The modified shuttle test is a field test that avoids the ceiling effect, and there are no reports of a multidimensional assessment concerning physical activity in asthmatic patients. Thus, the aim was to evaluate functional capacity by MST, additionally to perform a multidimensional assessment as physical activity in daily life, muscle strength, and cytokine levels in children and adolescents with asthma, and to correlate these variables.
MethodThis cross-sectional study included volunteers aged between 6 and 18 years who were divided into two groups: asthma group (n=43) that received regular treatment and control group (n=24). Functional capacity was evaluated by distance walked during the MST; physical activity in daily life was evaluated using an accelerometer by the number of steps. Quadriceps femoris strength was evaluated by load cell.
ResultsDistance walked was lower for the asthma group (790m [222m]) when compared with the control group (950m [240m]; p=0.007); however, the number of steps was similar between the two groups (asthma group: 7743 [3075]; control group: 7181 [3040]; p=0.41), and both groups were classified as sedentary behavior. There was no difference in muscle strength. Tumor necrosis factor-α differed, but interleukin levels were similar between groups. Quadriceps strength was correlated to distance walked (r=0.62; p<0.001) and tumor necrosis factor-α to the number of steps taken (r=−0.54, p=0.005).
ConclusionChildren and adolescents undergoing regular asthma treatment showed reduced functional capacity and sedentary behavior. The lower the quadriceps strength, the shorter the distance walked; the higher the tumor necrosis factor-α levels, the lower their daily physical activity levels.
O Teste Shuttle Modificado é um teste clínico de campo que evita o efeito-teto e não existem relatos de avaliação multidimensional com relação à atividade física em pacientes com asma. Assim, o objetivo era avaliar a capacidade funcional pelo shuttle teste, adicionalmente, realizar avaliação multidimensional através da a atividade física na vida cotidiana, a força muscular e os níveis de citocina em crianças e adolescentes com asma, a fim de correlacionar essas variáveis.
MétodoEste estudo transversal incluiu voluntários entre seis a 18 anos, os quais foram divididos em dois grupos: o grupo com asma (n=43), que recebeu tratamento regular, e o grupo de controle (n=24). A capacidade funcional foi avaliada pela distância percorrida durante o Teste Shuttle Modificado, ao passo que a atividade física na vida cotidiana foi avaliada utilizando um acelerômetro pelo número de passos. A força muscular do quadríceps femoral foi avaliada por uma célula de carga.
ResultadosA distância percorrida foi menor no grupo com asma (790m [222 m]) em comparação com o grupo de controle (950 m [240 m]; p=0,007), contudo o número de passos foi semelhante nos dois grupos (grupo com asma: 7.743 [3.075]; grupo de controle: 7.181 [3.040]; p=0,41) e ambos os grupos foram classificados como sedentários. Não houve diferença na avaliação da força muscular. O fator de necrose tumoral-α apresentou divergências, porém os níveis de interleucina foram semelhantes entre os grupos. A força muscular do quadríceps foi correlacionada com a distância percorrida (r: 0,62; p<0,001) e o fator de necrose tumoral-α, ao número de passos dados (r=−0,54, p=0,005).
ConclusãoCrianças e adolescentes que recebem tratamento regular de asma apresentaram redução da capacidade funcional e comportamento sedentário. Quanto menor a força muscular do quadríceps, menor a distância percorrida; quanto maiores os níveis de fator de necrose tumoral-α, menores seus níveis diários de atividade física.
The primary characteristic of asthma is chronic inflammation of the lungs. In asthmatic patients, increased airway resistance and lung elastic recoil increase ventilation demand and exertional dyspnea, which can potentially cause exercise intolerance.1,2 However, some studies have shown no difference in exercise capacity, based on oxygen uptake, between asthma patients and their healthy peers, which has been justified by nutritional adequacy,3 maintenance of daily activities, condition of treatment,4,5 and severity of the disease.6 However, other studies have shown reduced aerobic capacity not only among children with uncontrolled asthma7,8 but also among children undergoing regular treatment who exhibit controlled symptoms.9
The evaluation of functional capacity is of great interest because it is representative of the ability to perform physical activities that are part of daily life. In this context, the modified shuttle test (MST)10 is appropriate for evaluating pediatric patients, because it is performed at a wide range of speeds, which prevents floor or ceiling effects.11 Although functional capacity among asthma patients has been evaluated in previous research, the MST has rarely been used. Ahmaidi et al.12 performed a shuttle run test among asthmatic children, which requires a higher initial speed (8.0km/h), creating performance difficulty for severely affected patients. In contrast, Augusto et al.13 used an incremental shuttle test with similar speeds as the MST, although it is a shorter test which may result in a ceiling effect. Finally, Gomes et al.14 used the MST but did not allow volunteers to run. These studies prompted the research question: “Based on the MST, what is the functional capacity status of children with asthma who are undergoing regular treatment?”.
