The incidence and prevalence of inflammatory bowel disease (IBD) in pediatric patients are increasing. Currently, the diagnostic method for IBD is inconvenient, expensive, and difficult. S100A12, a type of calcium-binding protein, detected in the feces of patients with IBD has recently been suggested as a promising diagnostic tool. Hence, the authors aimed to evaluate the accuracy of fecal S100A12 in diagnosing IBD in pediatric patients by performing a meta-analysis.
MethodsThe authors performed a systematic literature search in five electronic databases for eligible studies up to July 15, 2021. Pooled diagnostic accuracies of fecal S100A12 were analyzed as the primary outcomes. Secondary outcomes were standardized mean difference (SMD) of fecal S100A12 levels between IBD and non-IBD groups and a comparison of diagnostic accuracies between fecal S100A12 and fecal calprotectin.
ResultsSeven studies comprising 712 children and adolescents (474 non-IBD controls and 238 IBD cases) were included. Fecal S100A12 levels were higher in the IBD group than in the non-IBD group (SMD = 1.88; 95% confidence interval [CI] = 1.19–2.58; p < 0.0001). Fecal S100A12 could diagnose IBD in pediatric patients with a pooled sensitivity of 95% (95% CI = 88%–98%), specificity of 97% (95% CI = 95%–98%), and area under the receiver operating summary characteristics (AUSROC) curve of 0.99 (95% CI = 0.97–0.99). Fecal S100A12 specificity and AUSROC curve values were higher than those of fecal calprotectin (p < 0.05).
ConclusionFecal S100A12 may serve as an accurate and non-invasive tool for diagnosing pediatric IBD.
Inflammatory bowel disease (IBD) is a group of chronic disorders of the digestive tract, including Crohn's disease (CD) and ulcerative colitis (UC), which have recently become more common among children and adolescents. In recent years, there has been an increase in the incidence and prevalence of IBD in both industrialized and developing countries.1 The prevalence of pediatric IBD in the United States has increased by 133% over 9 years.2 In Asian countries, the annual incidence is 11.4/100,000 person-years. This was supported by an analysis of trends over time, which showed an increased incidence of pediatric IBD.3
Childhood is a period of crucial physical and emotional development. IBD in children is often associated with a more aggressive disease course, including a greater tendency of engendering extensive disease and requiring early immunomodulation therapy.4,5 IBD in children is also associated with anemia,6,7 developmental disorders,8,9 performance drop,9 or depression.10 The relationship between genetics and diet in this disease has been widely studied.11-15 It is important to screen children for symptoms and risks to prevent disease worsening and complications.
Endoscopic evaluation with biopsy remains the standard criterion for IBD diagnosis. Histopathological findings are essential to determine disease severity and differentiate between UC and CD.16 However, endoscopic procedures are invasive, uncomfortable, and expensive. Moreover, endoscopy is tricky, especially in pediatric patients, because children need to be hospitalized and often have problems with bowel preparation.17 Therefore, developing non-invasive diagnostic tools for the early detection of IBD in pediatric patients is essential. Fecal markers, a group of substances released by inflamed mucosa, are potential diagnostic tools for IBD.18,19 Calprotectin is one of the widely used fecal markers.20,21 Unfortunately, a previous meta-analysis found that fecal calprotectin has a lower specificity in diagnosing pediatric IBD than adult IBD.17
Calcium-binding protein S100A12 (abbreviated as S100A12 or calgranulin-c) is a biological marker of IBD that can be found in the feces of patients with IBD.22 This compound is derived from the infiltration of neutrophils in the inflamed intestinal mucosa.18 The use of the fecal S100A12 has shown excellent accuracy in diagnosing IBD. However, to our knowledge, no systematic review and meta-analysis have discussed the use of fecal S100A12 as a non-invasive diagnostic tool for pediatric IBD. Therefore, this study aimed to evaluate the accuracy of fecal S100A12 in diagnosing IBD in pediatric patients.
Materials and methodsStudy protocol and reference guidelinesThe protocol of this systematic review and meta-analysis has been previously registered on the International Prospective Register of Systematic Reviews (PROSPERO) (https://www.crd.york.ac.uk/prospero/) with the registration number CRD42021273493.23 This study was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 guidelines.24
Data search strategyA systematic and computerized data search was performed in PubMed, Scopus, Web of Science, ProQuest, and CINAHL (via EBSCOhost) for published studies from inception to July 15, 2021. Keywords were constructed using Medical Subject Headings (MeSH) terms and other free-text terms, which included ‘S100A12’, ‘inflammatory bowel disease’, ‘pediatric’, ‘child’, and ‘adolescent’. The detailed search terms used in each database are listed in Supplementary Material, Table S1. The overall study selection process was conducted independently by two investigators (BS and VV). Any discrepancies in the results were resolved by a consensus involving a third investigator (AP).
