To describe the success rate and the complications after procedures to diagnose abdominal non-Hodgkin's lymphoma in children and adolescents.
MethodsA retrospective cross-sectional study was conducted with a population consisting of children and adolescents with abdominal non-Hodgkin's lymphoma diagnosed between September 1994 and December 2012. The sample comprised of 100 patients who underwent 113 diagnostic procedures, including urgent surgery (n=21), elective surgery (n=36), and non-surgical diagnosis (n=56).
ResultsThe most frequent procedures were laparotomy (46.9%) and ultrasound-guided core biopsy (25.6%). The rate of diagnostic success was 95.2% for urgent surgeries; 100% for elective surgeries and 82.1% for non-surgical procedures (p<0.05). The rates of complication during the three diagnosis procedures considered were significant (p<0.001; 95.2% of the urgent surgeries, 83.8% of the elective surgeries, and 10.7% of the non-surgical procedures). The length of time before resuming a full diet and starting chemotherapy was significantly reduced for patients who underwent non-surgical procedures when compared with the other procedures (p<0.001).
ConclusionNon-surgical procedures for the diagnosis of pediatric abdominal non-Hodgkin's lymphoma are an effective option with low morbidity rate, allowing an earlier resumption of a full diet and chemotherapy initiation. Furthermore, non-surgical procedures should also be considered for obtaining tumor samples from patients with extensive disease.
Descrever a taxa de sucesso e as complicações dos procedimentos para o diagnóstico de linfoma não Hodgkin abdominal em crianças e adolescentes.
MétodosEstudo retrospectivo transversal em uma população de crianças e adolescentes com linfoma não Hodgkin abdominal diagnosticada entre setembro de 1994 e dezembro de 2012. A amostra foi composta por 100 pacientes submetidos a 113 procedimentos diagnósticos, inclusive cirurgia de urgência (n = 21), cirurgia eletiva (n = 36) e diagnóstico não cirúrgico (n = 56).
ResultadosOs procedimentos mais frequentes foram laparotomia (46,9%) e biópsia guiada por ultrassonografia (25,6%). A taxa de sucesso diagnóstico foi de 95,2% para cirurgias de urgência; 100% para cirurgias eletivas e 82,1% para procedimentos não cirúrgicos (p <0,05). Houve diferença significativa entre as taxas de complicação associadas aos três grupos (p <0,001; 95,2% das cirurgias urgentes, 83,8% das cirurgias eletivas e 10,7% dos procedimentos não cirúrgicos). O tempo decorrido até o reinício da dieta plena e o início a quimioterapia foi significativamente reduzido para os pacientes submetidos a procedimentos não cirúrgicos quando comparados com os outros procedimentos (p < 0,001).
ConclusãoOs procedimentos não cirúrgicos para o diagnóstico do linfoma não Hodgkin abdominal pediátrico são uma opção efetiva com baixa taxa de morbidade, permitem uma retomada mais precoce de uma dieta plena e início de quimioterapia. Em pacientes com doença extensa, os procedimentos não cirúrgicos também devem ser considerados para a obtenção de amostras tumorais.
Pediatric lymphomas are the third most common malignant neoplasm of childhood and adolescence in developed countries and the second most common in developing countries, after leukemia.1,2
There is no consensus regarding the best way to obtain material for a histopathological study in pediatric non-Hodgkin's lymphoma (NHL) when different variables, such as diagnostic accuracy and morbidity–mortality following the procedure, are taken into consideration.3–5 Surgical biopsy of lymph nodes and extra-nodal tissue is considered to be the gold standard for lymphoma diagnosis. Nevertheless, image-guided core needle biopsy (CNB) has recently been considered as a suitable alternative to surgery, as it is less expensive, less invasive, and has fewer associated complications, particularly in the case of non-superficial masses.4,6–8 Although there is some evidence of the benefits of non-surgical procedures in diagnosing NHL in adults, there is a lack of literature exclusively concerning children and adolescents.
This study aimed to describe the success rate and complications for each type of procedure used in histological and cytological diagnosis of children and adolescents with abdominal NHL.
MethodsStudy designThis was a retrospective, observational and cross-sectional study. The study was approved by the Human Research Ethics Committee (CAAE 11825013.1.0000.5201). The studied population comprised children and adolescents with abdominal NHL who were diagnosed between September 1994 and December 2012. Over this period of time, a total of 262 NHL patients were admitted to the pediatric oncology service. Of these, 177 patients aged 0–18 years and diagnosed with abdominal NHL were included in this study, while seven patients who had been diagnosed through extra-abdominal biologic material and 70 with incomplete or missing main medical records (defined by pathologic results and complications of the diagnostic procedure) were excluded from this study.
