to investigate the rate of positivity for immunoglobulin M anti-Toxoplasma gondii (Toxo-IgM) in newborns with congenital toxoplasmosis, and the age when these antibodies become negative.
Methodspatients with congenital toxoplasmosis who started monitoring in a congenital infection clinic between 1998 and 2009 were included. Inclusion criteria were routine maternal or neonatal serological screening; diagnostic confirmation by persistence of immunoglobulin G anti-Toxoplasma gondii at age ≥ 12 months, and Toxo-IgM screening in the neonatal period. To calculate the frequency of positive Toxo-IgM, cases detected by neonatal screening were excluded. For the study of the age when Toxo-IgM results became negative, patients with negative Toxo-IgM since birth and those in whom it was not possible to identify the month when the negative result was achieved were excluded.
Resultsamong the 28 patients identified through maternal screening, 23 newborns had positive Toxo-IgM (82.1%, 95% CI: 64.7-93.1%). When adding the 37 patients identified by neonatal screening, Toxo-IgM was positive in the first month of life in 60 patients, and it was possible to identify when the result became negative in 51 of them. In 19.6% of patients, these antibodies were already negative at 30 days of life; and in 54.9%, at 90 days. Among the 65 patients included in the study, 40 (61.5%) had some clinical alteration.
Conclusionseven with high sensitivity methods, newborns with congenital toxoplasmosis can have negative Toxo-IgM at birth. In those who have these antibodies, the positive period may be quite short. It is important not to interrupt the monitoring of infants with suspected congenital toxoplasmosis simply because they present a negative Toxo-IgM result.
investigar a taxa de positividade para imunoglobulina M anti-Toxoplasma gondii (Toxo-IgM) em recém-nascidos com toxoplasmose congênita, e a idade de negativação desses anticorpos.
Métodosforam incluídos pacientes com toxoplasmose congênita que iniciaram acompanhamento em uma clínica de infecções congênitas entre 1998 e 2009. Os critérios de inclusão foram toxoplasmose congênita detectada por triagem sorológica materna ou neonatal de rotina, confirmação diagnóstica por persistência de imunoglobulina G anti-Toxoplasma gondii com ≥12 meses e pesquisa de Toxo-IgM no período neonatal. Para o cálculo da frequência de positividade da Toxo-IgM foram excluídos os detectados por triagem neonatal. Para o estudo da época de negativação da Toxo-IgM foram excluídos os pacientes com Toxo-IgM negativa desde o nascimento e aqueles em que não foi possível identificar o mês da negativação.
Resultadosentre 28 pacientes detectados por triagem materna, 23 recém-nascidos tiveram Toxo-IgM positiva (82,1%, IC 95%: 64,7-93,1%). Somando-se os 37 pacientes detectados por triagem neonatal, a Toxo-IgM foi positiva no primeiro mês de vida em 60 pacientes e em 51 foi possível identificar a época de negativação. Em 19,6% dos pacientes esses anticorpos já eram negativos aos 30 dias e em 54,9% aos 90 dias. Entre os 65 pacientes incluídos no estudo, 40 (61,5%) apresentaram alguma alteração clínica.
Conclusõesmesmo com métodos de alta sensibilidade, recém-nascidos com toxoplasmose congênita podem ter Toxo-IgM negativa ao nascer. Nos que apresentam esses anticorpos, o período de positividade pode ser bastante fugaz. É importante não interromper o monitoramento dos lactentes com suspeita de toxoplasmose congênita por apresentarem Toxo-IgM negativa.
