Evaluate the effect of probiotics on the symptoms, duration of disease, and the occurrence of new episodes of upper and lower respiratory infections in healthy children.
SourcesIn order to identify eligible randomized controlled trials, two reviewers accessed four electronic databases [MEDLINE/PubMed, Scopus (Elsevier), Web of Science, and Cochrane (Cochrane VHL)], as well as ClinicalTrials.gov until January 2015. Descriptors were determined by using the Medical Subject Headings tool, following the same search protocol.
Summary of the findingsStudies showed to be heterogeneous regarding strains of probiotics, the mode of administration, the time of use, and outcomes. The present review identified 11 peer-reviewed, randomized clinical trials, which analyzed a total of 2417 children up to 10 incomplete years of age. In the analysis of the studies, reduction in new episodes of disease was a favorable outcome for the use of probiotics in the treatment of respiratory infections in children. It is noteworthy that most of these studies were conducted in developed countries, with basic sanitation, health care, and strict, well-established and well-organized guidelines on the use of probiotics. Adverse effects were rarely reported, demonstrating probiotics to be safe.
ConclusionsDespite the encouraging results – reducing new episodes of respiratory infections – the authors emphasize the need for further research, especially in developing countries, where rates of respiratory infections in children are higher when compared to the high per capita-income countries identified in this review.
Avaliar o efeito do uso de probióticos na redução dos sintomas, da duração da doença e da ocorrência de novos episódios de infecções respiratórias superior e inferior em crianças saudáveis.
Fontes de dados: Com a finalidade de identificar ensaios clínicos randomizados elegíveis, dois revisores acessaram quatro bases de dados eletrônicas [Medline/PubMed, Scopus (Elsevier), Web of Science e Cochrane (The Cochrane Library)], além do ClinicalTrials.gov, até Janeiro de 2015. Foram utilizados descritores, por meio da ferramenta Medical Subject Headings, seguindo um mesmo protocolo de busca.
Síntese dos dados: Os estudos apresentaram grande heterogeneidade em relação às cepas de probióticos, à forma de administração, ao tempo de uso e aos desfechos. Identificamos 11 ensaios clínicos randomizados, revisados por pares, que analisaram um total de 2.417 crianças até 10 anos incompletos. Na análise dos estudos, redução de novos episódios de doença foi o desfecho favorável ao uso dos probióticos no tratamento das infecções respiratórias na criança. Importante salientar que essas pesquisas foram realizadas, em sua maioria, em países desenvolvidos, com condições de saneamento, de assistência à saúde e de regulamentação rigorosa ao uso de probióticos bem estabelecidos e organizados. Quanto aos efeitos adversos, pouco relatados, configuram os probióticos como seguros.
Conclusões: Apesar do resultado encorajador - redução de novos episódios de infecções respiratórias - destacamos a necessidade de pesquisas futuras, principalmente em países em desenvolvimento, onde as taxas de infecções respiratórias na criança são maiores quando comparadas aos dos países de elevada renda per capita identificados nesta revisão.
Respiratory tract infections are common in children and significantly contribute to pediatric morbidity and mortality worldwide.1 The economic and social impact of these infections is significant and constitutes an important challenge for public health, due to high costs concerning treatment, hospitalizations, school absenteeism, and loss of working days by parents and caregivers.2
The great variety of etiological agents, the inappropriate and large-scale use of antibiotics, increased bacterial resistance, and reduced availability of vaccines for most viruses and bacteria challenge the appearance of efficient and adequate therapies for the treatment of this disease.3
Since their introduction by Metchnikoff in 1907,4 probiotics have been increasingly used to benefit the human host's immune system.5 Defined by the World Health Organization (WHO) and the Food and Agriculture Organization of the United Nations (FAO) as “live microorganisms that, when administered in adequate amounts as part of food, confer beneficial effects to the host through his intestinal flora,”6 probiotics have found widespread use in the respiratory, gastrointestinal, and urogenital tracts; in allergic and autoimmune diseases; and in cancer.7–11
Recent systematic reviews and meta-analyses have reported a positive, albeit modest, effect of probiotics in respiratory tract infection prevention,12–17 but only one meta-analysis evaluated the effectiveness of probiotics on the duration of respiratory diseases in children and adults, restricted to the randomized clinical trials that used only probiotics of the Lactobacillus and Bifidobacterium genus.18
Thus, the objective of this systematic review was to explore and describe clinical trials that have as primary endpoint the effect of probiotics on the reduction, duration, and occurrence of new episodes of upper and lower respiratory infections, and as a secondary outcome, the possible adverse events due to the use of these supplements in healthy children, considering different probiotic strains.
MethodsResearch protocolThis systematic review was performed through a research protocol that was written to guide the implementation of all stages, using methodological approaches described in the Cochrane Handbook for Systematic Reviews19 and reported in accordance with the information items for systematic reviews and meta-analyses.20
Eligibility criteriaEligible studies for inclusion in this systematic review were randomized clinical trials (RCTs) of any duration (phase III studies) comparing strains of probiotics, single or combined, consumed by any form of administration, with placebo or “no treatment” in apparently healthy children (from birth to 10 incomplete years of age), who developed acute upper or lower respiratory infection at some point during the study. Open or blind trials were eligible, provided that patients were randomized.
The probiotic strains could be administered at any dose, whether or not combined with other functional ingredients (such as prebiotics and vitamins) or antibiotics, provided that the comparison included the same products, so that the overall effect could be attributed to the used probiotics. To be eligible for inclusion, studies had to be published in Portuguese, English, or Spanish languages and the results had to show one or more than one of the study objectives: decrease in disease symptoms, decrease in the duration, decrease of occurrence of new episodes, and the presence of any adverse event.
Exclusion criteria were: clinical trials with follow-up losses >20%; animal studies; studies on respiratory infection prevention; studies in children that had some type of acquired or congenital immune deficiency, or chronic illness; publications such as comments, editorials, or letters; studies with results from other affected organs other than the respiratory tract; duplicated studies, annals of congresses, inappropriate study designs (for instance: observational studies, non-randomized studies) and studies in languages other than those previously mentioned. Each identified article was initially analyzed by title and abstract, and the eligible articles were selected for full reading.
Definitions of search termsInitially, the following terms and keywords were used: (probiotics) AND (respiratory tract infections) AND (infant) AND (children), with the following definitions: probiotics – all strains of bacteria and/or yeast potentially beneficial to the host, administered by any vehicle; respiratory tract infections – upper (common cold, otitis media, pharyngitis, and sinusitis) and lower (bronchitis and pneumonia); infant AND children – all children from birth to 10 years of age, characterizing the exclusion of adolescents.