Data collection and analysisThe following clinical and epidemiological variables were collected: age at the time of diagnosis (years), tumor size (greatest axis of the mass in centimeters), histological type in accordance with the World Health Organization criteria,9 stage in accordance with the Murphy Staging of Tumor,10 diagnostic method, tumor location, and number of procedures required for diagnosis. The data were grouped according to diagnostic method: surgical methods, including laparotomy and laparoscopic biopsy, which were divided into urgency surgery group (when acute surgical abdomen was present) or elective surgery group (with purely diagnostic indication, in the absence of acute abdomen presentation); and non-surgical methods, including ultrasound (US), computed tomography (CT)-guided CNB, non-guided CNB, paracentesis, and endoscopic biopsy. Within these groups, the collected variables were: diagnostic success (defined by diagnostic confirmation by pathology/cytology), complications related to the diagnostic method (classified in grades of severity from I to V),11 number of samples collected in CNB, indication and surgical finding in urgent surgeries, time before resuming a full diet (days), and time before chemotherapy initiation (days).
Specimens sent to the pathology department were processed as follows: for tissue specimens from surgical and non-surgical methods, cytological analysis was performed by touch imprint, followed by histopathology examination of hematoxylin and eosin-stained slices and immunohistochemistry (IHC) analysis. Peritoneal fluid was evaluated by cytological analysis after cytocentrifugation and, since 2003, by flow cytometry and immunophenotyping.
The classification of procedural complication modified by Dindo et al. consists of five grades of severity. Grade I included any deviation from the normal postoperative. The allowed therapeutic regimens are drugs (such as antiemetics, antipyretics, analgesics, and diuretics), electrolytes, and physiotherapy. This grade also includes wound infections opened at the bedside. Grade II comprises complications requiring pharmacological treatment with drugs other than those allowed for grade I complications (blood transfusions and total parenteral nutrition are also included). Grade III complications are defined as those requiring surgical, endoscopic, or radiological intervention. Grade IV describes life-threatening complications requiring intensive care management. Grade V indicates the death of a patient.11
Statistical analysisData analysis was performed using Stata 12.1 software (StataCorp, College Station, TX, USA). The Z-test was used for analysis of diagnostic success and complications. The chi-squared (χ2) test was used to compare the proportion of complications of each grade of severity between different diagnostic procedures. Fisher's exact test was used for comparison between the groups, when the expected number of subjects within a category was sufficiently small. This analysis was followed by use of the Marascuilo procedure for comparison within groups. Kruskal–Wallis with the Bonferroni post-test were used to assess the significance of the differences found among the different groups regarding the time before resuming a full diet and the time before chemotherapy initiation. Prevalence ratios were calculated through adjustments to Poisson regression models with the option of robust standard deviation. The relation between the number of fragments in CNB and positive diagnosis was assessed with the chi-squared test for linear trends. A p-value<0.05 was considered significant.
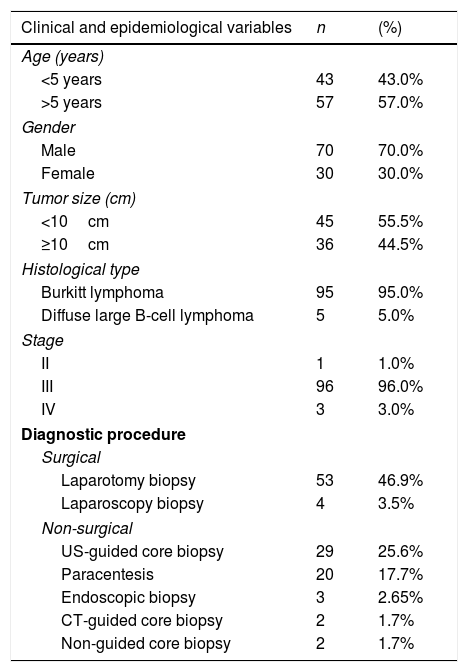
ResultsThe sample consisted of 100 patients, who had undergone 113 diagnostic procedures, including 57 surgeries and 56 non-surgical procedures. There was a predominance of male patients (2.3:1). Ages ranged from 1 to 16 years, with a mean of 6.3 (±3.5) years. At the time of diagnosis, 43% of patients were under 5 years of age. Burkitt lymphoma (BL) was the predominant histological type of NHL (95%). Cases of lymphoblastic lymphoma were not reported. Stage III cancer was predominant in 96% of the cases (Table 1). The most common tumor locations were the following: small intestine (64%), mesentery (59%), retroperitoneum (26%), colon (21%), pelvis (20%), liver (17%), and kidney (10%). Other locations (28 patients) included other abdominal and non-abdominal organs.
