a meta-analysis in April of this year comparing propofol to several other sedation regimens for procedures in 249 children from six meta-analyses. Although propofol resulted in a higher proportion of hypotension, without the need for interventions in addition to the infusion of small aliquots of crystalloid solution, it generated the same proportion of AEs and was as effective as procedures carried out with other drugs.
Propofol was last assessed by Cochrane in 2011,30 more specifically in NBs. As seen in Table 2, the vast majority of studies were performed after this date. This meta-analysis involved only one clinical trial with 66 newborns, and at the time the authors concluded that there was no recommendation for routine use of propofol in this population.
Scheiermann et al.31 published a meta-analysis comparing inhaled and intravenous anesthetics for pediatric surgeries. The authors state that the results may have been biased by the heterogeneity of the selected articles, and that the occurrence of postoperative nausea and vomiting was lower in the group receiving intravenous anesthesia (OR 0.68; 95 %CI: 0.48-0.98, p = 0.04). Schaefer et al.32 also evaluated 558 patients with nausea and vomiting in this scenario (due to oculovagal reflex) during strabismus surgery, in two groups – one receiving propofol as the only sedative and another receiving only preoperative antiemetic prophylaxis. There was no difference in AEs between groups.
Table 3 shows the summary of articles involving the use of propofol in children.33–59
Summary of recommendations for imaging exams- •
In pediatric patients submitted to MRI with propofol and sevoflurane, the incidence of EA is significantly lower; their use should be strongly encouraged (Level I).
- •
There is no evidence that propofol, when compared to midazolam, has a lower incidence of respiratory AEs based on airway diameter measurement (Level II).
- •
The use of propofol at doses of 1−2 mg/kg has been shown to be safe for imaging exams such as MRI and EGD, and should be strongly considered for its short duration and low prevalence of AEs (Level I).
- •
The use of propofol as a continuous infusion for procedures outside the operating room should be limited to anesthesiologists; in bolus infusions, it should be performed only by trained personnel with readily available emergency equipment (Level I).
- •
Table 4 shows the main indications for propofol use in children, with emphasis on imaging exams.38,43,60–66
Main indications of propofol use in children, with emphasis on imaging exams.
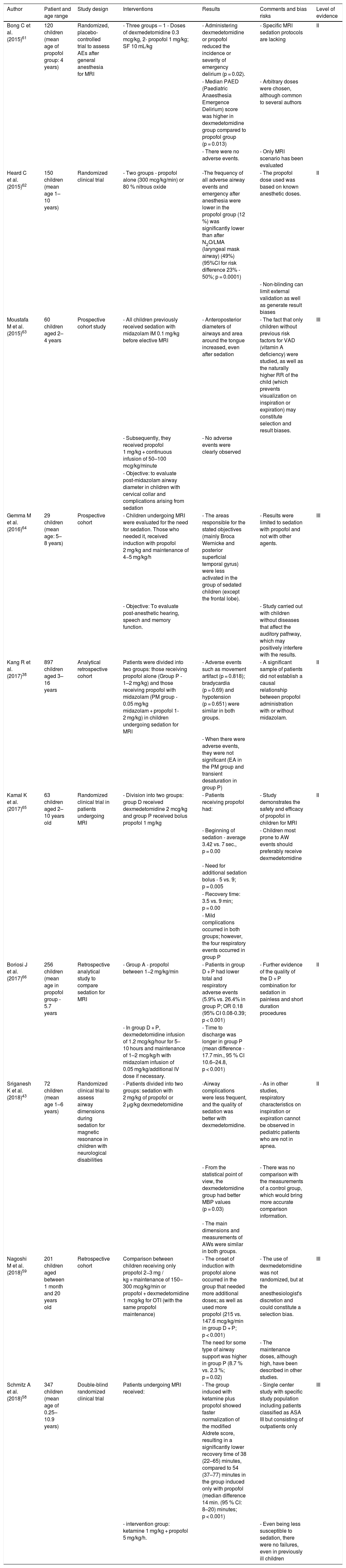
| Author | Patient and age range | Study design | Interventions | Results | Comments and bias risks | Level of evidence |
|---|---|---|---|---|---|---|
| Bong C et al. (2015)61 | 120 children (mean age of propofol group: 4 years) | Randomized, placebo-controlled trial to assess AEs after general anesthesia for MRI | - Three groups – 1 - Doses of dexmedetomidine 0.3 mcg/kg, 2- propofol 1 mg/kg; SF 10 mL/kg | - Administering dexmedetomidine or propofol reduced the incidence or severity of emergency delirium (p = 0.02). | - Specific MRI sedation protocols are lacking | II |
| - Median PAED (Paediatric Anaesthesia Emergence Delirium) score was higher in dexmedetomidine group compared to propofol group (p = 0.013) | - Arbitrary doses were chosen, although common to several authors | |||||
| - There were no adverse events. | - Only MRI scenario has been evaluated | |||||
| Heard C et al. (2015)62 | 150 children (mean age 1–10 years) | Randomized clinical trial | - Two groups - propofol alone (300 mcg/kg/min) or 80 % nitrous oxide | -The frequency of all adverse airway events and emergency after anesthesia were lower in the propofol group (12 %) was significantly lower than after N2O/LMA (laryngeal mask airway) (49%) (95%CI for risk difference 23% - 50%; p = 0.0001) | - The propofol dose used was based on known anesthetic doses. | II |
