Intimins are protein adhesins of enteropathogenic Escherichia coli and enterohemorrhagic E. coli capable of inducing attachment and effacement lesions in enterocytes. Anti-intimin antibodies are important for the protection from enteropathogenic E. coli and enterohemorrhagic E. coli infections because these antibodies inhibit bacterial adhesion and impair the initial step of the pathogenesis. We studied the transfer of maternal anti-intimin antibodies from healthy Brazilian mothers to their newborns through the placenta and colostrum.
MethodsSerum immunoglobulin G and secretory immunoglobulin A antibodies against conserved and variable regions of intimins α, β, and γ were analyzed using an enzyme linked-immunosorbent assay in the blood and colostrum from 45 healthy women as well as cord blood serum samples from their newborns.
ResultsThe concentrations of antibodies reactive with α intimin were significantly lower than those of anti-γ and anti-conserved intimin antibodies in the colostrum samples. IgG serum antibodies reactive with all the subtypes of intimins were transferred to the newborns, but the concentrations of anti-conserved intimin serum antibodies were significantly higher in mothers and newborns than concentrations of antibodies against variable regions. The patterns of IgG transfer from mothers to newborns were similar for all anti-intimin antibodies. These values are similar to the percentage transference of total IgG.
ConclusionsAnti-intimin antibodies are transferred from mothers to newborns through the placenta, and reinforce the protection provided by breastfeeding against diarrheagenic E. coli infections.
As Intiminas são adesinas proteicas de Escherichia coli enteropatogênicas e enterohemorrágicas capazes de induzir as lesões “attaching and effacing” nos enterócitos. Anticorpos anti-intiminas são importantes para a proteção contra infecções por E. coli enteropatogênica e E. coli enterohemorrágica porque estes anticorpos inibem a adesão bacteriana impedindo o passo inicial do mecanismo patogênico destas bactérias. Nós estudamos a transferência de anticorpos maternos anti-intiminas de mães brasileiras saudáveis para os seus recém-nascidos através da placenta e do colostro.
MétodosAnticorpos séricos da classe IgG e secretórios da classe IgA reativos com as porções conservada e variáveis das intiminas α, β e γ foram analisados pelo teste imunoenzimático no sangue e no colostro de 45 parturientes saudáveis e no sangue de cordão umbilical dos seus respectivos recém-nascidos.
ResultadosAs concentrações de anticorpos reativos com intimina vα foram significativamente mais baixas que as dos anticorpos anti-γ e anti-intimina conservada nas amostras de colostro. Anticorpos IgG séricos reativos com todas as intiminas foram transferidos para os recém-nascidos, mas as concentrações de anti-intimina conservada foram significativamente mais altas tanto nas mães como nos recém-nascidos do que os anticorpos reativos com as regiões variáveis das intiminas. O padrão de transferência de IgG das mães para os recém-nascidos foi muito semelhante para todos os anticorpos anti-intiminas. Os valores de porcentagem de transferência foram semelhantes à transferência de IgG total.
ConclusõesAnticorpos anti-intimina são transferidos das mães para os recém-nascidos pela placenta e corroboram a proteção contra infecções por Escherichia coli diarreiogênicas conferida pelo aleitamento materno.