Distribution of clinical and epidemiological variables in patients with abdominal non-Hodgkin's lymphoma.
| Clinical and epidemiological variables | n | (%) |
|---|---|---|
| Age (years) | ||
| <5 years | 43 | 43.0% |
| >5 years | 57 | 57.0% |
| Gender | ||
| Male | 70 | 70.0% |
| Female | 30 | 30.0% |
| Tumor size (cm) | ||
| <10cm | 45 | 55.5% |
| ≥10cm | 36 | 44.5% |
| Histological type | ||
| Burkitt lymphoma | 95 | 95.0% |
| Diffuse large B-cell lymphoma | 5 | 5.0% |
| Stage | ||
| II | 1 | 1.0% |
| III | 96 | 96.0% |
| IV | 3 | 3.0% |
| Diagnostic procedure | ||
| Surgical | ||
| Laparotomy biopsy | 53 | 46.9% |
| Laparoscopy biopsy | 4 | 3.5% |
| Non-surgical | ||
| US-guided core biopsy | 29 | 25.6% |
| Paracentesis | 20 | 17.7% |
| Endoscopic biopsy | 3 | 2.65% |
| CT-guided core biopsy | 2 | 1.7% |
| Non-guided core biopsy | 2 | 1.7% |
US, ultrasound; CT, computed tomography.
Among the patients, 12 underwent two diagnostic procedures; one of these was subjected to three diagnostic procedures. The most widely used diagnostic methods were laparotomy, US-guided CNB, and paracentesis (Table 1). Urgent surgeries were performed in 21 cases (36.8%), while 36 patients were subjected to elective surgery (63.1%). Intussusception was the major indication and surgical finding (50%) in urgent surgeries. Among these patients, three already had intestinal perforation. A perforated tumor was the second most frequent isolated finding (31.8%). Enterectomy involving the tumor was performed in 18 patients (85.7%), with 12 of them having primary anastomosis. Two patients with intussusception were subjected to reduction and incisional biopsy of the lesions.
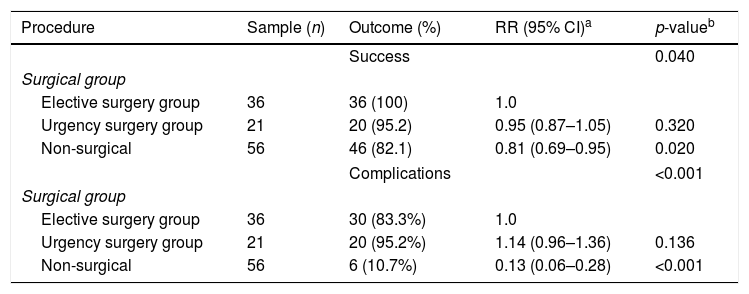
The diagnostic success rate for laparotomy was 98.1%, while that of CNB stood at 82.8%. When comparing the methods employed (urgent surgery, elective and non-surgery), a significant difference was observed in favor of surgical biopsy for confirmation of diagnosis (p<0.05; Table 2).
Diagnostic success and complications rates for diagnostic procedures in patients with abdominal non-Hodgkin's lymphoma.
| Procedure | Sample (n) | Outcome (%) | RR (95% CI)a | p-valueb |
|---|---|---|---|---|
| Success | 0.040 | |||
| Surgical group | ||||
| Elective surgery group | 36 | 36 (100) | 1.0 | |
| Urgency surgery group | 21 | 20 (95.2) | 0.95 (0.87–1.05) | 0.320 |
| Non-surgical | 56 | 46 (82.1) | 0.81 (0.69–0.95) | 0.020 |
| Complications | <0.001 | |||
| Surgical group | ||||
| Elective surgery group | 36 | 30 (83.3%) | 1.0 | |
| Urgency surgery group | 21 | 20 (95.2%) | 1.14 (0.96–1.36) | 0.136 |
| Non-surgical | 56 | 6 (10.7%) | 0.13 (0.06–0.28) | <0.001 |
Among the patients subjected to CNB (n=32), 33 procedures were carried out. The number of fragments obtained ranged from one to seven, with a mean of 2.87 (±1.49). The data was divided into four classes according to the number of fragments extracted (1, 2, 3, ≥4), performed respectively in four, 13, seven, and nine procedures. A progressive increase in the success rate of the method was achieved the more fragments were extracted (75%, 76.9%, 85.7%, and 100%, respectively). The authors assessed the association between success rate of diagnosis and number of extracted fragments in patients who underwent CNB and found a significant linear trend (p=0.04).