Suspected congenital toxoplasmosis can occur in several situations: a) clinical or laboratory evidence of toxoplasmosis acquired by the mother during pregnancy; b) fetal sonographic abnormalities; c) clinical manifestations in the infant; and d) neonatal screening, which consists of the routine investigation of immunoglobulin (Ig)-M anti-Toxoplasma gondii (Toxo-IgM) in capillary blood. In any of these situations, diagnosis confirmation requires a number of clinical and laboratory examinations.1,2
Treatment of congenital toxoplasmosis is indicated even in subclinical cases with laboratory diagnosis, which consists primarily of IgG anti-T. gondii (Toxo-IgG) and Toxo-IgM in serum. Although there are other serological tests that can contribute to the diagnosis, such as specific IgE and IgA tests, and Western blot IgG/IgM for mother/child pairs, Toxo IgG and Toxo-IgM are the most often used, and they are frequently the only tests available, especially in Brazil.1–6
As IgM does not cross the placental barrier, the presence of Toxo-IgM in the newborn serum indicates congenital infection. Conversely, it is known that newborn infants with congenital toxoplasmosis can have negative Toxo-IgM, although the frequency of this finding is controversial.7–14 Information regarding the prevalence of positive results for Toxo-IgM in the newborn, as well as the age at which it tends to become negative in infants with congenital toxoplasmosis, is essential when confirming a suspected diagnosis.
This study aimed to demonstrate the dynamics of Toxo-IgM in neonates and infants with confirmed congenital toxoplasmosis. First, this study sought to investigate the frequency these antibodies are detected in the newborn; and second, to determine, in cases with positive Toxo-IgM, at what age these results become negative. The possible associations between the serological aspects and some clinical variables were also investigated.
MethodsThis cohort study included children whose monitoring started between January of 1998 and December of 2009 in the Congenital Infection Clinic of Hospital São Lucas, Porto Alegre, state of Rio Grande do Sul, Brazil. The project was approved by the Research Ethics Committee of the Pontifícia Universidade Católica do Rio Grande do Sul and informed consent was obtained from all parents or guardians.
Inclusion criteria were: 1) congenital toxoplasmosis confirmed by positive Toxo-IgG≥ 12 months of age; 2) suspected by routine serological screening (maternal or neonatal); and 3) Toxo-IgM test since the first month of life.
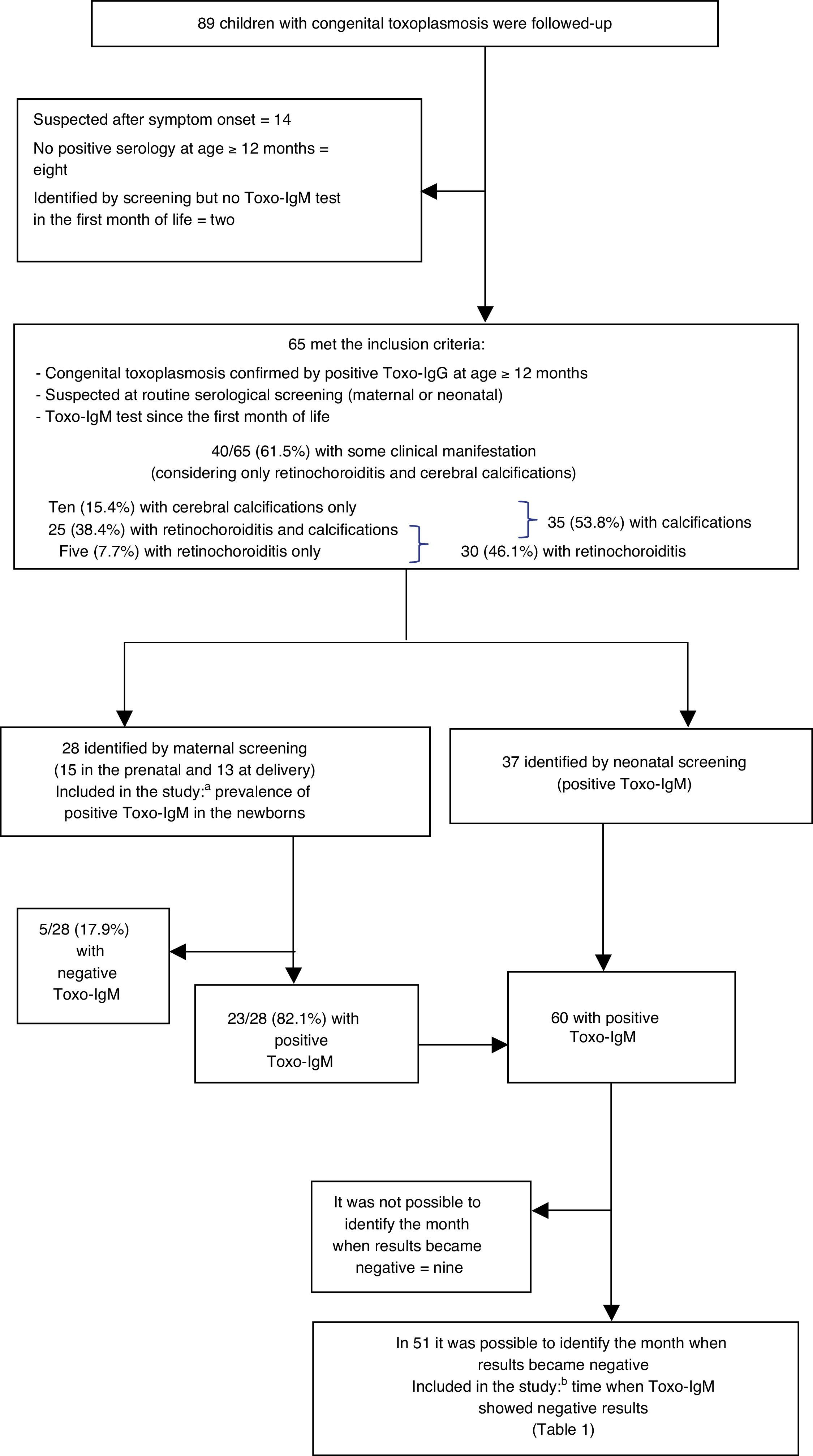