In the developing world, the morbidity and mortality related to pediatric bacterial diarrhea are largely attributed to the diarrheagenic form of Escherichia coli (DEC). Newborns and infants up to 1 year of age are particularly vulnerable to diarrhea caused by DEC when they have not been breastfed.1
Epidemiological studies among Brazilian children have revealed the presence of DEC strains in feces not only of children with diarrhea but also in those of healthy children. Typical (tEPEC) and atypical (aEPEC) forms of enteropathogenic E. coli have been detected among these isolates from children in both rural and metropolitan areas.2,3 Enterohemorrhagic E. coli (EHEC) is an enteric pathogen that is genetically and phenotypically related to EPEC, but distinguishable by its toxin production. Both kinds of bacterial strains produce attaching and effacing lesions (A/E) in the gut mucosa, resulting in intimate contact of the bacteria with the host cell. This phenomenon leads to protein phosphorylation, cell membrane destruction, and expression of bacterial genes, clustered in a genomic island called locus of enterocyte effacement (LEE).1 One of the most important proteins encoded by LEE is intimin, a 94-kDa outer-membrane protein involved in intimate bacterial-cell attachment. The intimin molecule is composed of 939 amino acid residues (aa) and has two functional regions: the N-terminal portion, which is highly conserved and is inserted into the bacterial external membrane, and the C-terminal 280 aa portion, which is variable and whose polymorphisms determine the various intimin subtypes.4,5 At least 27 subtypes of intimin have been described, but only a few have been implicated in a disease in humans. The prevalence of intimin subtypes varies among tEPEC, aEPEC, and EHEC, in different geographic regions and periods, as demonstrated by epidemiological studies. Intimins α, β, and γ were among the prevalent intimin subtypes in Brazil in the early 2000.1,6–8
Some studies have identified anti-intimin antibodies in the serum of children and adults infected with EPEC or EHEC.9,10 The authors have identified anti-intimin antibodies in the serum of healthy adults and children,11–13 as well as in the colostrum of healthy Brazilian mothers.14–18 Similar findings have been described for American and Mexican women.19 Nonetheless, the transfer of maternal antibody subtypes reactive with intimin to newborns through the placenta has not yet been described.
Antibodies from colostrum inhibit bacterial adhesion to HEp-2 cells in vitro; the same anti-EPEC antibodies were found in the feces of breastfeed newborns as present in their mother's colostrum,15 pointing to a possible mechanism by which breastfeeding confers protection onto newborns against infections caused by EPEC and EHEC.14,16,17 In addition, secretory and serum anti-intimin antibodies have been shown to inhibit bacterial adhesion to cultured cells and have been implicated in protection against infection15,17,20–22; therefore, the transfer of anti-intimin antibodies could protect the fetus and newborn against infection. In the present study, the authors assessed the presence of anti-intimin antibodies in the serum and colostrum from healthy mothers and in the umbilical cord of their newborn babies using purified proteins obtained from recombinant bacteria expressing the conserved (cons) and variable regions of intimins α (vα), β (vβ), and γ (vγ).4 By simultaneously measuring concentrations of these antibodies in serum samples obtained from the mothers and babies, their possible placental transfer was also assessed.
Materials and methodsBiological samples from human subjectsThis study's protocol was approved by the ethics committee of the Hospital Israelita Albert Einstein (HIAE) and of the Universidade de São Paulo, Brazil (CEP number: 06/434); all procedures were in accordance with the 1964 Helsinki declaration, and a written informed consent was obtained from all the participating mothers.
The samples were obtained at HIAE, a private Hospital serving families of a medium to high socioeconomic level, from July to October 2006. Serum, colostrum, and cord blood serum were obtained from 45 healthy mothers and their healthy newborn babies, corresponding to 37 cesarean deliveries. The inclusion criteria were as follows: healthy mothers, without any pathologies recorded during pregnancy or labor, and with negative results on serological tests for HIV, HTLV I/II, hepatitis B and C, Chagas disease, syphilis, toxoplasmosis, and cytomegalovirus. Maternal and cord blood were obtained immediately after delivery and colostrum samples were collected up to 72h after delivery. All mothers were aged between 22 and 36 years (mean age 31.2), and the total serum immunoglobulin G (IgG) ranged from 508.8 to 1389.1mg/dL (mean 800.3mg/dL). The mothers were all well-nourished, predominantly primiparous, with medium to high educational and socioeconomic status, living in good sanitary conditions in the city of São Paulo. The newborn babies were healthy, with adequate weight for the gestational age, 37–41 gestational weeks (mean 38), weighting between 2630 and 3945g (mean 3298g).
A control colostrum pool was prepared with equal volumes of 20 samples collected from healthy women, and a control serum pool was prepared with equal volumes of 100 blood samples collected from healthy adult donors. The total secretory immunoglobulin A (SIgA) concentration of the colostrum pool was determined by a capture enzyme linked-immunosorbent assay (ELISA) using a commercially available standard (Sigma, USA). A serum pool with a known immunoglobulin G (IgG) concentration served as a standard. Samples with low and high concentrations of antibodies were included in each ELISA plate as an internal control of the assay.