| - Non-blinding can limit external validation as well as generate result biases | ||||||
| Moustafa M et al. (2015)63 | 60 children aged 2–4 years | Prospective cohort study | - All children previously received sedation with midazolam IM 0.1 mg/kg before elective MRI | - Anteroposterior diameters of airways and area around the tongue increased, even after sedation | - The fact that only children without previous risk factors for VAD (vitamin A deficiency) were studied, as well as the naturally higher RR of the child (which prevents visualization on inspiration or expiration) may constitute selection and result biases. | III |
| - Subsequently, they received propofol 1 mg/kg + continuous infusion of 50–100 mcg/kg/minute | - No adverse events were clearly observed | |||||
| - Objective: to evaluate post-midazolam airway diameter in children with cervical collar and complications arising from sedation | ||||||
| Gemma M et al. (2016)64 | 29 children (mean age: 5–8 years) | Prospective cohort | - Children undergoing MRI were evaluated for the need for sedation. Those who needed it, received induction with propofol 2 mg/kg and maintenance of 4−5 mg/kg/h | - The areas responsible for the stated objectives (mainly Broca Wernicke and posterior superficial temporal gyrus) were less activated in the group of sedated children (except the frontal lobe). | - Results were limited to sedation with propofol and not with other agents. | III |
| - Objective: To evaluate post-anesthetic hearing, speech and memory function. | - Study carried out with children without diseases that affect the auditory pathway, which may positively interfere with the results. | |||||
| Kang R et al. (2017)38 | 897 children aged 3–16 years | Analytical retrospective cohort | Patients were divided into two groups: those receiving propofol alone (Group P - 1–2 mg/kg) and those receiving propofol with midazolam (PM group - 0.05 mg/kg midazolam + propofol 1-2 mg/kg) in children undergoing sedation for MRI | - Adverse events such as movement artifact (p = 0.818); bradycardia (p = 0.69) and hypotension (p = 0.651) were similar in both groups. | - A significant sample of patients did not establish a causal relationship between propofol administration with or without midazolam. | II |
| - When there were adverse events, they were not significant (EA in the PM group and transient desaturation in group P) | ||||||
| Kamal K et al. (2017)65 | 63 children aged 2–10 years old | Randomized clinical trial in patients undergoing MRI | - Division into two groups: group D received dexmedetomidine 2 mcg/kg and group P received bolus propofol 1 mg/kg | - Patients receiving propofol had: | - Study demonstrates the safety and efficacy of propofol in children for MRI | II |
| - Beginning of sedation - average 3.42 vs. 7 sec., p = 0.00 | - Children most prone to AW events should preferably receive dexmedetomidine | |||||
| - Need for additional sedation bolus - 5 vs. 9; p = 0.005 | ||||||
| - Recovery time: 3.5 vs. 9 min; p = 0.00 | ||||||
| - Mild complications occurred in both groups; however, the four respiratory events occurred in group P | ||||||
| Boriosi J et al. (2017)66 | 256 children (mean age in propofol group - 5.7 years | Retrospective analytical study to compare sedation for MRI | - Group A - propofol between 1−2 mg/kg/min | - Patients in group D + P had lower total and respiratory adverse events (5.9% vs. 26.4% in group P; OR 0.18 (95% CI 0.08-0.39; p < 0.001) | - Further evidence of the quality of the D + P combination for sedation in painless and short duration procedures | II |
| - In group D + P, dexmedetomidine infusion of 1.2 mcg/kg/hour for 5–10 hours and maintenance of 1–2 mcg/kg/h with midazolam infusion of 0.05 mg/kg/additional IV dose if necessary. | - Time to discharge was longer in group P (mean difference - 17.7 min., 95 % CI 10.6–24.8, p < 0.001) | |||||