Exclusion criteria were determined according to the two specific study objectives. To calculate the rate of Toxo-IgM positivity in the newborn, patients identified by neonatal screening (which must obligatorily have positive Toxo-IgM result) were excluded. To demonstrate the time when Toxo-IgM results became negative, patients who never had a positive Toxo-IgM result were excluded, as well as those in whom it was not possible to identify the month when the result became negative (as there is a very large gap between the last positive and the first negative test) (Fig. 1). The month when Toxo-IgM became negative was considered to be that when the first test was negative, provided that the interval to the last positive test did not exceed two months.
Distribution of inclusion and exclusion criteria for the study. Patients who started follow-up between 1998 and 2009 in the Congenital Infection Outpatient Clinic of Hospital São Lucas, Porto Alegre, RS, Brazil. Toxo-IgM: Immunoglobulin M anti-Toxoplasma gondii, analyzed by immunoenzymatic capture assay.
a Prevalence of positivity for immunoglobulin M anti-Toxoplasma gondii (Toxo-IgM) in the newborn.
b Time of the negative results for Toxo-IgM.
The studied variables were the time of screening, presence of Toxo-IgM in the first month of life, age when the Toxo-IgM became negative, gestational age, maternal and infant treatment, presence of cerebral calcifications, and presence of retinochoroiditis within the first year of life. Suspicion due to maternal screening occurred when pregnant woman with negative tests showed seroconversion or serology consistent with recent toxoplasmosis (positive Toxo-IgM and/or low Toxo-IgG avidity) detected in the prenatal period or at the time of hospitalization for childbirth. In these cases, serological tests in newborns were performed in peripheral blood samples during their stay in the maternity ward, and repeated at varying intervals according to clinical indication.
The start of treatment for congenital toxoplasmosis was indicated in the presence of one or more of the following situations: a) positive Toxo-IgM in the newborn; b) typical clinical manifestations of congenital toxoplasmosis; c) increased Toxo-IgG during the first months of life.
The method used for Toxo IgG and Toxo-IgM identification in serum was the enzyme linked fluorescent assay (ELFA; BioMérieux - Marcy l’Etoile,France), whose cutoffs for IgM are index value<0.55 for negativity and >0.65 for positivity. A fluorometric enzyme immunoassay (FEIA; Ani LabSystems - Helsinki, Finland) was used to test Toxo-IgM on filter paper. The presence of retinochoroiditis in newborns was assessed by indirect ophthalmoscopy, and calcifications were investigated by computed tomography or ultrasound.