Pulmonary chronic disease is also associated with reduced physical activity in daily life (PADL),15,16 reduced muscle strength,17 and worse inflammatory profile.18 However, to the best of the authors’ knowledge, a multidimensional assessment that includes evaluations of functional capacity, PADL, muscle strength, and inflammatory profile has not been performed among children and adolescents with asthma undergoing regular treatment. Thus, two research questions were proposed for this study: “Does chronic disease reduce the physical activities of asthmatic patients?” and “Are there associations among functional capacity, physical activity, muscle strength, and serum cytokines”. Thus, the aim of this study was to evaluate the functional capacity, physical activity in the daily life, muscle strength, and inflammatory profile in asthmatic children and adolescents who were undergoing regular monitoring for the disease. Moreover, the authors also attempted to examine the correlation of peripheral muscle strength, cytokines, PADL, and functional capacity.
Patients and methodsParticipantsThis cross-sectional study enrolled subjects aged 6–18 years. Study subjects were divided into an asthma group (AG) and a control group (CG). AG subjects were recruited from a tertiary referral center of a pediatric department. The inclusion criteria were: subjects who had been diagnosed with asthma at least 6 months previously (Global Initiative for Asthma [GINA] steps 1–5)19 and who were regularly using controller medication for at least the previous 3 months. Those with irregular or no use of controller asthma medication and any exacerbation in the previous 4 weeks were excluded. The CG was matched by age and sex to the AG; the CG comprised the children of the employees of the University. The subjects were excluded of the CG if they had chronic disease or acute pulmonary disease in the previous 4 weeks, showed abnormalities in the lung function test (<80% of the predicted value). Subjects were enrolled in the study after their legal guardians had read, agreed to, and signed the informed consent form and after the subject himself/herself signed the informed assent form. The current study was approved by the Ethical Committee under No. 738192/2014.
Study design and protocolThis study was performed at the pulmonary rehabilitation laboratory. The protocol involved 2 days of evaluation. On the first day, the following procedures were conducted: serum cytokine sample, asthma control questionnaire, spirometry (pre- and post-bronchodilator), and the modified shuttle test (MST). On the second day, peripheral muscle strength was measured. On that day, the subjects received the accelerometer, which was returned after 7 days.
This study was conducted from April 2014 to November 2015.
Asthma control questionnaireThe Asthma Control Test (ACT) is a questionnaire for assessing asthma control based on patient perspective; the ACT is used for subjects >12 years of age, while the children ACT (C-ACT) is used for children aged 4–11 years.20 The ACT assesses activity limitation, shortness of breath, and nighttime symptoms over the previous 4 weeks. The answer options range from 1 (worst) to 5 (best). The highest possible score was 25 for the ACT and 27 for the C-ACT. Asthma is considered to be controlled when the score is over 20, partially controlled when the score is between 16 and 19, and uncontrolled when the score is ≤15.20
Cytokines evaluationInterleukins (IL) 4, 5, 10, 13, and 17, as well as TNFα, were analyzed and evaluated using MILLIPLEX® MAP (#MMHMAG-44K, Merck Millipore Corporation, Darmstadt, Germany). The data were interpreted using XPONENT® software (Luminex, IL, USA), and the samples were expressed as pg/mL.
Lung functionSpirometry was performed using the ULTIMA CPX equipment (MedGraphics Corporation®, MN, USA). The technical procedure, acceptance criteria, and reproducibility were according to the ATS/ERS statement.21 The AG subjects repeated the test after bronchodilation (salbutamol 400μg). The forced vital capacity (FVC), expiratory forced volume at 1st second (FEV1), FEV1/FVC, and forced expiratory flow at 25–75% of the FVC (FEF25–75) were recorded.22
MSTThe MST was performed in a 10-m-long corridor according to the original description.10 This is an externally cadenced test, dictated by an audible signal wherein the speed increases every minute ranging from 1.79 to 10.2km/h. There are 15 levels in this test and the volunteer could walk/run during the test. The test finished when the subject was unable to reach the extremities two consecutive times, if he/she needed to stop because of fatigue or breathlessness, or if the SpO2 dropped below 82%.23 The test was performed twice in the same day, with a 30-min interval. Heart rate (HR) and SpO2 were continuously evaluated. Blood pressure (BP), modified Borg lower limb fatigue, and modified Borg dyspnea were evaluated at the beginning and at the end of the test.24 The MST was performed after 400μg of bronchodilator, for AG, to achieve the best performance and avoid bronchospasm induced by exercise. The distance walked (DW) of the best test (longest distance covered) was the outcome of the MST and was expressed in meters and as percentage of the predicted value.25
All subjects performed the MST connected to a system for gas exchange analyses (VO2000; MedGraphics Corporation®, St. Paul, USA). The VO2, pulmonary carbon dioxide production (VCO2), minute ventilation (VE), and VE/MVV (minute ventilation to maximal voluntary ventilation) were measured.