Confirmation of the NHL diagnosis was not possible in 11 (9.7%) of the procedures, involving 10 patients. They were paracentesis (n=5), US-guided CNB (n=5), and laparotomy (n=1), including one nonspecific histiocytic reaction process, two cases where paracentesis fluid could not be retrieved, and eight inconclusive results by the pathologist/cytologist report. The diagnostic method used after the first failure was laparotomy in seven cases, paracentesis in one case, and US-guided CNB in two cases. One patient was subjected to a laparoscopy after two inconclusive US-guided CNBs.
Among the unsuccessful US-guided biopsies, four were analyzed by immunohistochemistry. They were considered insufficient for diagnostic purposes because they contained inflammatory or degenerative cellular changes associated with unclear immunohistochemical patterns. Flow cytometry and immunophenotypic analysis were not possible in three specimens. The tissue extracted by laparotomy was not subjected to immunohistochemistry analysis.
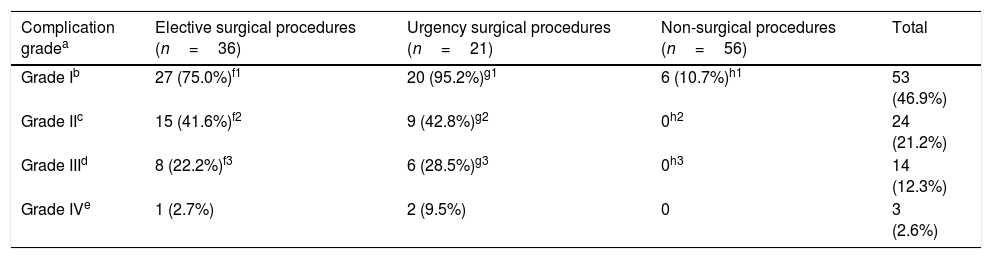
Some type of complication was reported in 56 procedures. A significant difference was observed among the urgent surgery, elective, and non-surgical groups (p<0.01, Table 2). The most frequently reported complication was pain (49 patients, 43.3%). Six patients developed partial obstruction and two of them needed to undergo surgery. Three patients presented complications related with intestinal dehiscence (two of them were treated with ostomy, and the third patient with an enterocutaneous fistula). Two laparoscopies were converted into laparotomies, one due to technical issues and the other because the patient had a cardiorespiratory arrest. The number and severity of complications associated with the procedure were higher among surgical procedures (elective and urgency). Complication grades II, III, and IV were not associated with non-surgical procedures. Analysis by Marascuilo test of the severity of complications did not differ between the two surgical groups in grades I, II, and III. However, a significant difference was observed between them and the non-surgical group for these degrees (p<0.001) for grade I and II and for grade III (p=0.006). Because of the small number of grade IV complications (n=3), it was not possible to demonstrate any statistical difference among the groups. No grade V was described (Table 3).
Complications due to procedures for diagnosis in patients with abdominal non-Hodgkin's lymphoma.
| Complication gradea | Elective surgical procedures (n=36) | Urgency surgical procedures (n=21) | Non-surgical procedures (n=56) | Total |
|---|---|---|---|---|
| Grade Ib | 27 (75.0%)f1 | 20 (95.2%)g1 | 6 (10.7%)h1 | 53 (46.9%) |
| Grade IIc | 15 (41.6%)f2 | 9 (42.8%)g2 | 0h2 | 24 (21.2%) |
| Grade IIId | 8 (22.2%)f3 | 6 (28.5%)g3 | 0h3 | 14 (12.3%) |
| Grade IVe | 1 (2.7%) | 2 (9.5%) | 0 | 3 (2.6%) |
In 105 procedures, it was possible to determine the interval between the procedure and the resumption of a full diet (0–48 days). The authors noted a significant association between the diagnostic procedure and the length of time needed to resume a full diet (p<0.001). Comparisons between groups revealed that the time needed to resume a full diet was, in general, shorter in patients who underwent a non-surgical procedure when compared to the elective surgery group (p<0.001), as well as to the urgent surgery group (p<0.001). Furthermore, patients subjected to elective surgery needed a significantly less time to resume a full diet compared to those who underwent urgent surgery (p<0.005; Table 4).