Eligibility criteriaStudies were included on meeting the following criteria: (1) used an observational design (cohort study, cross-sectional, or case-control study); (2) included a pediatric population aged < 18 years; (3) evaluated the role of fecal S100A12 in pediatric IBD; and (4) evaluated fecal S100A12 levels in the non-IBD and IBD groups or fecal S100A12 accuracy parameters (sensitivity and specificity) for diagnosing pediatric IBD. The exclusion criteria were as follows: (1) duplicated records; (2) records with irrelevant titles or abstracts; (3) articles with irretrievable full text; (4) review articles, case reports, case series, letters to editors, or conference abstracts; and (5) non-English studies.
Data extraction and quality assessmentThe following relevant data were extracted from each included study: first author, year of publication, study location, study design, characteristics of the study population, diagnostic criteria for pediatric IBD, fecal S100A12 assay, sample size, age, fecal S100A12 values in the non-IBD and IBD groups, and cut-off, and diagnostic accuracy parameters. Data extraction was conducted by two authors independently (BS and VV) and was further validated by a third author (AP).
The quality of diagnostic studies was assessed using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool.25 The QUADAS-2 tool evaluates the risk of bias and concerns regarding the applicability of studies in four domains: (1) patient selection, (2) index test, (3) reference standard, and (4) flow and timing. Each domain was judged as ‘high,’ ‘low,’ or ‘unclear.’ The quality of non-diagnostic studies was assessed using the Newcastle-Ottawa Scale (NOS) for case-control studies because all the non-diagnostic studies used a case-control design. The quality of studies was categorized as ‘high’ if the total NOS score was 7–9, ‘moderate’ if it was 4–6, and ‘low’ if it was 0–3. Two investigators independently performed the quality assessments (BS and AP); any disagreements were resolved by a third independent investigator (VV).
Statistical analysisStatistical analyses were conducted using Review Manager version 5.4 (The Cochrane Collaboration, The Nordic Cochrane centre, Copenhagen, Denmark), STATA version 16.0 (Stata Corporation, College Station, TX, USA), and Meta-DiSc version 1.4.26 The authors performed a meta-analysis of standardized mean difference (SMD) to compare the levels of fecal S100A12 in the non-IBD versus IBD groups and diagnostic test accuracy (DTA) meta-analysis of fecal S100A12 in the diagnosis of pediatric IBD. For conducting the meta-analysis of SMD, data reported in median and interquartile range (IQR) or range was extrapolated into mean and standard deviation (SD) using methods suggested by Wan et al.27 and Luo et al.28 Given the variability in the population characteristics, IBD diagnostic criteria, and fecal S100A12 assay methods between studies, a random-effects model for the meta-analysis was primarily applied. Publication bias was assessed qualitatively using the funnel plot and quantitatively using Egger's regression test.29 Sensitivity analysis was performed using the leave-one-out method.
DTA meta-analysis using a bivariate method was carried out to obtain the pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic score, diagnostic odds ratio (DOR), and area under the summary receiver operating characteristic (AUSROC) curve, along with their corresponding 95% confidence intervals (CI). Spearman's correlation analysis was conducted to detect the presence of a threshold effect. The threshold effect is one of the causes of heterogeneity in DTA meta-analysis, which arises owing to the variability of cut-off values used between the studies. A positive Spearman correlation coefficient with p < 0.05 indicated a significant threshold effect.30 Publication bias was assessed quantitatively using Deeks' funnel plot.31 Additionally, the authors performed Z-tests32 to indirectly compare the pooled sensitivities, specificities, and AUSROC curve values of fecal S100A12 obtained from this study with fecal calprotectin obtained from previous meta-analyses by Henderson et al.17 and Holtman et al.33 Furthermore, the authors performed subgroup and meta-regression analyses to determine the cause of heterogeneity and other covariates that might affect the pooled result. Subgroup analyses were performed based on study location, study design, type of pediatric IBD diagnostic criteria, and fecal S100A12 assay method, while meta-regression analyses were performed for sample size and mean population age.
Heterogeneity between studies was assessed using Cochran's Q statistics and quantified using I2 statistics, where I2 values of 0%, 25%, 50%, and 75% indicated negligible, low, moderate, and high heterogeneity, respectively. A p < 0.05 was used to indicate statistical significance in all analyses.