Study research strategyThe electronic search was carried out in January 2015 in the following databases: MEDLINE/PubMed, Scopus (Elsevier), Web of Science (Thomson Reuters Scientific), and Cochrane VHL, with search strategies adapted to each database:
MEDLINE/PubMed(“probiotics”[MeSH Terms] OR “probiotics”[All Fields]) AND (“respiratory tract infections”[MeSH Terms] OR (“respiratory”[All Fields] AND “tract”[All Fields] AND “infections”[All Fields]) OR “respiratory tract infections”[All Fields]) AND (“infant”[MeSH Terms] OR “infant”[All Fields]) AND (“child”[MeSH Terms] OR “child”[All Fields] OR “children”[All Fields]).
Subsequently, to detail respiratory tract infections: (“probiotics”[MeSH Terms] OR “probiotics”[All Fields]) AND (“common cold”[MeSH Terms] OR (“common”[All Fields] AND “cold”[All Fields]) OR “common cold”[All Fields]) AND (“child”[MeSH Terms] OR “child”[All Fields] OR “children”[All Fields]). The search for the other terms was carried out by sequentially substituting the word “common cold” with “otitis media,” “sinusitis,” “pharyngitis,” “bronchitis,” and “pneumonia.”
Scopus (Elsevier)(“Probiotics” AND “respiratory tract infections” AND “infant” AND “children”), followed by sequentially substituting “respiratory tract infections” by “common cold,” “otitis media,” “sinusitis,” “pharyngitis,” “bronchitis,” and “pneumonia.”
Web of Science (Thomson Reuters Scientific)Articles were selected from 1945 to January 2015 using the following search strategy: (Probiotics* AND respiratory tract infections* AND infant* AND Children*), followed by sequentially substituting respiratory tract infections* with common cold*, otitis media*, sinusitis*, pharyngitis*, bronchitis*, and pneumonia*.
Cochrane VHL(Probiotics and respiratory tract infections and infant AND children) followed by sequentially substituting respiratory tract infections with common cold, otitis media, sinusitis, pharyngitis, bronchitis, and pneumonia.
A total of 52 searches were performed in the databases, thirteen in each, using the terms separately and sequentially, for better accuracy and precision.
Data extractionTwo stages of the process were used to identify and select studies: first, two reviewers (GVA and MHO) independently identified the titles and abstracts of each study to assess whether they met the inclusion criteria. Second, the selected articles were obtained as full-text versions and then were independently reviewed, to determine the inclusion and exclusion criteria. Any discrepancies were resolved by consensus and/or consulting a third reviewer. Whenever possible, the authors were contacted by e-mail, in case of doubt, in the absence of specific data, or to obtain additional information.
Information on ongoing or recently completed studies, investigation, and original research reported in the gray literature was obtained through research on trial registries (ClinicalTrials.gov) and the main conference proceedings were selected (three years before the research date).
Additionally, a manual search was performed through the references of the pre-selected studies and reviews published on the subject.
The following data were collected: characteristics of included studies, such as clinical condition, intervention, and comparison details; risk of bias assessment; and quality criteria of the selected studies. The decrease in symptoms, duration of disease episodes, and the possibility of reducing new episodes of respiratory tract diseases were analyzed as primary outcomes; whether the use of probiotics triggered an adverse event was analyzed as a secondary outcome.
Quality assessment and risk of biasThe studies were also evaluated for the overall risk of bias (low, high, or unclear) based on Cochrane Collaboration's risk-of-bias tool.21 For the purpose of this review, a study was considered as having a “low risk of bias” when all major quality criteria (i.e., randomization method, allocation concealment, and masking/blinding), as well as other additional criteria (similarity between the intervention and comparison groups, withdrawal of patients from studies, and intention-to-treat analysis) were adequately met; an “unclear risk of bias” when most of the key criteria were not reported or were not clear; and a “high risk of bias” when one or more of the main criteria were not properly met. The “some risk of bias” category was attributed when all aspects of the key criteria were adequate, but (1) an intention-to-treat analysis was not performed and when a criterion was not met, or (2) when two key criteria were adequate, but the intention-to-treat analysis was not performed.
When analyzing the quality of the randomized studies, this review used GRADE (Grading of Recommendations Assessment, Development, and Evaluation) recommendations.22 Due to the great heterogeneity of the clinical trials, study data were assessed qualitatively, without the use of meta-analysis, following the PRISMA (Preferred Reporting Items for systematic Reviews and Meta-Analyses) guidelines.23 To avoid publication bias, unpublished studies were identified, but did not meet the inclusion criteria of this systematic review.