Data were recorded prospectively in medical records, entered in an Excel file (Microsoft Corp. - USA), and analyzed through Epi Info 3.5.1 (Centers for Disease Control and Prevention - Atlanta, United States). Absolute frequencies and percentages were described, possible associations between variables were investigated by chi-squared test or Fisher's exact test, correlations were assessed using Pearson's correlation, and comparison between medians were performed by the Mann-Whitney/Wilcoxon test, considering significant a p-value <0.05.
ResultsIncluded patientsInclusion criteria were met by 65 patients among 89 children with congenital toxoplasmosis whose monitoring at the Clinic of Congenital Infections started during that period. Twenty-four patients were not included; in 14 the suspected diagnosis occurred at symptom onset, eight had no serology at 12 months of age or older, and two patients that met the initial criteria lacked Toxo-IgM test in the first month of life.
Of the 65 patients included, 28 were detected by maternal screening (15 in the prenatal period and 13 at delivery) and 37 by neonatal screening. The number of patients excluded for each study objective, according to the criteria mentioned in the methodology, is described below.
Clinical manifestationsAmong the 65 patients included, 40 (61.5%; 95% confidence interval [CI]: 49.3% to 72.7%) had some clinical alteration within the first year of life, considering only retinochoroiditis and cerebral calcifications (without including other central nervous system abnormalities, such as ventricular dilation). Cerebral calcifications and retinochoroiditis were detected in 25 patients (38.4%), ten (15.3%) had only calcifications, four had only retinochoroiditis, and one patient had retinochoroiditis and did not undergo imaging examination; for statistical purposes, this patient was added to those that had only retinochoroiditis, totaling five cases (7.7%) in this category (Fig. 1). Therefore, cerebral calcifications were seen in 35 patients (53.8%; 95% CI: 41.6% to 65.6%) and retinochoroiditis was detected in 30 (46.1%; 95% CI: 34.3% to 58.3%) within the first year of life.
Toxo-IgM positivity in the neonatal periodTo calculate the prevalence of positive Toxo-IgM in the newborn, the 37 patients identified by neonatal screening were excluded. Among the 28 patients in whom clinical suspicion arose due to maternal serology, 20 had positive Toxo-IgM on the first day of life, confirmed after over one week (71.4%; 95% CI: 52.8% to 85.7%), while eight had negative Toxo-IgM on the first day (28.6%; 95% CI: 14.2% too 47.1%). In three of these, Toxo-IgM became positive after the second week, and Toxo-IgG became positive shortly after. The mothers of these patients had evidence of T. gondii infection that had occurred a few days before delivery. Therefore, of the 28 patients, five never had positive Toxo-IgM (17.9%; 95% CI: 6.8% to 35.2%), whereas 23 had positive Toxo-IgM in the neonatal period (82.1%; 95% CI: 64.7% to 93.1%) (Fig. 1). In this study, no cases of false Toxo-IgM positivity were identified.
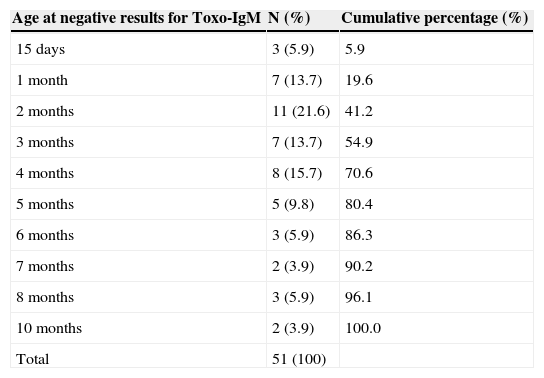
Time when Toxo-IgM results became negativeThe 23 patients detected by maternal serological tests that showed positive Toxo-IgM (after excluding the five who never tested positive), in addition to the 37 detected by newborn screening, comprise the 60 newborns with positive Toxo-IgM in the first month of life. To study the time when Toxo-IgM results became negative, nine patients for whom the month when results became negative could not be identified had to be excluded, since the interval between the first negative and the last positive test was >two months (Fig. 1). Thus, it was possible to demonstrate the month in which Toxo-IgM became negative in 51 patients (Table 1).