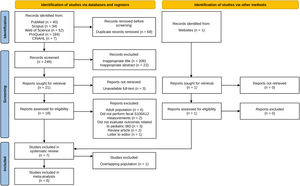
ResultsSelection of studiesInitial searches of five databases yielded 317 records, and 249 records remained after removing duplicates. Of these, 231 were excluded due to irrelevant titles, abstracts, or no fulltext available. The remaining 18 articles were reviewed thoroughly based on the eligibility criteria. Twelve articles were excluded because of the following reasons: adult population (n = 4), did not perform fecal S100A12 measurements (n = 2), did not evaluate outcomes related to pediatric IBD (n = 3), review articles (n = 2), and a letter to the editor (n = 1). Additionally, the authors identified one eligible study from outside the databases. Accordingly, seven studies34-40 were included in this systematic review. However, one study by Pham et al.38 was further excluded from the meta-analysis because of its overlapping population with other studies. The entire study selection process is shown in Figure 1.
Characteristics of the included studiesThe seven included studies comprised 712 children and adolescents aged < 18 years, of whom 474 were non-IBD controls and 238 were patients with IBD. Of the overall patients with IBD, 172 were of CD, 60 were of UC, and 6 were of IBD-unclassified (IBDU) types. Most were case-control studies, and two—by Sidler et al.35 and Heida et al.37 — were cross-sectional studies. The diagnostic criteria for IBD and fecal S100A12 assay methods varied among studies. The characteristics of the included studies are summarized in Table 1.
Summary of the main characteristics of included studies in the systematic review.
| Author, Year | Study Location | Study Design | Population Characteristics | IBD Diagnostic Criteria | Fecal S100A12 Assay | Sample Size | Agea | AUC (95% CI) | Cut-Off | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-IBD | IBD | |||||||||||
| CD | UC | IBDU | ||||||||||
| de Jong et al., 200634 | Australia | Case-control | Case: Children diagnosed with IBD at the hospitalControl: Healthy children from families of hospital staff | Standard criteria with clinical, endoscopic, histological, and imaging findings | In-house ELISA | 25 | 22 | 1 | 0 | 8.98 ± 4.28 | 0.98 | 10 mg/kg (pre-defined) |
| Sidler et al., 200835 | Australia | Cross-sectional | Children presenting with GI symptoms who were suspected of having organic bowel disease, which required further examinationCase: Diagnosed with IBDControl: Diagnosed other than IBD | Standard criteria with clinical, endoscopic, histological, and imaging findings | In-house ELISA | 30 | 30 | 1 | 0 | 11.11 ± 3.52 | 0.99 (0.97–1.01) | 10 mg/kg (pre-defined) |
| Heida et al., 201736 | Netherlands | Case-control | Case: Children diagnosed with IBD at the hospitalControl: Healthy school children | European Society for Pediatric Gastroenterology Hepatology and Nutrition | Inflamark® ELISA | 122 (one outlier was excluded from accuracy analysis) | 21 | 19 | 1 | 12.24 ± 3.73 | 0.97(0.93–1.00) | 0.75 μg/g (pre-defined) |
| Heida et al., 201837 | Netherlands and Belgium | Cross-sectional | Children presenting with persistent diarrhea for > 4 weeks or chronic/recurrent abdominal painCase: Diagnosed with IBDControl: Diagnosed other than IBD | IBD Risk Stratifier (Endoscopy with biopsy or clinical follow-up) | Inflamark® ELISA | 244 | 52 | 39 | 2 | N/A | N/A | 0.75 μg/g (pre-defined) |
| Pham et al., 201038 | Australia | Case-control | Case: Children diagnosed with CD at the hospitalControl: Same control population as Sidler et al., 200,835 | Standard criteria with clinical, endoscopic, histological, and imaging findings | In-house ELISA | 30 | 13 | 0 | 0 | N/A | N/A | N/A |
| Ehn, 201139 | Sweden | Case-control | Case: children diagnosed with IBD at the hospitalControl: Healthy children from outpatient clinic, pediatric ward, or family of hospital staff | Standard criteria | In-house ELISA | 38 | 7 | 0 | 3 | 5.08 ± 5.15 | N/A | N/A |
| Nylund et al., 201140 | United States | Case-control | Case: Children diagnosed with CD at the hospitalControl: Healthy children from local communities | Standard diagnostic criteria with clinical, endoscopic, and radiographic findings | Double sandwich ELISA | 15 | 27 | 0 | 0 | N/A | N/A | N/A |
Data are presented in mean ± SD.
AUC, area under curve; CD, Crohn's disease; CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; GI, gastrointestinal; IBD, inflammatory bowel disease; IBDU, inflammatory bowel disease unclassified; N/A, not available; SD, standard deviation; UC, ulcerative colitis.