| Sriganesh K et al. (2018)43 | 72 children (mean age 1–6 years) | Randomized clinical trial to assess airway dimensions during sedation for magnetic resonance in children with neurological disabilities | - Patients divided into two groups: sedation with 2 mg/kg of propofol or 2 μg/kg dexmedetomidine | -Airway complications were less frequent, and the quality of sedation was better with dexmedetomidine. | - As in other studies, respiratory characteristics on inspiration or expiration cannot be observed in pediatric patients who are not in apnea. | II |
| - From the statistical point of view, the dexmedetomidine group had better MBP values (p = 0.03) | - There was no comparison with the measurements of a control group, which would bring more accurate comparison information. | |||||
| - The main dimensions and measurements of AWs were similar in both groups. | ||||||
| Nagoshi M et al. (2018)59 | 201 children aged between 1 month and 20 years old | Retrospective cohort | Comparison between children receiving only propofol 2−3 mg / kg + maintenance of 150–300 mcg/kg/min or propofol + dexmedetomidine 1 mcg/kg for OTI (with the same propofol maintenance) | - The onset of induction with propofol alone occurred in the group that needed more additional doses; as well as used more propofol (215 vs. 147.6 mcg/kg/min in group D + P; p < 0.001) | - The use of dexmedetomidine was not randomized, but at the anesthesiologist's discretion and could constitute a selection bias. | III |
| The need for some type of airway support was higher in group P (8.7 % vs. 2.3 %; p = 0.02) | - The maintenance doses, although high, have been described in other studies. | |||||
| Schmitz A et al. (2018)58 | 347 children (mean age of 0.25–10.9 years) | Double-blind randomized clinical trial | Patients undergoing MRI received: | - The group induced with ketamine plus propofol showed faster normalization of the modified Aldrete score, resulting in a significantly lower recovery time of 38 (22–65) minutes, compared to 54 (37–77) minutes in the group induced only with propofol (median difference 14 min. (95 % CI: 8–20) minutes; p < 0.001) | - Single center study with specific study population including patients classified as ASA III but consisting of outpatients only | III |
| - intervention group: ketamine 1 mg/kg + propofol 5 mg/kg/h. | - Even being less susceptible to sedation, there were no failures, even in previously ill children |
MRI, magnetic resonance imaging; RR, respiratory rate; EA, emergency agitation; MBP, mean blood pressure; AWs, airways; OTI, orotracheal intubation.
This review has some limitations to be described. The main one among them is the enormous comprehensiveness of the subject, with many publications. It would be more concise to separate a clinical scenario, for instance, EGDs, to reduce the number of analyzed articles. However, good-quality systematic reviews in each of the analyzed scenarios have been published in large numbers. Moreover, even with the independent reading of the articles’ abstracts by the two authors, a possible selection bias of the article is established, which can make the final findings and conclusions incomplete.
ConclusionsPropofol, although an old hypnotic drug, has been studied with increasing interest in children, and several articles confirm its efficacy and safety, especially for short-term sedations and when it is not possible to stop assessing the patient’s level of consciousness for a short period of time. However, its use by a trained team can be recommended, ready to respond to the several potentially severe adverse events, especially airway emergencies. Its ideal dose, as well as the several applications, are still subjects of study and fields to be explored.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Mekitarian Filho E, Riechelmann MB. Propofol use in newborns and children: is it safe? A systematic review. J Pediatr (Rio J). 2020. https://doi.org/10.1016/j.jped.2019.08.011
Study conducted at Universidade Cidade de São Paulo (UNICID), São Paulo, SP, Brazil.