Age when negative results were observed for immunoglobulin M anti-Toxoplasma gondii in 51 infants with confirmed congenital toxoplasmosis who showed a positive result for these antibodies in the first month of life.
| Age at negative results for Toxo-IgM | N (%) | Cumulative percentage (%) |
|---|---|---|
| 15 days | 3 (5.9) | 5.9 |
| 1 month | 7 (13.7) | 19.6 |
| 2 months | 11 (21.6) | 41.2 |
| 3 months | 7 (13.7) | 54.9 |
| 4 months | 8 (15.7) | 70.6 |
| 5 months | 5 (9.8) | 80.4 |
| 6 months | 3 (5.9) | 86.3 |
| 7 months | 2 (3.9) | 90.2 |
| 8 months | 3 (5.9) | 96.1 |
| 10 months | 2 (3.9) | 100.0 |
| Total | 51 (100) |
Toxo-IgM, Immunoglobulin M anti-Toxoplasma gondii tested by an immunoenzymatic capture assay.
None of the mothers of the patients identified through maternal screening at delivery or neonatal screening had received treatment for toxoplasmosis. Among the 15 mothers whose toxoplasmosis was diagnosed in the prenatal period, 12 had received some type of treatment before delivery. Considering all 28 patients detected by maternal screening, it was observed that among the 12 mothers who received treatment, three newborns (25%) had never had positive Toxo-IgM, whereas of the 16 mothers who did not receive treatment, two newborns (12%) showed the same characteristic (odds ratio [OR] 2.33; 95% CI: 0.28- 22.25; p=0.4).
Treatment of the infantAfter excluding the five infants who never had positive IgM, treatment start was earlier in those whose identification was through maternal screening than in those who were identified by neonatal screening. In the first, the median age at start of treatment was 5.5 days (interquartile range: 4-23; minimum 1, maximum 52), whereas in the latter the median was 45 days (interquartile range: 31-62; minimum 17, max 275; p=0.0003). However, when comparing the groups identified through maternal or neonatal screening in relation to the time when Toxo-IgM became negative, there was no significant difference.
In those with suspected diagnosis at the maternal screening, median age of negative result was 3 months (interquartile range: 2-6, minimum 1, maximum 10), whereas in those detected by neonatal screening, the median age was 4 months (interquartile range: 2–6; minimum 0, maximum 10; p=0.3752).
Considering all 51 infants in whom it was possible to identify the time when Toxo-IgM became negative, linear regression showed no correlation between age in days at start of treatment and the age at the negative result (Pearson's correlation coefficient=0.03).
In one of the five patients that never had positive IgM, treatment was started on the second day of life, but in four of them the start occurred later (between 1 and 4 months). In six of the patients whose negative Toxo-IgM results occurred within 1 month of age, the only positive sample was the one collected at the routine neonatal screening. These mothers and their newborns had not received any treatment for toxoplasmosis. In four cases, who were asymptomatic at the first clinical evaluation, as well as in two other patients (part of the five who had never had a positive Toxo-IgM result), treatment was started based on the Toxo-IgG increase after the third month of life.
Other variablesThere was no association of other variables (presence of clinical manifestations and gestational age) with the positivity or negativity of Toxo-IgM at birth or age when the results became negative.
DiscussionIn addition to assessing the positivity rate of Toxo-IgM in newborns with confirmed congenital toxoplasmosis, this study demonstrate, for the first time by means of a cohort design, the age at which these antibodies, when present in the neonatal period, become negative, which often occurred early. This information is important for the proper management of cases of suspected congenital toxoplasmosis, since the clinical investigation must be thorough in these infants, and monitoring cannot be interrupted, even in the absence of Toxo-IgM in serum.