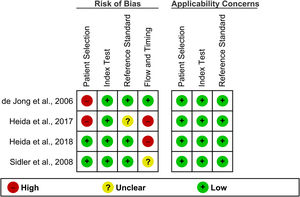
The results of the quality assessment of the diagnostic studies using the QUADAS-2 tool are presented in Figure 2. In the patient selection domain, two studies[34,36] had a high risk of bias because of the case-control design. All the studies had a low risk of bias in the index test domain. One study[36] was judged to have an unclear risk of bias in the reference standard domain because of unclear information on whether the diagnosis of IBD was made without knowledge of the fecal S100A12 results. In the flow and timing domain, two studies[36,37] had high risk, and one[35] had an unclear risk of bias because the interval from the start of IBD diagnosis to fecal S100A12 measurement was either significant or not clearly reported. All studies had low concerns regarding their applicability in all domains. According to the NOS, all non-diagnostic studies[38–40] were of high quality (NOS score = 7) (Supplementary Material, Table S2).
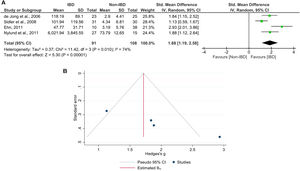
SMD meta-analysis of fecal S100A12Four studies[34,35,39,40] involving 91 pediatric patients with IBD and 108 non-IBD controls were included in this meta-analysis (Figure 3A). The results showed that the fecal levels of S100A12 in the pediatric IBD group were significantly higher than those in the non-IBD group (SMD = 1.88; 95% CI = 1.19–2.58; p < 0.0001). The heterogeneity level was high (I2 = 74%). The funnel plot demonstrated an asymmetrical distribution of studies (Figure 3B), and Egger's test showed a significant result (p = 0.0007), indicating potential publication bias. The sensitivity analysis revealed no substantial influence on the significance of the pooled effect size when each study was excluded from the analysis.
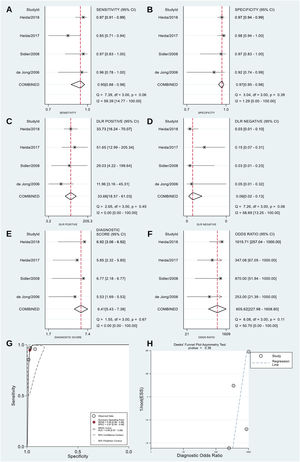
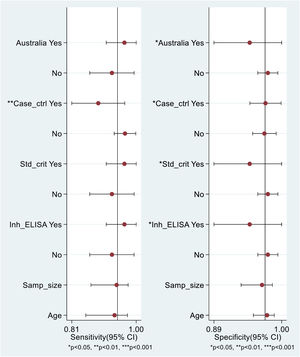
DTA meta-analysis of fecal S100A12Four diagnostic studies[34–37] were included in this meta-analysis. The result revealed that fecal S100A12 could detect pediatric IBD with a pooled sensitivity of 95% (95% CI = 88%–98%), specificity of 97% (95% CI = 95%–98%), PLR of 33.66 (95% CI = 18.57–61.03), NLR of 0.06 (95% CI = 0.02–0.13), a diagnostic score of 6.41 (95% CI = 5.43–7.38), DOR of 605.62 (95% CI = 227.98–1608.80), and AUSROC curve of 0.99 (95% CI = 0.97–0.99) (Figure 4A–G). The Spearman's coefficient was +0.316 (p = 0.68), indicating no threshold effect. Deeks' funnel plot showed no potential publication bias (p = 0.36) (Figure 4H). The results of the subgroup analyses revealed significant differences in fecal S100A12 diagnostic accuracy between studies conducted in Australia and other than Australia (p < 0.05), case-control and cross-sectional studies (p < 0.05), studies using standard and other IBD diagnostic criteria (p < 0.05), and studies using in-house ELISA and other types of ELISA (p < 0.05). Meta-regression analysis showed that differences in sample size and mean population age between studies had no significant effect on overall fecal S100A12 diagnostic accuracy (Figure 5).
Meta-analysis of the diagnostic accuracy of fecal S100A12 for identifying pediatric IBD. (A) Sensitivity. (B) Specificity. (C) PLR. (D) NLR. (E) Diagnostic score. (F) DOR. (G) AUSROC curve. (H) Deeks’ funnel plot.