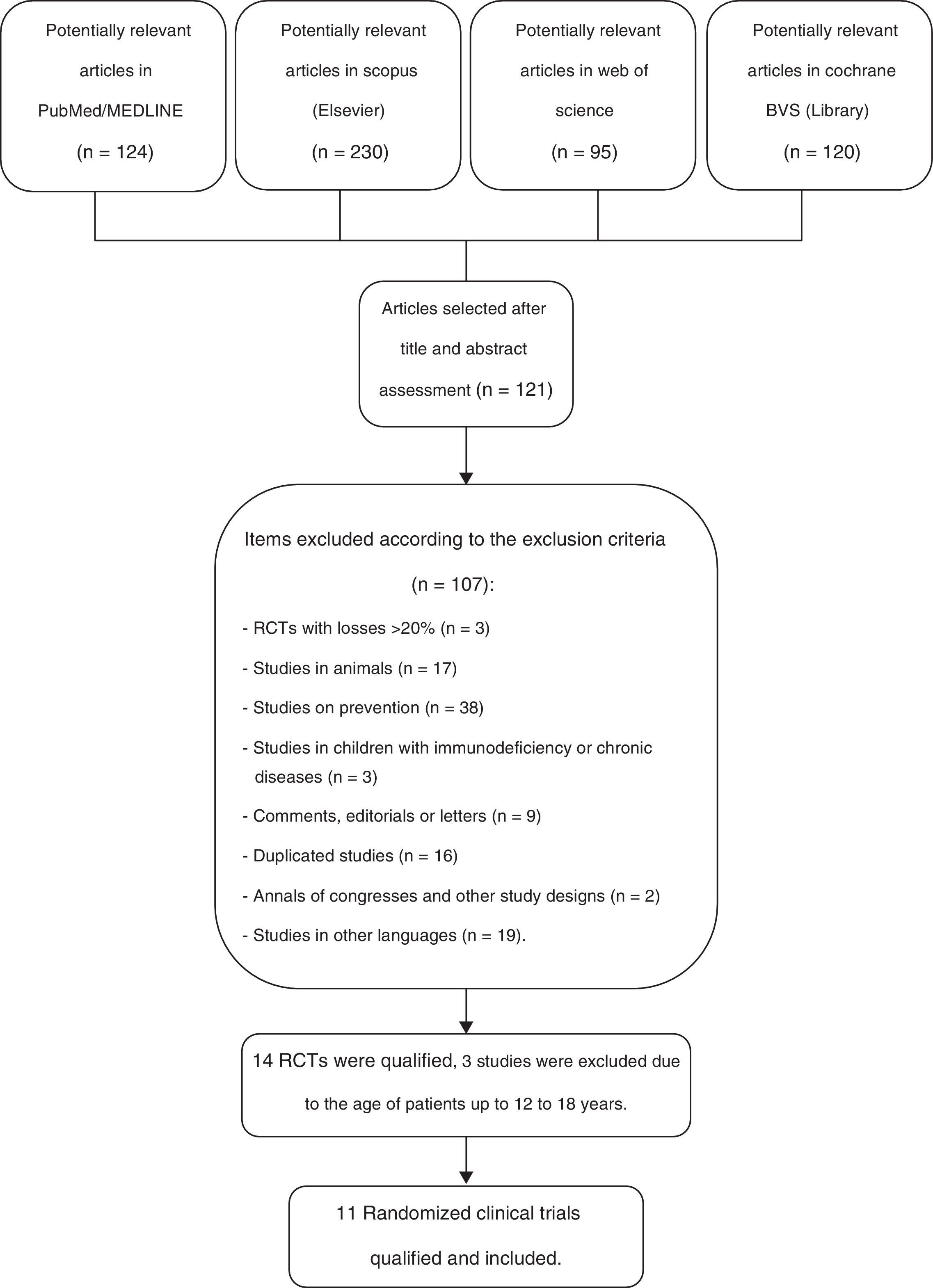
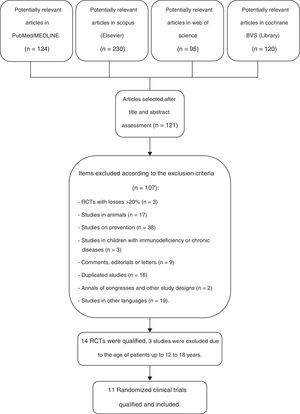
ResultsOf a total number of 569 citations identified in the four major electronic databases (PubMed/MEDLINE, Scopus [Elsevier], Web of Science, and Cochrane VHL), 11 peer-reviewed, randomized controlled trials (RCTs) were included, which analyzed a total of 2417 children from birth to 10 incomplete years of age. The inclusion criteria were met by eleven RCTs, which were used to identify the primary and secondary outcomes of this systematic review. The study selection process is shown in Fig. 1.
Flow diagram of the selection process of randomized clinical trials for inclusion in the systematic review.23
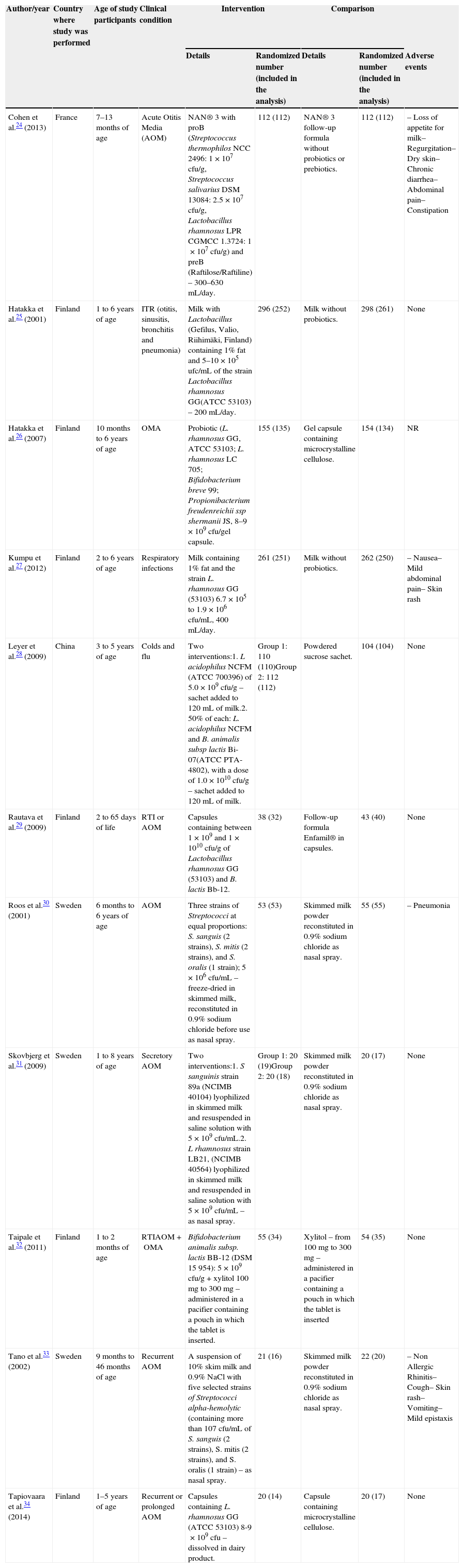
Eleven RCTs were analyzed for the author/year, country of study, age of participants, clinical condition, intervention and comparison details, randomized numbers, and numbers included in the analysis and adverse events, as described in Table 1. The following clinical trials were included in this systematic review: Cohen et al.,24 Hatakka et al.,25 Hatakka et al.,26 Kumpu et al.,27 Leyer et al.,28 Rautava et al.,29 Roos et al.,30 Skovbjerg et al.,31 Taipale et al.,32 Tano et al.,33 and Tapiovaara et al.,34 which were performed in their totality in two countries: Finland and Sweden, whereas the other trials were performed in two different countries: China and France. The duration of treatment with probiotics ranged from ten days to 12 months, although most trials were performed for approximately six to seven months during the winter months.