Time before the resumption of a full diet and start of chemotherapy.
| Time before resumption of full diet | ||||
|---|---|---|---|---|
| Procedure | Sample (n) | Median (days) (p25–p75a) | Min–Max (days) | p-value |
| Surgical group | ||||
| Elective surgery group | 31 | 1 (1–2) | 0–16 | |
| Urgency surgery group | 18 | 6.5 (6–10) | 4–48 | |
| Non-surgical | 56 | 0 (0–0) | 0–21 | |
| Total | 105 | 0 (0–4) | 0–48 | <0.001b |
| Time before chemotherapy initiation | ||||
|---|---|---|---|---|
| Surgical group | ||||
| Elective surgery group | 35 | 5 (3–6) | 1–26 | |
| Urgency surgery group | 20 | 8 (7–12) | 5–24 | |
| Non-surgical | 43 | 2 (0–5) | 0–18 | |
| Total | 98 | 5 (1–7) | 0–26 | <0.001c |
The time interval between the confirmation of diagnosis and chemotherapy initiation was recorded for 98 patients, ranging from 0 to 26 days. A significant association was observed between the diagnostic procedure and the chemotherapy initiation (p<0.001). The Kruskal–Wallis test with Bonferroni post-test showed that the time needed to start chemotherapy was, in general, shorter for patients who underwent non-surgical procedures when compared with those in the elective surgery group (p<0.005), as well as with those in the urgent surgery group (p<0.001). The same conclusion applies to patients of the elective surgery group when compared with the urgent surgery group (p<0.001; Table 4).
DiscussionAlthough similar studies exist, none of them exclusively analyzed patients under 18 years in a single center,4–7,12,13 with such a large sample.3,14,15 Furthermore, the authors also addressed some aspects not always emphasized by other studies, as the stratification of complications, lower-grade complications, and length of time before starting chemotherapy in association to the diagnostic method.
In the present study, only one patient presented a localized disease (stage II), and over 40% of the patients had lesions bigger than 10cm. The size of the abdominal mass has been previously correlated to poor prognosis, as well as to the number of extra-nodal sites.16 It was frequently observed that, in addition to the involvement of the small intestine, other extra-nodal sites in the abdomen and the extra-abdomen were involved concomitantly. This extra-nodal involvement is typical of pediatric lymphoma in contrast to that observed in adults, which primarily involves nodal structures.14,17,18
In the past, it was suggested that resection of the primary tumor would extend life expectancy among NHL children, particularly in the case of BL.19 Currently, there is a common understanding that NHL is a systemic disease; therefore, extensive surgeries are no longer recommended due to complications and delay on chemotherapy initiation.14,20
In the present study, the main intra-surgical finding in urgent procedures was intussusception, similarly to what had been described by Vural et al.14 (30.4%) and Attarbaschi et al.20 (47%). Other complications often cited by other authors include obstruction, perforation, and hemorrhage.14,20 Due to the urgent indication for acute abdomen, which represents a more complex medical situation, this group presented the highest rate of complications associated with the diagnostic method, as well as a delay in the chemotherapy initiation.
Open biopsy is usually required for a successful diagnosis of NHL, and other techniques can be used when the condition of the patient contraindicates the surgical procedure. However, the quickest and least invasive procedure should be used in diagnosing NHL presenting as life-threatening and fast-growing tumors, while several studies have shown the successful use of CNB in diagnosing lymphomas.4,6,8,14
In the present study, the tumor samples of the surgical groups were better at providing sufficient pathological material for the diagnosis of pediatric NHL when compared with non-surgical techniques. Laparotomy was the most preferred alternative when the first non-invasive procedure failed. However, they also accounted for the largest number and for the most serious complications arising from the diagnostic procedures. No deaths were directly linked to the procedures, notwithstanding the fact that surgery led to increased morbidity and delays to the resumption of diet and start of chemotherapy. Several case reports described an increase in complications14 and in mortality-associated renal insufficiency3,21 and delays in chemotherapy initiation.14
Laparoscopy showed a good diagnostic success rate, but all the patients presented complications, including one patient with a severe neurological sequela. The complications in the present sample were more frequent and more serious than those described in other series.12 The authors regret the lack of studies on pediatric NHL and laparoscopy, which would have contributed to a better understanding of the role of such procedures for this specific population. However, it can be assumed that, because the present patients were at an advanced stage of the disease, the formation of pneumoperitoneum would contribute to an unfavorable outcome, but only further investigations will be able to clarify this issue.