Regarding the proportion between positivity and negativity for Toxo-IgM in newborns with congenital toxoplasmosis, the data in the literature are quite discrepant, partly because of the different sensitivities of the methods used and partly due to population characteristics.7–14 To achieve accuracy when testing Toxo-IgM, it is necessary to use a capture method, with high sensitivity and specificity.1
In the state of Goiás, Rodrigues et al.14 evaluated 28 infants with congenital toxoplasmosis in relation to the positivity of specific IgM antibodies. Using two immunoenzymatic capture methods, it was observed that 16 (57%; 95% CI: 38% to 74%) had negative Toxo-IgM.14 A French study observed that, of 103 patients with congenital toxoplasmosis, 31 (30%; 95% CI: 21% to 39%) had negative Toxo-IgM in the first month of life, also performed by enzyme immunoenzymatic capture method.10 The positivity rate found in the present study is among the highest when compared to these and other published data;7–15 yet, it was evident that up to one-third of the newborns with congenital toxoplasmosis in this population may be negative for Toxo-IgM even using a highly sensitive method.16
Factors that may influence the presence or absence of Toxo-IgM in the newborn include concentration of maternal antibodies and treatment during pregnancy. It has been demonstrated that the treatment of pregnant women decreases the rate of positive Toxo-IgM in the newborn.8,17 Although the present study found a 2.33-OR for the effect of maternal treatment on Toxo-IgM negativity in the newborn, the confidence interval was not statistically significant, so this effect cannot be ruled out or confirmed in the study population. Bessières et al.12 found no difference in Toxo-IgM positivity in the newborn when comparing two types of maternal treatment (spiramycin or pyrimethamine+sulfadoxine).
Three newborns of mothers with very recent infection who tested negative on the day of birth showed later seroconversion. The risk of infections going unnoticed at the end of gestation has been highlighted in the literature.18 When maternal infection is very recent, the newborn should be retested in two weeks, if the serology performed at birth shows a negative result.
This cohort study confirms that the period during which Toxo-IgM remains positive in infants with congenital toxoplasmosis is very restricted: over half of infants with positive Toxo-IgM in the neonatal period already tested negative at three months of age. Gilbert et al.13 and Olariu et al.15 also demonstrated that Toxo-IgM positivity decreases with age; however, the design of those studies was cross-sectional, calculating the rate of negativity in infants by age groups, without longitudinal follow-up.
In the present study, it was observed that, in 20% of newborns, Toxo-IgM was already negative at 1 month of age, when serum confirmatory tests are performed in most patients with positive Toxo-IgM at the neonatal screening. Although not included in the national program, neonatal screening for congenital toxoplasmosis is a reality in Brazil, where most health insurance plans provide for its performance.19–21 At least six patients in this cohort would be diagnosed only after months or years, after symptom onset, if the neonatal screening test had been considered a false positive due to the early negative Toxo–IgM test, preventing the indication for full clinical investigation, as well as the monitoring of Toxo-IgG. Pediatricians should be warned to not to consider a negative Toxo-IgM result in infants as evidence of absence of congenital infection. These infants should undergo a thorough medical investigation; and when this is normal, Toxo–IgG should be monitored monthly until a total negative result or confirmatory diagnosis is attained.
Other tests, such as specific IgA test (which, together with Toxo-IgM screening, moderately increase sensitivity) and molecular biology tests, can be performed to attempt confirmation of the diagnosis as early as possible.1,10,11,15
Although it is well known that treatment in infants decreases Toxo-IgG levels,1,2 this study indicated that the same does not occur with Toxo-IgM levels. The analyses showed no influence of the treatment on Toxo-IgM duration.
Some clinical data were collected aiming to investigate their association with the dynamics of Toxo-IgM in the infant. Although the present study did not aim to assess the prevalence of clinical manifestations in congenital toxoplasmosis, and subjected to the biases inherent to the inclusion criteria employed, the authors believe the fact that the study did not include patients with diagnostic suspicion due to symptom onset and used a strict diagnostic confirmation criterion makes this a representative investigation of the studied population by offering relevant information, provided that its limitations are taken into account.