Characteristics of randomized clinical trials and adverse events.
| Author/year | Country where study was performed | Age of study participants | Clinical condition | Intervention | Comparison | |||
|---|---|---|---|---|---|---|---|---|
| Details | Randomized number (included in the analysis) | Details | Randomized number (included in the analysis) | Adverse events | ||||
| Cohen et al.24 (2013) | France | 7–13 months of age | Acute Otitis Media (AOM) | NAN® 3 with proB (Streptococcus thermophilos NCC 2496: 1×107cfu/g, Streptococcus salivarius DSM 13084: 2.5×107cfu/g, Lactobacillus rhamnosus LPR CGMCC 1.3724: 1×107cfu/g) and preB (Raftilose/Raftiline) – 300–630mL/day. | 112 (112) | NAN® 3 follow-up formula without probiotics or prebiotics. | 112 (112) | – Loss of appetite for milk– Regurgitation– Dry skin– Chronic diarrhea– Abdominal pain– Constipation |
| Hatakka et al.25 (2001) | Finland | 1 to 6 years of age | ITR (otitis, sinusitis, bronchitis and pneumonia) | Milk with Lactobacillus (Gefilus, Valio, Riihimäki, Finland) containing 1% fat and 5–10×105ufc/mL of the strain Lactobacillus rhamnosus GG(ATCC 53103) – 200mL/day. | 296 (252) | Milk without probiotics. | 298 (261) | None |
| Hatakka et al.26 (2007) | Finland | 10 months to 6 years of age | OMA | Probiotic (L. rhamnosus GG, ATCC 53103; L. rhamnosus LC 705; Bifidobacterium breve 99; Propionibacterium freudenreichii ssp shermanii JS, 8–9×109cfu/gel capsule. | 155 (135) | Gel capsule containing microcrystalline cellulose. | 154 (134) | NR |
| Kumpu et al.27 (2012) | Finland | 2 to 6 years of age | Respiratory infections | Milk containing 1% fat and the strain L. rhamnosus GG (53103) 6.7×105 to 1.9×106cfu/mL, 400mL/day. | 261 (251) | Milk without probiotics. | 262 (250) | – Nausea– Mild abdominal pain– Skin rash |
| Leyer et al.28 (2009) | China | 3 to 5 years of age | Colds and flu | Two interventions:1. L acidophilus NCFM (ATCC 700396) of 5.0×109cfu/g – sachet added to 120mL of milk.2. 50% of each: L. acidophilus NCFM and B. animalis subsp lactis Bi-07(ATCC PTA-4802), with a dose of 1.0×1010cfu/g – sachet added to 120mL of milk. | Group 1: 110 (110)Group 2: 112 (112) | Powdered sucrose sachet. | 104 (104) | None |
| Rautava et al.29 (2009) | Finland | 2 to 65 days of life | RTI or AOM | Capsules containing between 1×109 and 1×1010cfu/g of Lactobacillus rhamnosus GG (53103) and B. lactis Bb-12. | 38 (32) | Follow-up formula Enfamil® in capsules. | 43 (40) | None |
| Roos et al.30 (2001) | Sweden | 6 months to 6 years of age | AOM | Three strains of Streptococci at equal proportions: S. sanguis (2 strains), S. mitis (2 strains), and S. oralis (1 strain); 5×106cfu/mL – freeze-dried in skimmed milk, reconstituted in 0.9% sodium chloride before use as nasal spray. | 53 (53) | Skimmed milk powder reconstituted in 0.9% sodium chloride as nasal spray. | 55 (55) | – Pneumonia |
| Skovbjerg et al.31 (2009) | Sweden | 1 to 8 years of age | Secretory AOM | Two interventions:1. S sanguinis strain 89a (NCIMB 40104) lyophilized in skimmed milk and resuspended in saline solution with 5×109cfu/mL.2. L rhamnosus strain LB21, (NCIMB 40564) lyophilized in skimmed milk and resuspended in saline solution with 5×109cfu/mL – as nasal spray. | Group 1: 20 (19)Group 2: 20 (18) | Skimmed milk powder reconstituted in 0.9% sodium chloride as nasal spray. | 20 (17) | None |
| Taipale et al.32 (2011) | Finland | 1 to 2 months of age | RTIAOM+OMA | Bifidobacterium animalis subsp. lactis BB-12 (DSM 15 954): 5×109cfu/g+xylitol 100mg to 300mg – administered in a pacifier containing a pouch in which the tablet is inserted. | 55 (34) | Xylitol – from 100mg to 300mg – administered in a pacifier containing a pouch in which the tablet is inserted | 54 (35) | None |
| Tano et al.33 (2002) | Sweden | 9 months to 46 months of age | Recurrent AOM | A suspension of 10% skim milk and 0.9% NaCl with five selected strains of Streptococci alpha-hemolytic (containing more than 107cfu/mL of S. sanguis (2 strains), S. mitis (2 strains), and S. oralis (1 strain) – as nasal spray. | 21 (16) | Skimmed milk powder reconstituted in 0.9% sodium chloride as nasal spray. | 22 (20) | – Non Allergic Rhinitis– Cough– Skin rash– Vomiting– Mild epistaxis |
| Tapiovaara et al.34 (2014) | Finland | 1–5 years of age | Recurrent or prolonged AOM | Capsules containing L. rhamnosus GG (ATCC 53103) 8-9×109cfu – dissolved in dairy product. | 20 (14) | Capsule containing microcrystalline cellulose. | 20 (17) | None |
RTI, respiratory tract infection; NR, not reported; AOM, acute otitis media; cfu, colony-forming unit.
NAN 3®, Enfamil® are follow-on milks suitable for infants.
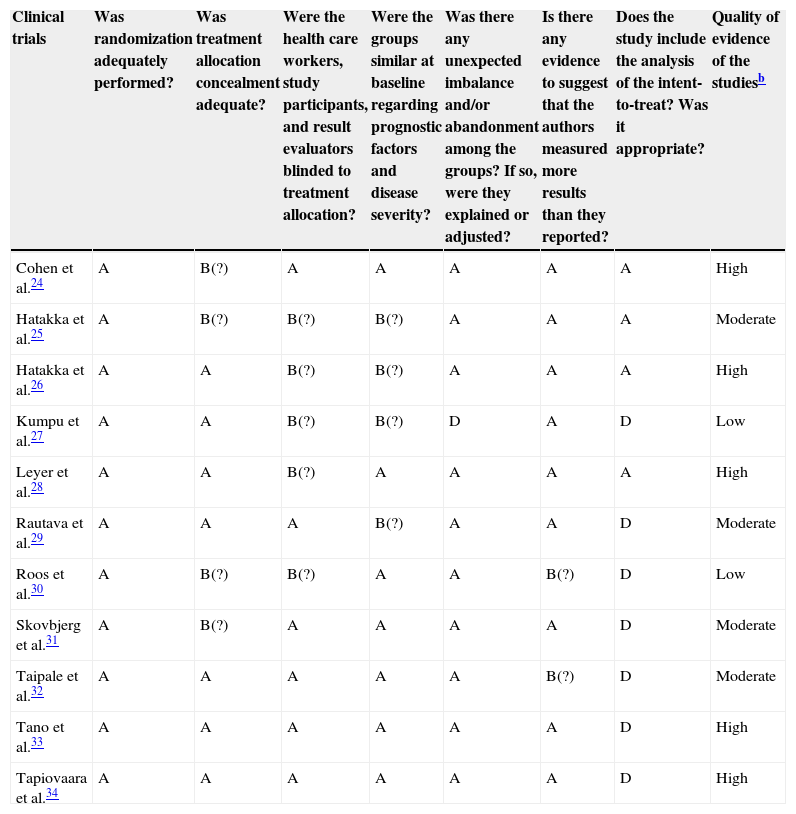
Study quality assessment is summarized in Table 2, showing that all trials used correct randomization methods, such as a randomization list generated by computer or by a random number. Appropriate allocation concealment was reported by most studies, including the use of sealed envelopes,24,25,27,30,31,33 and/or use of encoded containers/packages that were identical in appearance.26,28,29,32,34 Patients were included sequentially, according to the randomization list, and blinding was correctly performed in seven trials.26–29,32–34 Among the eleven trials that were identified as double-blinded, detailed descriptions of the blinding methods were provided by six RCTs.24,29,31–34
Biasa risk and quality criteria assessment in selected studies.