AntigensPurified intimins were obtained from cultures of recombinant bacteria expressing each of the conserved and variable regions of intimins α, β, and γ, as previously described.18 Briefly, the recombinant E. coli M15 bacteria transformed with plasmids pFLvα, pFLvβ, pFLvγ, or pFLcons were analyzed by PCR to confirm the presence of the insert after cloning of the plasmid vector. Fragments of the eae gene, which encodes intimin, were sequenced for comparison with standard strains registered in GenBank. Protein expression was induced by culturing the bacteria with isopropyl-β-d-thiogalactopyranoside, followed by extraction in a French press and centrifugation. The proteins were finally purified by immobilized metal (Ni) ion adsorption chromatography (Qiagen, USA) and the products were analyzed by sodium dodecyl sulphate polyacrylamide gel electrophoresis.
Anti-intimin antibodiesThe concentrations of anti-intimin serum IgG and secretory IgA (SIgA) were determined by ELISA based on the Fomsgaard technique.23 Concentrations of specific anti-intimin antibodies were determined in comparison with total IgG and IgA concentrations, as described elsewhere.18 For standardization, half of a microtiter 96-well ELISA plate was coated with anti-IgG as a capture antibody (2.5μg/mL) and the other half was coated with each of the purified intimins obtained from recombinant bacteria expressing cons, vα, vβ, and vγ4 at the same concentration (2.5μg/mL). Appropriate serial dilutions of the control serum pool (with known total IgG concentration determined by nephelometry) were added on both sides of the plate. The assay was developed with an anti-IgG conjugate. The optical density values were established and the concentration of anti-intimin antibodies was determined by comparison with the standard curve of total IgG. This standard procedure was also performed for SIgA in a colostrum pool. Then, the individual serum and colostrum samples in serial dilutions starting from 1:10 were analyzed in ELISA plates coated with the recombinant intimins, with the serum or colostrum pool used to construct the standard curve. Samples with high and low concentrations of antibodies were included in each plate as an internal control. The total IgG concentrations in maternal and cord serum samples were measured by an immunoturbidimetric assay (Abbott Diagnostics, USA). The placental transfer percentages of the total and intimin-specific IgG antibodies were defined in each assay as follows:
Statistical analysisAll results were subjected to statistical analysis including descriptive analyses with box plot graphs, non-parametric ANOVA with Friedman's multiple comparison test, and the Spearman correlation test, at a 95% confidence level, using the GraphPad Prism software (Graph Pad Software Inc., USA).
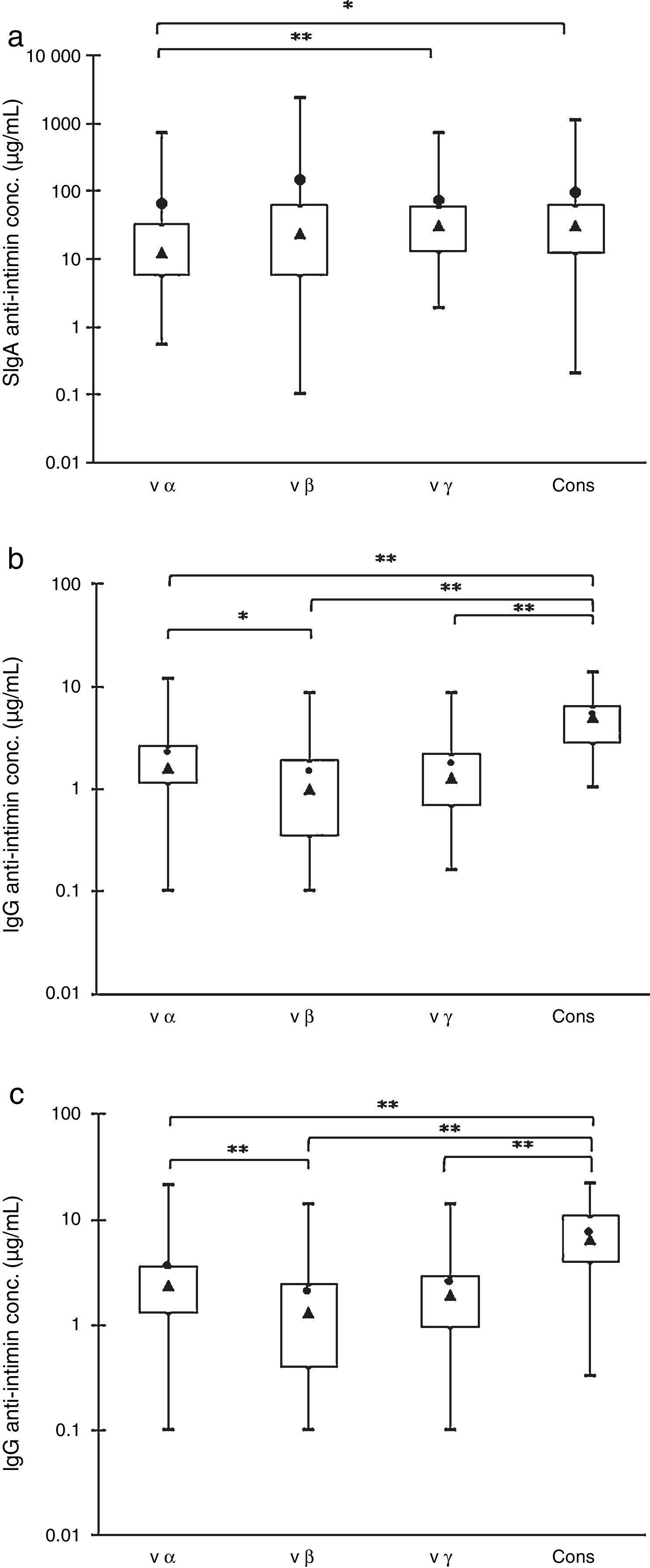
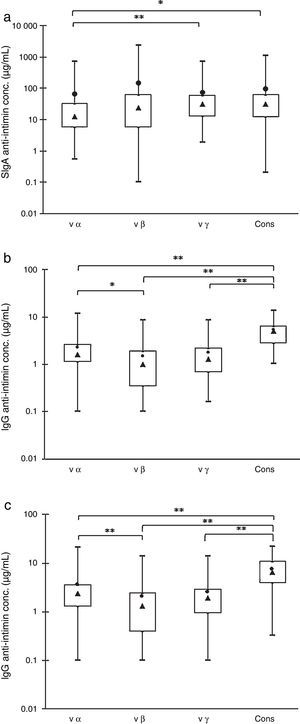
ResultsFig. 1 shows the concentrations of antibodies reactive with the four purified recombinant antigens (cons, vα, vβ, and vγ) present in 45 colostrum samples, 45 maternal serum samples, and 45 cord serum samples from their respective newborns. The box plots show the maximal and minimal values, 25% and 75% percentiles, medians, and means.
Box-plot distributions of samples by their anti-intimin concentrations: maximal and minimal values (top and bottom lines outside the box, respectively), means (●) and medians (▴), and 75% and 25% quartiles (top and bottom sides of the box, respectively). (a) SIgA antibodies in colostrum samples. (b) IgG antibodies in maternal serum samples. (c) IgG antibodies in newborns’ serum samples. Significant differences: *p<0.05, **p<0.01.
The analyses of colostrum samples by the Friedman test for multiple comparisons showed that the concentrations of anti-vα IgA antibodies were significantly lower than those of anti-vγ and anti-cons, but there were no statistically significant differences within the other pairs of values (Fig. 1A). The concentrations of IgA antibodies varied widely, and the means above the medians indicated that it was not a normal distribution.
The comparison of specific IgG revealed that the concentration of anti-cons was significantly higher when compared with the other subtypes in the mothers’ and newborns’ serum samples. The anti-vα antibody levels were higher than those of anti-vβ, but there were no significant differences between these two subtypes and anti-vγ antibodies (Fig. 1B and C). These results were confirmed by the frequency distribution analysis (data not shown).