The pooled sensitivity and specificity of fecal calprotectin were obtained from Henderson et al.,17 whereas the AUSROC curve value was obtained from Holtman et al.33 (Table 2). The results showed no significant difference (Z = 0.99; p = 0.32) between the pooled sensitivities of fecal S100A12 and calprotectin (95% vs. 97.8%). The pooled specificity of fecal S100A12 was significantly higher (Z = 3.12; p = 0.002) than that of fecal calprotectin (97% vs. 68.2%). Additionally, the AUSROC curve value of fecal S100A12 was significantly higher (Z = 2.91; p = 0.004) than that of fecal calprotectin (0.99 vs. 0.95).
Comparison of the diagnostic accuracy of fecal S100A12 and fecal calprotectin for identifying pediatric IBD.
| Diagnostic Accuracy Parameter | Fecal S100A12 | Fecal Calprotectin | Comparison Result | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Studies | Total Sample | Pooled Value | 95% CI | SE | Author, Year | Number of Studies | Total Sample | Pooled Value | 95% CI | SE | Z | p | |
| Sensitivity | 4 | 608 | 95% | 88%–98% | 2.55 | Henderson et al., 201417 | 8 | 715 | 97.8% | 94.7%–99.6% | 1.25 | 0.99 | 0.32 |
| Specificity | 97% | 95%–98% | 0.77 | 68.2% | 50.2%–86.3% | 9.21 | 3.12 | 0.002a | |||||
| AUC | 0.99 | 0.97–0.99 | 0.01 | Holtman et al., 201733 | 6 | 593 | 0.95 | 0.93–0.98 | 0.01 | 2.91 | 0.004a | ||
`The study results showed higher S100A12 levels in the IBD group than in the non-IBD group. Fecal S100A12 also had a very high AUSROC curve value of 0.99. This value is categorized as an outstanding accuracy (0.90–1.00). A good diagnostic tool should have a PLR > 10 and an NLR < 0.1.41 Based on the study results, S100A12 feces met both values. The comparison of fecal S100A12 with fecal calprotectin showed a significant difference in AUSROC curve values and specificity, with no difference in sensitivity values. Thus, indicating that fecal S100A12 has a better overall diagnostic potential than fecal calprotectin. This finding is consistent with previous studies, which stated that calprotectin had moderate specificity, whereas the specificity of S100A12 was very good.42-45
Moreover, S100A12 is an antimicrobial that plays a role in initiating a pro-inflammatory response in the digestive tract.46 It is one of the receptors for advanced glycation end product (RAGE) ligands.47,48 Activated RAGE induces the production of pro-inflammatory mediators such as tumor necrosis factor (TNF)-α and further promotes the release of S100A12 from neutrophils.49 The accumulation of inflammatory mediators, such as TNF- α, is closely related to the pathogenesis of IBD.50,51 S100A12 derived from neutrophil infiltration in the inflamed intestine is then found in feces and is used as a fecal biologic marker.15
Fecal S100A12 showed better AUSROC curve values and specificity than fecal calprotectin in pediatric IBD because the specific expression of S100A12 was limited to the activation of neutrophils and monocytes.18,52 However, other findings suggest that the AUSROC curve value, sensitivity, and specificity of the two fecal markers are equally good in adult patients.53 Fecal calprotectin levels were found to vary in children aged younger than 10 years — having higher levels than those in children aged older than 10 years.54 Therefore, it is necessary to adjust the cut-off when using fecal calprotectin for diagnosing pediatric IBD.55 Fecal S100A12 levels are relatively constant throughout age, except in those younger than 12 months old.56 The results of the meta-regression, with age as a covariate, further suggested that the diagnostic ability of fecal S100A12 is uniform across a range of ages of pediatric patients.
To our knowledge, the present study is the first to analyze the pooled SMD values and diagnostic accuracy parameters of fecal S100A12 for diagnosing pediatric IBD. This study has several limitations. First, the present study found significant heterogeneity in the pooled SMD meta-analysis results. Second, this study did not separate CD and UC groups. S100A12 may have different roles in CD and UC due to the different roles of RAGE in the two types of IBD.47 Separation of patient groups could not be performed because there were no studies that separately analyzed the two types of IBD. Third, other clinical conditions were still not assessed. Finally, the number of included studies and total sample size was still limited.
ConclusionsThis study demonstrated an increase in the fecal levels of S100A12 in pediatric patients with IBD and supported its use as a non-invasive tool to facilitate the diagnosis of pediatric IBD with excellent accuracy. Given the limitations of the present study, the authors encourage more large research evaluating fecal S100A12 for diagnosing pediatric IBD, ideally for separate populations of CD and UC, and directly comparing its diagnostic accuracy with fecal calprotectin to corroborate the authors’ findings.