| Clinical trials | Was randomization adequately performed? | Was treatment allocation concealment adequate? | Were the health care workers, study participants, and result evaluators blinded to treatment allocation? | Were the groups similar at baseline regarding prognostic factors and disease severity? | Was there any unexpected imbalance and/or abandonment among the groups? If so, were they explained or adjusted? | Is there any evidence to suggest that the authors measured more results than they reported? | Does the study include the analysis of the intent-to-treat? Was it appropriate? | Quality of evidence of the studiesb |
|---|---|---|---|---|---|---|---|---|
| Cohen et al.24 | A | B(?) | A | A | A | A | A | High |
| Hatakka et al.25 | A | B(?) | B(?) | B(?) | A | A | A | Moderate |
| Hatakka et al.26 | A | A | B(?) | B(?) | A | A | A | High |
| Kumpu et al.27 | A | A | B(?) | B(?) | D | A | D | Low |
| Leyer et al.28 | A | A | B(?) | A | A | A | A | High |
| Rautava et al.29 | A | A | A | B(?) | A | A | D | Moderate |
| Roos et al.30 | A | B(?) | B(?) | A | A | B(?) | D | Low |
| Skovbjerg et al.31 | A | B(?) | A | A | A | A | D | Moderate |
| Taipale et al.32 | A | A | A | A | A | B(?) | D | Moderate |
| Tano et al.33 | A | A | A | A | A | A | D | High |
| Tapiovaara et al.34 | A | A | A | A | A | A | D | High |
Cochrane risk-of-bias tool was used to assess the risk of bias for each study - A, low risk; B, low risk with some areas of uncertainty (?); C, high risk; D, unclear risk.
GRADE – High quality, it is very unlikely that confidence in the estimate will change; Moderate, Further research is likely to have a significant impact on the confidence of the effect; Low, Further research is likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate; Very low, There is a very high level of uncertainty regarding the estimate.
In general, the eleven clinical trials were considered as having a “low” risk of bias, with a few studies showing allocation concealment and blinding with “low” risk with some areas of uncertainty. As all quality criteria were well-indicated, some studies showed that the intent-to-treat analysis had an unclear risk, either because it was not performed or because it was not mentioned in more than half of the trials.27,29–34 No trial showed “high” risk of bias in the main analyzed criteria.
In the clinical trials including “common cold,” “flu,” “respiratory tract infections,” and “acute otitis media,” all authors of the studies reported clear descriptions of the signs, symptoms, and diagnoses of these conditions. In six of the trials,24–26,28,32,34 a physician confirmed the diagnosis of infection, and in the other five trials,27,29–31,33 the signs and/or symptoms were reported by the participants in a diary, with the diagnosis confirmed by a study investigator's opinion.
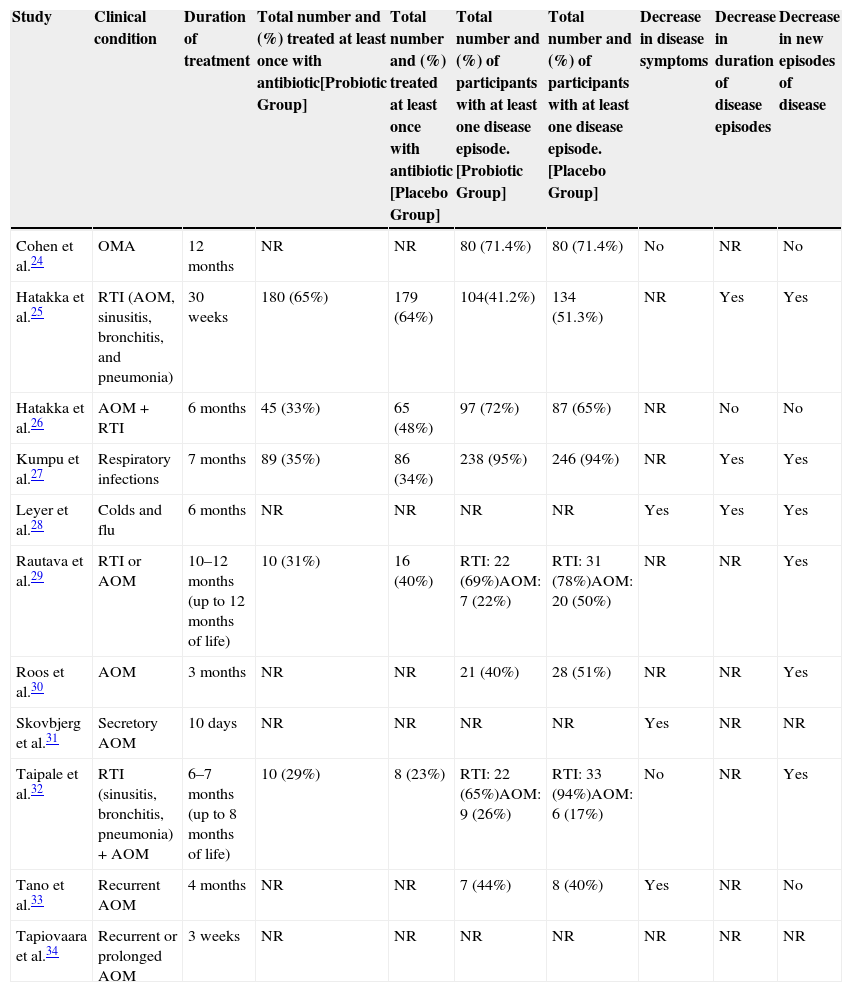
In the included trials, three main outcomes were reported: decrease in disease symptoms, decrease in the duration of disease episodes, and decrease in new disease episodes. The outcomes of studies in children are reported in Table 3, which also shows the total number and percentage of patients that used antibiotics during the study, both in the probiotic group and in the placebo groups, and the total number and percentage of patients that had at least one disease episode.