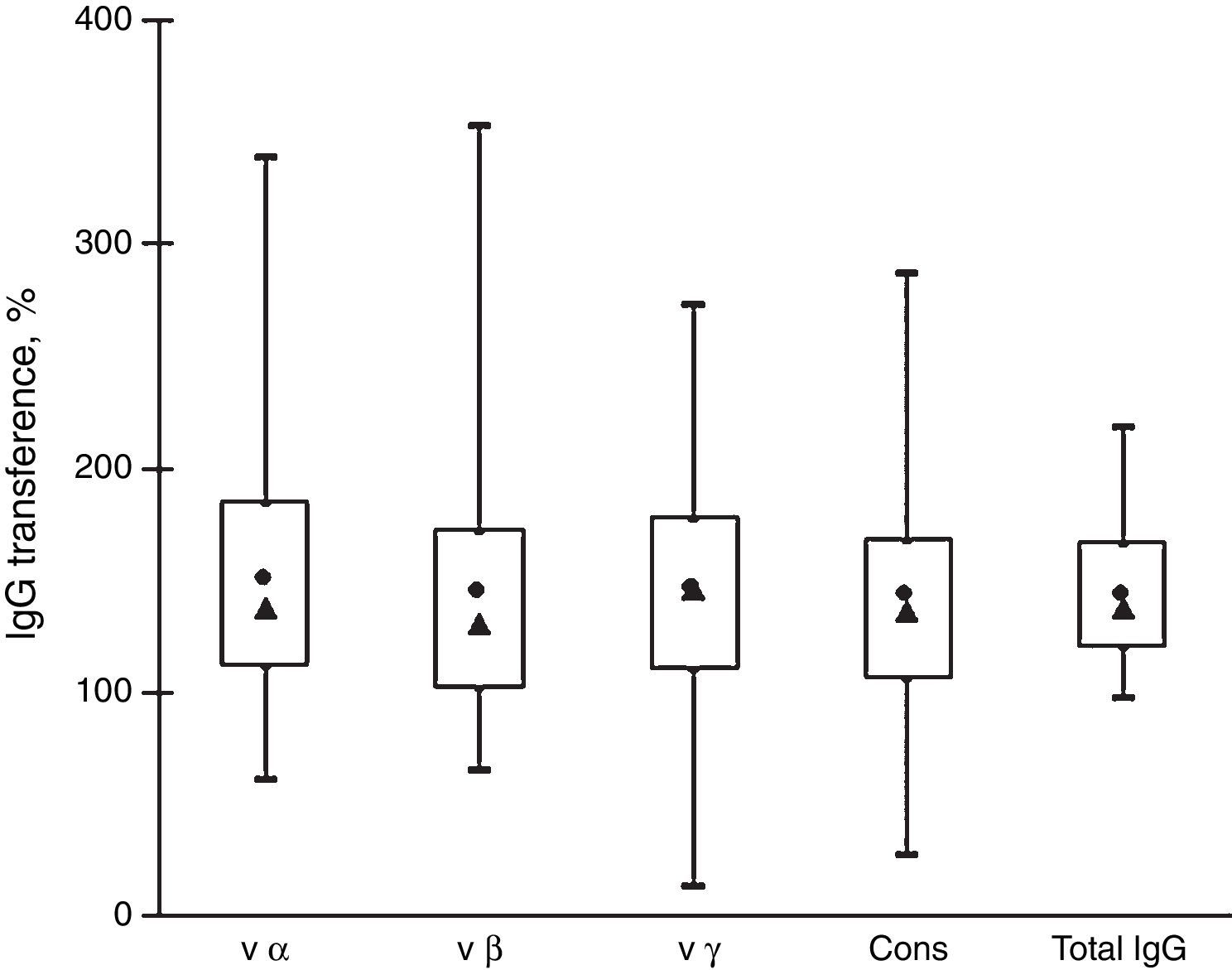
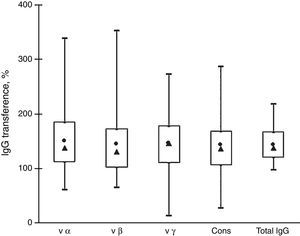
Fig. 2 shows that the IgG transfer from mothers to newborns was very similar for all the antibody subtypes analyzed. The mean transference percentage of anti-intimin antibodies ranged from 142% to 149% and the medians, from 130% to 146%. These values are similar to the percentage of transference of total IgG (mean=143%, median=137%). There were no significant differences among the transference percentages of antibodies reactive with the various intimin subtypes.