The prevalence of clinical manifestations in this sample of patients (more than 60% with retinochoroiditis and/or cerebral calcifications) appears to confirm the higher morbidity of the infection in Brazilian and South American children in general, when compared to children from other continents, such as Europe and North America, where clinical manifestations are described at birth in approximately 40% of infected newborns.20,22–26
Vasconcelos-Santos et al.20 observed a higher prevalence of retinochoroiditis, 79.8% (95% CI: 73.4% to 85.1%) in a population of infants with congenital toxoplasmosis detected by neonatal screening in the state of Minas Gerais, Brazil. In that study, ophthalmological assessment was decisive in 15.7% of infants who no longer showed positive Toxo-IgM at the confirmatory serology, similar to the situation observed in the present study.20 Melamed et al.,27 evaluating 44 infants with congenital toxoplasmosis, some of whom belonged to the present cohort, observed 31 (70.4%) cases of eye lesion, 29 (65.9%) of which were retinochoroiditis. Eye lesions may become evident within the months following birth, not only due to the appearance of new lesions, but also due to greater facility to visualize the peripheral retina. Differences in clinical presentation of congenital toxoplasmosis among populations can be explained by genetic factors of the host and parasite.20,28,29 This reason adds importance to studies in different geographical areas.
In the study by Vasconcelos-Santos et al.,20 the prevalence of cerebral calcifications was 20.5%; neuroimaging studies included radiographs and ultrasound. Other studies that also detected a lower prevalence of cerebral calcifications than that of the present study generally included skull radiographs among the neuroimaging tests, which have known low sensitivity to detect calcifications.26,30 In the present study, neuroimaging studies were performed by computed tomography and/or ultrasound, a characteristic that was taken into account when including part of this same cohort in comparative studies with European children, demonstrating that most Brazilian patients had undergone skull CT, which can increase the detection of calcifications.15,24,31
This study observed no association between Toxo-IgM positivity in the newborn and clinical manifestations of congenital toxoplasmosis, as did studies by other authors.1,11,13,15 A higher prevalence of negative Toxo-IgM in symptomatic children would be expected, as there is a greater tendency toward negativity in newborns in whom fetal infection occurred at earlier times of gestation, and who tend to be more severely affected and show evident changes on physical examination.
Wallon et al.,11 Bessières et al.,12 and Gilbert et al.13 demonstrated that the detection of Toxo-IgM in newborns was lower in cases of maternal seroconversion that occurred in the first and second trimesters of gestation. There are two possible reasons for the gestational age of maternal infection to influence the presence of Toxo-IgM in the newborn: first, in earlier infection cases, the time between placental and fetal infection is longer, allowing increased passage of maternal antibodies to the fetus and inhibiting fetal production of these antibodies. Second, the early infection would allow time for fetal Toxo-IgM to have reached its peak during intrauterine life and already be negative at birth.
In European studies, it is possible to assess the moment of maternal infection because prenatal routine assessment includes monthly serology, which does not occur in Brazil; thus, the seroconversion trimester usually cannot be exactly identified. This limitation can also be found in the United States, where no routine prenatal serology is performed.15
It can be concluded that, even when tested using serological methods with high sensitivity, up to one-third of infants with congenital toxoplasmosis may be negative for Toxo-IgM at birth. In cases of maternal infection that occurred very close to the time of delivery, newborns can show positive serology for toxoplasmosis a few days or weeks after birth. In most newborns or infants with positive Toxo-IgM, the period of positivity is quite brief. Infected children with positive Toxo-IgM in the neonatal screening may already be negative at the time of confirmatory testing, which should not be initially regarded as false positivity of the screening test. It is important not to interrupt the monitoring of infants with suspected congenital toxoplasmosis due to a negative Toxo-IgM result.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Lago EG, Oliveira AP, Bender AL. Presence and duration of anti-Toxoplasma gondii immunoglobulin M in infants with congenital toxoplasmosis. J Pediatr (Rio J). 2014;90:363–9.
Institution and study setting: Hospital São Lucas, Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Rio Grande do Sul.