Results of the primary outcomes of the studies.
| Study | Clinical condition | Duration of treatment | Total number and (%) treated at least once with antibiotic[Probiotic Group] | Total number and (%) treated at least once with antibiotic [Placebo Group] | Total number and (%) of participants with at least one disease episode. [Probiotic Group] | Total number and (%) of participants with at least one disease episode. [Placebo Group] | Decrease in disease symptoms | Decrease in duration of disease episodes | Decrease in new episodes of disease |
|---|---|---|---|---|---|---|---|---|---|
| Cohen et al.24 | OMA | 12 months | NR | NR | 80 (71.4%) | 80 (71.4%) | No | NR | No |
| Hatakka et al.25 | RTI (AOM, sinusitis, bronchitis, and pneumonia) | 30 weeks | 180 (65%) | 179 (64%) | 104(41.2%) | 134 (51.3%) | NR | Yes | Yes |
| Hatakka et al.26 | AOM+RTI | 6 months | 45 (33%) | 65 (48%) | 97 (72%) | 87 (65%) | NR | No | No |
| Kumpu et al.27 | Respiratory infections | 7 months | 89 (35%) | 86 (34%) | 238 (95%) | 246 (94%) | NR | Yes | Yes |
| Leyer et al.28 | Colds and flu | 6 months | NR | NR | NR | NR | Yes | Yes | Yes |
| Rautava et al.29 | RTI or AOM | 10–12 months (up to 12 months of life) | 10 (31%) | 16 (40%) | RTI: 22 (69%)AOM: 7 (22%) | RTI: 31 (78%)AOM: 20 (50%) | NR | NR | Yes |
| Roos et al.30 | AOM | 3 months | NR | NR | 21 (40%) | 28 (51%) | NR | NR | Yes |
| Skovbjerg et al.31 | Secretory AOM | 10 days | NR | NR | NR | NR | Yes | NR | NR |
| Taipale et al.32 | RTI (sinusitis, bronchitis, pneumonia)+AOM | 6–7 months (up to 8 months of life) | 10 (29%) | 8 (23%) | RTI: 22 (65%)AOM: 9 (26%) | RTI: 33 (94%)AOM: 6 (17%) | No | NR | Yes |
| Tano et al.33 | Recurrent AOM | 4 months | NR | NR | 7 (44%) | 8 (40%) | Yes | NR | No |
| Tapiovaara et al.34 | Recurrent or prolonged AOM | 3 weeks | NR | NR | NR | NR | NR | NR | NR |
RTI, respiratory tract infection; NR, not reported; AOM, acute otitis media.
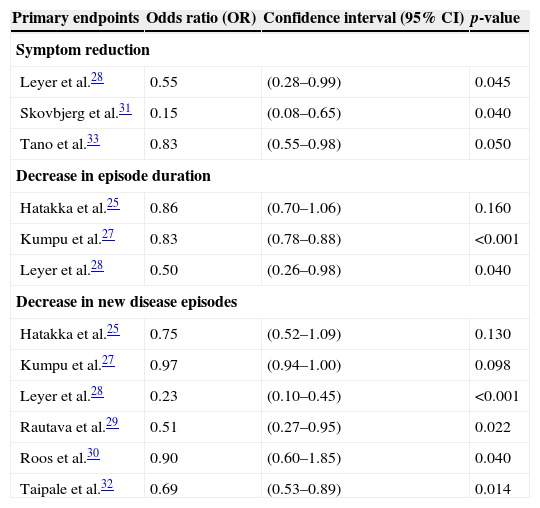
For better assessment of the primary outcomes, Table 4 adds values of odds ratios (OR), 95% confidence intervals (95% CI), and p-values extracted from the selected articles that reflect the positive results of the probiotic groups when compared to placebo groups in the randomized clinical trials.
Values of the association measures of positive primary endpoints in the probiotic groups of the selected studies.
| Primary endpoints | Odds ratio (OR) | Confidence interval (95% CI) | p-value |
|---|---|---|---|
| Symptom reduction | |||
| Leyer et al.28 | 0.55 | (0.28–0.99) | 0.045 |
| Skovbjerg et al.31 | 0.15 | (0.08–0.65) | 0.040 |
| Tano et al.33 | 0.83 | (0.55–0.98) | 0.050 |
| Decrease in episode duration | |||
| Hatakka et al.25 | 0.86 | (0.70–1.06) | 0.160 |
| Kumpu et al.27 | 0.83 | (0.78–0.88) | <0.001 |
| Leyer et al.28 | 0.50 | (0.26–0.98) | 0.040 |
| Decrease in new disease episodes | |||
| Hatakka et al.25 | 0.75 | (0.52–1.09) | 0.130 |
| Kumpu et al.27 | 0.97 | (0.94–1.00) | 0.098 |
| Leyer et al.28 | 0.23 | (0.10–0.45) | <0.001 |
| Rautava et al.29 | 0.51 | (0.27–0.95) | 0.022 |
| Roos et al.30 | 0.90 | (0.60–1.85) | 0.040 |
| Taipale et al.32 | 0.69 | (0.53–0.89) | 0.014 |
Among the included studies, five RCTs24,28,31–33 reported on the occurrence of disease symptom reduction, defined as the reduction of signs and symptoms or persistence with no improvement of the disease, observed by the physician involved in the research or another doctor involved in the child's care, and based upon the patient's health diary, filled out by parents or caregivers. Data were categorized as “yes” when there was a decrease in signs and symptoms, usually shown as percentages in the studies, “no” when the perception of doctors or parents and caregivers did not identify improvement in disease presentation patterns, and “not reported” (NR) when the study did not describe this outcome.