Transfer of IgG anti-intimin antibodies and total IgG from mothers to newborns. Box-plot distribution of samples by their percentages of transference of anti-vα, anti-vβ, anti-vγ, and anti-cons and transfer of total IgG: maximal and minimal values (top and bottom lines outside the box, respectively), means (●) and medians (▴), and 75% and 25% quartiles (top and bottom sides of the box, respectively).
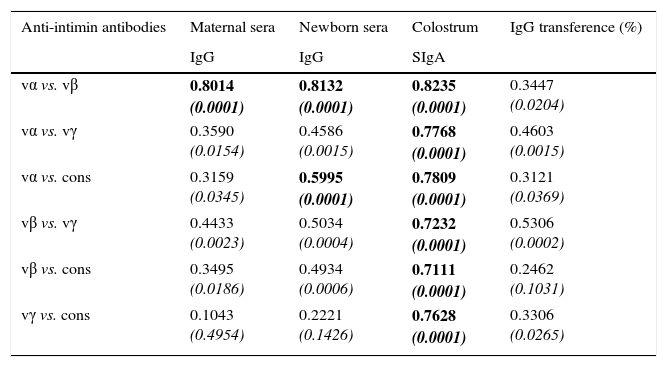
Table 1 shows results of the Spearman correlation tests. In maternal serum samples, there was a significant correlation between anti-vα and anti-vβ antibodies (p<0.0001). There was no statistically significant correlation between vγ and cons. The same pattern of correlation was observed in newborns’ serum samples. The correlation coefficients were high in all pairs analyzed for the colostrum samples.
Correlation coefficients of the concentrations of anti-intimin antibodies in serum (IgG) and colostrum (SIgA) samples and IgG transference percentage from mothers to newborns, in pairs of intimin types.
| Anti-intimin antibodies | Maternal sera | Newborn sera | Colostrum | IgG transference (%) |
|---|---|---|---|---|
| IgG | IgG | SIgA | ||
| vα vs. vβ | 0.8014 (0.0001) | 0.8132 (0.0001) | 0.8235 (0.0001) | 0.3447 (0.0204) |
| vα vs. vγ | 0.3590 (0.0154) | 0.4586 (0.0015) | 0.7768 (0.0001) | 0.4603 (0.0015) |
| vα vs. cons | 0.3159 (0.0345) | 0.5995 (0.0001) | 0.7809 (0.0001) | 0.3121 (0.0369) |
| vβ vs. vγ | 0.4433 (0.0023) | 0.5034 (0.0004) | 0.7232 (0.0001) | 0.5306 (0.0002) |
| vβ vs. cons | 0.3495 (0.0186) | 0.4934 (0.0006) | 0.7111 (0.0001) | 0.2462 (0.1031) |
| vγ vs. cons | 0.1043 (0.4954) | 0.2221 (0.1426) | 0.7628 (0.0001) | 0.3306 (0.0265) |
vα, variable region of intimin α; vβ, variable region of intimin β; vγ, variable region of intimin γ; cons, conserved region of intimin.
The coefficients were determined by the Spearman correlation test, at a 95% confidence level. p-Values are shown in italics; p-values<0.0001 are highlighted in bold.
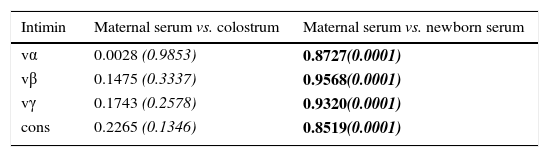
Table 2 shows significant correlation coefficients for all intimin types between maternal and newborn serum samples; therefore, the transference of antibodies from mothers to newborns was equally efficient for all the subtypes of intimin. Low correlation coefficients between maternal serum IgG and colostrum SIgA antibodies were observed, as the generation of serum IgG and secretory IgA antibodies proceeds via independent mechanisms.
Correlation coefficients of anti-intimin antibody concentrations between maternal serum (IgG) and colostrum (SIgA) samples, and between maternal and cord serum samples (IgG).
| Intimin | Maternal serum vs. colostrum | Maternal serum vs. newborn serum |
|---|---|---|
| vα | 0.0028 (0.9853) | 0.8727(0.0001) |
| vβ | 0.1475 (0.3337) | 0.9568(0.0001) |
| vγ | 0.1743 (0.2578) | 0.9320(0.0001) |
| cons | 0.2265 (0.1346) | 0.8519(0.0001) |
vα, variable region of intimin α; vβ, variable region of intimin β; vγ, variable region of intimin γ; cons, conserved region of intimin.