CNB, whether image-guided or not, presented a good diagnostic success rate and low rates of complications in relation to the other methods. Additionally, complications were treated clinically. The present findings were similar to those described in other series,4,5,8 which can even lead to no complications14 and up to 97%6 diagnostic success rate. In this subject, there is also a lack of literature focused exclusively on pediatric clients.
The present analysis further showed that there was no diagnostic improvement in extracting more than four fragments. Studies usually cite the removal of one to five fragments.4,5,8 For a patient with suspected lymphoma, Kerviler et al. recommend that three biopsies should be performed and fixed in formalin, and two additional biopsies should be frozen immediately in liquid nitrogen for the extraction of DNA and RNA. Furthermore, for aggressive lymphomas or Burkitt lymphoma, it is possible to make core imprints for a rapid cytological diagnosis.8
Some favorable aspects are associated with CNB, including reduced morbidity, shorter length of narcosis,7,15 shorter hospital stays, and earlier start of therapies.4 This is a less invasive technique, with better outcomes that result from technical improvements, the expertise of those involved, and the good quantity of pathological material, which is required for morphological and immunohistochemical analysis.4,5,14 However, a few aspects need to be better clarified, such as the needle gauge6,8 and the joint use of fine needle aspiration biopsy (FNAB).13,22,23
Among the image-supported methods, US is the most widely used due to its dynamic quality, which helps to prevent areas of necrosis during puncture.4,6,8 CT-guided biopsies can also be performed and can help in cases where tumors are not easily identified by US,5 but their disadvantages are the higher cost and exposure to ionizing radiation.24
Obtaining material through paracentesis produced positive outcomes and only one associated complication (5%) related to a patient's complaint of pain. According to Mann et al.15 diagnosis through cytology (including analysis of ascitic fluid, pleural, and FNAB) can be performed in most cases. However, the procedure requires an experienced cytopathologist for cytological interpretation and immunophenotype study. Two paracentesis fluid samples could not be retrieved, resulting in failure of the method. This adverse event could be decreased with imaging methods to guide the puncture procedure.25
The most frequent complication in this data series was pain. Postoperative pain, though not usually described as a complication in studies that deal with diagnostic procedures in NHL, is still one of the main complications. It has been related to considerable discomfort and suffering, particularly among children, and contributes to an increase in postoperative morbidity, as well as in poor diet acceptance, sleep disturbances, behavioral alterations, and vomiting.26,27
Pediatric NHLs are usually aggressive tumors with high rates of cell proliferation. Therefore, optimal quantity and quality of pathological material is the key to ensuring that all the required tests are promptly performed, so that a timely diagnosis can be made. Traditionally, excisional or incisional biopsy has been regarded as the gold standard interventional technique22; however, there is a notable increase in procedural complications and costs, as well as a greater potential for delay of treatment.3,6,14 Undoubtedly, in complex urgent cases, such as intussusceptions or perforation, surgery is imperative not only for diagnosis but also for therapy. In turn, non-surgical procedures are an effective option, allowing rapid diagnosis even with little quantity of material and the aid of ancillary studies (including cytofluorometry and molecular techniques). They present low morbidity in diagnosing pediatric NHL with abdominal involvement, which allows an early resumption of diet and chemotherapy initiation.
Nevertheless, more robust studies involving children and adolescents are still needed to corroborate these findings, so that the use of less invasive procedures can be recommended as the first line of inquiry in the diagnosis of abdominal NHL, as well as to clarify some aspects that the present study was not able to address due to design and sample size limitations.
Conflicts of interestThe authors declare no conflicts of interest.
The authors are grateful to Professor José Natal Figueiroa and Severino Ibánhez for their contributions on the early stages of this manuscript.
Please cite this article as: Aguiar AA, Lima LC, Araújo CC, Gallindo RM. Pediatric abdominal non-Hodgkin's lymphoma: diagnosis through surgical and non-surgical procedures. J Pediatr (Rio J). 2019;95:54–60.