Considering that all studies were randomized in 1:1 ratio in the probiotic and placebo groups, the numbers of participants are similar and, therefore, the perception of symptom improvement approximately reflects the differences in results in each group, considering that all had the same eligibility criteria defined in each study. The systematic review showed that the probiotic group of three RCTs had disease symptom reduction.28,31,33
In the study by Leyer et al.,28 in the single or combined probiotic groups, there was a reduction of fever of 53.0% and 72.7%, of cough of 41.4% and 62.1%, and of rhinorrhea of 28.2% and 58.8%, respectively, when compared to the placebo group. In the study by Skovbjerg et al.,31 using probiotic spray, the probiotic group had less fluid and more air in the middle ear, i.e., signs of improvement or healing in 36.8% vs. 5.8% in the placebo group. In the study by Tano et al.,33 which also used probiotic spray, there was a 10% reduction in otalgia and 12% reduction in middle ear secretion in the probiotic group, when compared to placebo.
When assessing the criterion symptom duration, most RCTs25–27,29,30,34 did not report data that could be used in the present review and two studies (Cohen et al. and Taipale et al.)24,32 showed no difference in symptom reduction in the probiotic and placebo groups.
Evaluation of disease episode durationThis was defined as the total sum of disease episode duration (in days) divided by the total number of disease episodes experienced by the study participants. The results showed that only three studies25,27,28 included this outcome in the analysis, as seven RCTs did not report this outcome.24,29–34 In the study by Hatakka et al.,25 the duration of the episodes was 4.9 days (95% CI: 4.4–5.5) vs. 5.8 days (95% CI: 5.3–6.4) in the probiotic and placebo groups, respectively; in the study by Kumpu et al.,27 it was 4.7 days (95% CI: 4.5–4.9) vs. 5.6 days (95% CI: 5.4–5.9), respectively; and in the study by Leyer et al.,28 the decrease was 32% in the probiotic group with single strain and 48% in the probiotic group with combined strains, when compared to the placebo group. Only one study, by Hatakka et al.,26 showed that the difference regarding the duration of acute otitis media (AOM) episodes was 5.6 days (95% CI: 3.5–9.4) vs. 6.0 days (95% CI: 4.0–10.5) in the probiotic and placebo groups, respectively, which did not reach statistical significance.
Assessment of the decrease in new episodes of the diseaseThis was characterized as “yes” when there was a decrease in new episodes of the disease or reduction in disease incidence and “no” when there was no statistical significance. Among the studies included in this outcome, six RCTs showed25,27–30,32 in their results that the probiotic group favored the decrease in new episodes of the disease when statistically compared to placebo. Two studies (Skovbjerg et al. and Tapiovaara et al.)31,34 did not report these data in their conclusions and three studies24,26,33 showed that the probiotic and placebo groups did not differ in the decrease of occurrence of new disease episodes.
When analyzing the need for antibiotic use during the occurrence of assessed bacterial diseases, five RCTs25–27,29,32 described the total number and percentage of patients treated at least once with antibiotics in the probiotic and placebo groups. In two studies (Hatakka et al. and Kumpu et al.),25,27 there was no difference between groups, in two other studies (Hatakka et al. and Rautava et al.),26,29 antibiotic prescription was more often observed in the placebo group; six studies did not report this outcome.24,26,30,31,33,34
In addition to the five trials that showed more frequent use of antibiotics in the placebo group, a study32 that compared Bifidobacterium animalis subsp. lactis BB-12 administered as tablets inserted into the pacifier with a placebo, in 69 children aged 1–2 months of age in Finland, showed that antibiotic use was increased in the probiotic group, with ten patients (29%), rather than in the placebo group, with eight patients (23%). The authors reported that this difference can be attributed to the fact that exclusive breastfeeding was higher in the placebo group than in the probiotic group, resulting in greater protection against the risk of respiratory infections. However, this result should be considered with some reserve.
Adverse eventsProbiotic administration appears to have a good safety profile, as most RCTs25,28,29,31,32,34 did not identify any adverse events and there was only one study with no report.26 Two clinical trials24,27 showed mild adverse events, such as loss of appetite for milk, regurgitation, dry skin, occasional abdominal pain, diarrhea, nausea, rash, and constipation. Two other studies30,33 that used the intervention and comparison with nasal spray showed one case that had pneumonia in the placebo group and was encouraged to discontinue treatment30; and in another study, the placebo and treatment groups were mildly affected by rhinitis, cough, rash, vomiting, and epistaxis, which may be inherent to the form and technique of the device used.33
DiscussionThis review identified a number of randomized controlled trials (RCTs), most of moderate-to-high quality, which evaluated the use of probiotics in upper and lower respiratory tract infections in children. The presentation, the doses, the different strains, the different mechanisms, and the time of probiotic administration caused these studies to display great heterogeneity and alterations in the sensitivity analysis, making it difficult to perform a concomitant meta-analysis. Analyzing the primary outcomes of this review regarding symptom reduction, time of disease duration, and of new episodes of the disease, the latter was shown to be the objective of most RCTs, demonstrating that in six studies there was a reduction of new episodes of respiratory infections, three others found no difference in results, and two did not report this outcome. It was also found that a small number of clinical trials showed adverse events with the use of probiotics, with mild cases that did not require hospital treatment.
Considering the values of the association measures of positive primary outcomes, this review shows that regarding symptom reduction, three clinical trials28,31,33 showed a trend toward statistical significance, with p-values close to 0.05, despite a greater confidence interval amplitude (95% CI) observed in studies by Leyer et al.28 and Skovbjerg et al.31 In the analysis of the reduction in disease episode duration, only the study by Kumpu et al.27 showed statistical significance; the study by Leyer et al.,28 although with a significant p-value of 0.04, demonstrated a wide 95% CI. Regarding the decrease of new disease episodes, of the six clinical trials,25,27–30,32 the studies by Leyer et al.28 and Taipale et al.32 showed significant p-values and confidence intervals, two other studies25,27 did not show significant data, and the studies by Rautava et al.29 and Roos et al.30 showed higher amplitude of the confidence interval, although the p-values had statistical significance.
Regarding the understanding of the term respiratory infection, it generally refers to upper and lower respiratory tract infections; however, term definitions showed variations between studies. In the study by Hatakka et al.,25 acute otitis media and sinusitis were reported as upper respiratory infections, whereas acute bronchitis and pneumonia were reported as lower respiratory infections. In another study, Kumpu et al.27 considered sinusitis, otitis, common cold, pneumonia, and bronchitis as respiratory infections, without specifying the frequency of each occurrence separately.
In many countries, children experience three to six episodes of respiratory infections per year and 40% of them may even suffer at least one episode of acute otitis media, which is one of the most common bacterial infections and complications, and one of the main reasons to treat individuals with antibiotics during early childhood.35,36 Thus, a decrease in new episodes of respiratory infections with shorter duration and symptom reduction could be of great clinical importance, with great impact on public health and positive economic consequences, particularly in developing countries.