Coefficients were determined by the Spearman correlation test, at a 95% confidence level. p-Values are shown in italics; p-values<0.0001 are highlighted in bold.
In this study, the authors provided evidence of placental and colostral transfer of anti-EPEC and anti-EHEC antibodies to newborn babies; these antibodies are likely to confer protection onto the neonates against diarrhea caused by these pathogens. Anti-intimin antibodies can inhibit the bacterial adhesion to epithelial cells, and this ability has been demonstrated for SIgA antibodies present in human colostrum and milk and serum antibodies from humans and immunized animals.13–17,22,24 Based on this property, intimins have been proposed as a potential candidate for a vaccine against EPEC and EHEC infection.20,21
Antibodies against the conserved and variable regions of intimins α, β, and γ were found in all serum and colostrum samples analyzed, probably due to the wide prevalence of EPEC in the Brazilian population. This study was conducted using samples collected from mothers from medium to high socioeconomic levels. The authors have demonstrated similar findings when analyzing samples collected from mothers of lower socioeconomic layers and living in poorly sanitized areas, suggesting that EPEC prevalence is independent of the socioeconomic status or hygienic conditions of the patients.11,12,16,18 Similar findings were reported by Araujo et al. regarding the detection of DEC in fecal samples of children from poor urban areas and in those of children receiving in high-socioeconomic-level private medical services.25
Healthy Brazilian children develop antibodies reactive to EPEC virulence-associated factors such as intimins.11 By the end of the first year of life, these infants exhibit serum and salivary anti-EPEC antibody repertoires equivalent to those of healthy adults in the same population. Intimins α, β, and γ are shared by EPEC (whether tEPEC or aEPEC) and EHEC strains, and are present in DEC found in domestic and farm animals that are in close contact with people, suggesting that intimin is a common antigen ubiquitously present in the Brazilian population.26
The anti-intimin antibody subtypes found in the samples analyzed in this study may inhibit bacterial adhesion, because this portion of the intimin molecule is responsible for interactions with the host cells, pointing to a role in protection against the bacteria.22
The statistical analysis of IgG detected in both maternal and newborn serum samples revealed that concentrations of antibodies against the conserved region of intimin were higher in comparison with antibodies to the variable regions of the subtypes α, β, and γ. The conserved region is common to all subtypes; therefore, it is reasonable to expect that all individuals will develop these antibodies regardless of the specific intimin subtype present in the contacting bacteria. Similar results were observed by Zapata-Quintanilla et al.18 in their analyses of serum samples from healthy adults (blood donors). Nevertheless, this profile was different in colostrum samples, where the concentration of antibodies reactive to vβ, vγ, and cons were all similar, but the anti-vα antibody concentration was lower than that of anti-vγ and anti-cons. This pattern was not identified by Zapata-Quintanilla et al.,18 who found lower anti-vγ levels. This discrepancy may be attributable to variations in the antibody repertoires among the mothers, as the characteristics of the donors in the study by Zapata-Quintanilla were different from those of the present study regarding the socio-economic level (public hospital versus private hospital, respectively), age (mean 24.6 vs. 31.2 years), type of delivery (24.4% vs. 82% cesarean section), and period of sample collection (September 1997 to February 1998 vs. July to October 2006). Epidemiological studies have indicated that the prevalence of intimin γ has increased with the emergence of aEPEC in recent years.1,3,6–8,25
Intimins are frequently found in bacteria isolated not only from patients with diarrhea, but also from healthy people and domestic animals in Brazil.1,6,7,26 Intimin α was detected in tEPEC and aEPEC strains. Intimin β is associated with tEPEC and aEPEC, as well as with EHEC, whereas intimin γ is found in aEPEC and EHEC. Isolation of these bacterial strains in Brazil can explain the presence of these antibodies in serum and colostrum samples. In spite of the low incidence of EHEC infection among Brazilian adults, anti-intimin γ antibodies are present and show a positive correlation with antibodies reactive with other intimin subtypes. The increasing frequencies of aEPEC strains in the environment may be responsible for the presence of anti-intimin γ antibodies among healthy Brazilians. Recent studies have implicated intimin subtypes β and γ in the induction of diarrhea in humans, and subtype β was found to be significantly more frequent in disease cases when compared with healthy carriers.8
There is a significant correlation between the concentration of anti-intimin α and β IgG antibodies in the maternal serum and newborns’ serum, which may be due to cross-reactions among IgG antibodies targeting these intimins. Indeed, the gene sequences of the variable portions of these intimins are 65% homologous,18 which explains the presence of cross-reacting antibodies. Therefore, this phenomenon implies that whatever the intimin subtype candidate for a vaccine, this subtype may raise specific antibodies but can also induce cross-reactive antibodies directed to the other subtypes.