Probiotics are live microorganisms offered as nutritional supplements that act in the host organism's intestine by regulating the intestinal flora or modulating the microbiota in other segments of the human body.37 Thus, they act by improving local and systemic immunity, competing with pathogens invading the local integrity and restoring the microorganisms that provide safety and maintenance of the individual's health. Many studies have shown the real benefits and safety of probiotic use in childhood,38–40 currently classified as belonging to the category “Generally Recognized as Safe” (GRAS) for consumption, as classified by the Food and Drug Administration (FDA), and routinely included in children's formula in some developed countries.41
Considering the complex results on probiotic use indicated in the scientific literature, it is emphasized that different mechanisms of action are expected in the human body: (a) microbiological functionality (due to competitive exclusion or active reduction of pathogens through the production of short chain fatty acids and organic acids, and production of bacteriocins and reactive oxygen species such as hydrogen peroxide) aiming to stabilize or improve microbial homeostasis in an area of the body and reduce the invasion and colonization by pathogens; (b) nutritional functionality (through the production of vitamins that act throughout the human host's organism); (c) physiological functionality (through improvement of intestinal transit and rheological properties of respiratory secretions), and d] immunological functionality (through the production of cytokines – interleukin (IL)-10 and interferon (INF)-γ, which beneficially modulate immunity in the respiratory mucosa).42,43
Through pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) and NOD-like receptors (NLRs), pathogen-associated molecular patterns (PAMPs) generate immune responses in dendritic cells, especially in Th1 or Regulatory T-cells (Treg), with production of IL-12 and IL-10, respectively, which have immune protection functions against viruses and bacteria and include tolerogenic functions, so there is no injury to the human host.44–46
Recently, members of the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN)47 and the American Academy of Pediatrics (AAP)48 reviewed the evidence for the use of probiotics in infants and children, and concluded that the probiotic formulas offered as supplements to healthy infants raised no safety concerns regarding the growth in stature and adverse effects. However, they did not observe data related to the safety of probiotic use in the long term, and did not identify homogeneity of doses, strains, and the time of use in RCTs.
Performing a search of all systematic reviews and meta-analyses in the literature related to the use of probiotics and respiratory tract infections, it was found that there were six systematic reviews targeted for prevention,12–17 which were very heterogeneous regarding the study population (children and adults), the assessed respiratory segment (upper and lower respiratory tract infections), and the type and strains of assessed probiotics.
Of these reviews, the study by Saeterdal et al.16 is a summary of the findings of the review by Hao et al.15 detailing the article tables that were not adequately analyzed. Three studies performed meta-analyses,12,13,15 identifying the beneficial effects of probiotics exclusively for the prevention of respiratory infections.
Among the reviews on prevention, Kang et al.12 concluded, based on the assessment of ten clinical trials in individuals of all ages, that there is a modest effect of probiotics on the prevention of common colds; Liu et al.13 analyzing four RCTs using only one probiotic strain, concluded that the administration of Lactobacillus rhamnosus GG has the potential to reduce the incidence of acute otitis media, upper respiratory infections, and antibiotic use; in the review by Vouloumanou et al.14 when assessing 14 RCTs, they concluded that probiotics may have a beneficial effect on symptom severity and duration, but do not seem to reduce the incidence of respiratory infections; the meta-analysis by Hao et al.15 also with 14 RCTs evaluating individuals of all ages, concluded that probiotics were better than placebo in reducing episodes of upper respiratory infections and antibiotic use.
In the recent study by Ozen et al.17 analyzing 14 RCTs performed in the pediatric population, they concluded that a minimum reduction of 5–10% in the incidence of upper airway infections would have a significant clinical and economic impact.
In the only meta-analysis that evaluated probiotics exclusively for the treatment of respiratory infections, King et al.18 included children aged 1–12 years, in addition to adults and the elderly, with this review analyzing studies with only two strains of probiotics. There were twenty RCTs, of which ten studies were conducted in children, and the results were evaluated in a generalized way, with a reduction of one day in disease duration. No comments from previous systematic reviews – exclusively related to the treatment of respiratory infections in children – provided summarized data on the reduction of disease symptoms, reduction in the duration of episodes, and new episodes of respiratory infections, whereas this review provides new evidence for these outcomes.
This review aimed to assess the best currently available evidence in the literature in order to elucidate the benefits of probiotics in the treatment of respiratory infections in healthy children. It included controlled and randomized clinical trials with well-defined protocols, while attempting to control for possible biases as much as possible. Quality of the studies was assessed using Cochrane Collaboration's risk-of-bias tool and GRADE,21,22 currently considered a more appropriate and accurate tool that the Jadad scale.49
In spite of the care taken in constructing this systematic review, some limitations have been identified: first, three clinical trials registered in ClinicalTrials.gov, including approximately 650 patients, are still in the ongoing phase and could not be included in the evaluation due to lack of conclusive data. They will be analyzed in a future update and thus will help identify the actual benefits obtained so far; second, while most of the study authors reported clear descriptions of signs and symptoms, diagnosis confirmed by a doctor was attained in only half of the trials. It is possible that acute infections may have been underdiagnosed or more often diagnosed in some of these clinical trials; that is, as it occurs with all appraisals of systematic reviews, it is possible that the addition of future publications can change the results; the third aspect to be considered is that the RCTs differed in relation to doses, the time of use, and administration forms. Clinical responses were observed after short-time use, as well as after prolonged periods of probiotic use, which leads to the conclusion that the desired effect depends on the infection complexity, the activated site, the probiotic strains used, and the concentrations administered as colony-forming units (CFUs) of probiotics.
Although some published studies have shown that probiotic administration promotes a beneficial effect in reducing the occurrence of new episodes of respiratory infections, mainly in those patients with a history of recurrent infections, it is observed that there are still many gaps in the knowledge, and thus, many unanswered questions regarding the most appropriate strain or strains of probiotics, required dose, administration regimens, optimal time of use, and the safety of prolonged use. It is necessary, in the exercise of pediatric practice, to establish standardized protocols for the use of probiotics in the treatment of major respiratory infections in children through guidelines and scientific committees. The authors also emphasize the need for further research, especially in developing countries, where rates of respiratory infections in childhood are higher when compared to the higher per capita income countries identified in this review.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: de Araujo GV, de Oliveira Junior MH, Peixoto DM, Sarinho ES. Probiotics for the treatment of upper and lower respiratory-tract infections in children: systematic review based on randomized clinical trials. J Pediatr (Rio J). 2015;91:413–27.