The concentrations of SIgA antibodies reactive with all types of intimins present in colostrum samples showed significant correlation coefficients, suggesting that there is a certain degree of cross-reaction among these antibodies. This situation may be explained by the elevated homology between the intimin molecules and by the lower specificity of colostrum SIgA antibodies as compared to serum IgG. Nevertheless, the higher concentrations of anti-intimin antibodies in colostrum samples when compared with serum samples support the crucial role of breastfeeding in protection of infants from DEC infection. In humans, milk SIgA antibodies are not significantly absorbed by the mucosa of the breastfed baby, but act as local defense. In a previous study, the authors have shown, using immunoblotting, that the reactivity of anti-EPEC SIgA antibodies of the colostrum samples was the same as the reactivity of anti-EPEC antibodies in the feces of the respective babies. In addition, SIgA antibodies isolated from colostrum by affinity chromatography inhibit EPEC adhesion to HEp-2 cells.15
The present results indicate that different anti-intimin antibodies are transferred from mothers to newborns with equal efficiency through the placenta. These results support the hypothesis that, in this case, the maternal–fetal transfer is not influenced by antibody specificity. The transference percentages of anti-intimin antibodies in the present study were found to be equivalent to the transference percentage of total IgG, which is approximately 140%. Similar findings have been reported by other authors.27,28
Although there is a high frequency of DEC isolates in Brazil, strains of EHEC are not as common as in other countries. It can be hypothesized that, besides environmental features such as climate and alimentary habits, people may develop EHEC immunity resulting from contact with other strains (e.g., DEC). Some studies revealed the presence of antibodies reactive with various lipopolysaccharide (LPS) serotypes, such as O111, O55, O157, and O26, in serum and colostrum samples from healthy Brazilians.12,13 Other studies have shown the maternal–fetal transfer of IgG antibodies reactive with O111, O16, and O6 LPS, as well as O157, to be associated with neonatal sepsis.29,30 These LPS antigens are commonly found in different pathotypes of E. coli that are agents of intestinal or systemic diseases. Taken together, these observations and the present work allow us to conclude that protection from EHEC can be conferred by contact with EPEC or other E. coli strains.
The present results confirm that maternal antibodies reactive with intimins are transferred to the newborns through the placenta, and that the presence of high concentrations of these antibodies in colostrum supports the protective role of breastfeeding.
FundingThe financial support was provided by FAPESP – 03/13250-3.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank all the mothers for their consent to donate the samples. They would also like thank the Maternity, the Milk Bank, and the Cryopreservation Laboratory of Hospital Israelita Albert Einstein, for their help with the sample collection; the Brazilian Division of Abbot Diagnosis, for the donation of the Immunoturbidimetric Kit for the analysis of IgG; Dr. Lucy B. Zapata-Quintanilla, for the help with experiments, Rosana Prisco, for the statistical analysis; and Dr. Yanira Riffo Vasquez, for review of the manuscript and her valuable suggestions (Institute of Pharmaceutical Science – King's College, London, United Kingdom).
Please cite this article as: Altman SP, Tino-De-Franco M, Carbonare CB, Palmeira P, Carbonare SB. Placental and colostral transfer of antibodies reactive with enteropathogenic Escherichia coli intimins α, β, or γ. J Pediatr (Rio J). 2017;93:568–75.
Study conducted at Instituto Butantan, São Paulo, SP, Brazil.