Peripheral muscle strengthThe maximum isometric voluntary contraction (MIVC) of the quadriceps femoris muscle was measured with the subjects seated on a leg extension machine (Carci®, São Paulo, Brazil) with load cell (EMG System model EMG800C, São José dos Campos, Brazil). The MIVC of the biceps brachii muscle was performed with the subjects seated on a chair. Three tests were performed for each muscle for 5s each, with a 1-min rest interval between consecutive measurements.26 The outcome was the greatest contraction value of quadriceps femoris and biceps brachii. The time of isometric endurance test was registered until 10% of drop off 60% of MIVC. The outcome was endurance time.
Physical activity in daily lifeThe subjects were monitored using an accelerometer (ActiGraph, GT3X, FL, USA) through seven consecutive days. Subjects were considered as successfully monitored when they used the accelerometer for at least 4 days for more than 12h/day.27 The outcomes were the number of steps per day (sedentary lifestyle was defined as <11,500steps/day),27,28 the time spent in sedentary/light physical activity (SLPA) was defined as 0–2295steps/day; and the time spent in moderate/vigorous physical activity (MVPA) defined as counts >2296steps/day.27
Data analysesThe sample size was calculated based on the DW at the MST and quadriceps muscle strength in a pilot study. The effect size for DW between groups was 0.89, with α=0.05, power of 80%, and n=21 per group. The effect size for quadriceps muscle strength was 1.5, with α=0.05, power of 80%, and n=8 per group. A moderate (r=0.6) correlation was observed between muscle strength and functional capacity, with α=0.05, power of 80%, and v=19 per group.
The data normality was analyzed using the Shapiro–Wilk test. Parametric data were presented as mean (SD) values, while nonparametric data were presented as median and interquartile (IQR 25–75%) ranges. To compare the AG and the CG, unpaired t test or Mann–Whitney was used. The AG was stratified according to the GINA19 steps as follows: intermittent/persistent asthma (GINA 1, 2, 3) or moderate/severe asthma (GINA 4 and 5). Analysis of variance (ANOVA) was performed with the Bonferroni test as a post hoc test to evaluate the differences in the severities of AG and CG. Pearson or Spearman correlation tests were used to correlate functional capacity, muscle strength, PADL, and inflammatory profile. The chi-square test was performed for desaturation during the exercise tests between the groups. SPSS (IBM SPSS Statistics for Windows, version 20.0., NY, USA) was the statistical software used in the study. A p<0.05 was considered statistically significant.
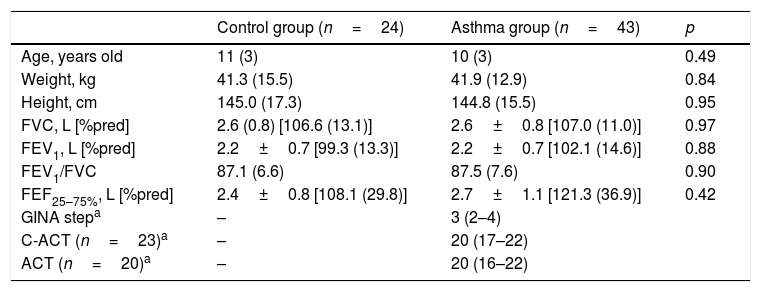
ResultsSixty-seven subjects with asthma were enrolled in this study. Of these, 12 dropped out of the study, 11 were excluded because they were physically active, and one was excluded because of an associated heart disease. Forty-three asthmatic subjects completed the protocol. For the control group (CG), 37 volunteers were invited to participate and 24 completed the study; 12 dropped out, and one was excluded because of failure to complete the tests. In the asthma group (AG), 20 (46%) volunteers were girls, while in the CG, 14 (58%) subjects were girls. Table 1 presents the characteristics of the groups.
Characteristics of the groups.
| Control group (n=24) | Asthma group (n=43) | p | |
|---|---|---|---|
| Age, years old | 11 (3) | 10 (3) | 0.49 |
| Weight, kg | 41.3 (15.5) | 41.9 (12.9) | 0.84 |
| Height, cm | 145.0 (17.3) | 144.8 (15.5) | 0.95 |
| FVC, L [%pred] | 2.6 (0.8) [106.6 (13.1)] | 2.6±0.8 [107.0 (11.0)] | 0.97 |
| FEV1, L [%pred] | 2.2±0.7 [99.3 (13.3)] | 2.2±0.7 [102.1 (14.6)] | 0.88 |
| FEV1/FVC | 87.1 (6.6) | 87.5 (7.6) | 0.90 |
| FEF25–75%, L [%pred] | 2.4±0.8 [108.1 (29.8)] | 2.7±1.1 [121.3 (36.9)] | 0.42 |
| GINA stepa | – | 3 (2–4) | |
| C-ACT (n=23)a | – | 20 (17–22) | |
| ACT (n=20)a | – | 20 (16–22) |
The data is represented as mean (SD).
In 37 (86%) subjects in the AG, the disease was controlled or partially controlled (ACT or C-ACT >16), and daily medications were as following: eight (19%) subjects were taking low doses of inhaled corticosteroids (ICS); 21 (49%) subjects were taking medium doses of ICS; seven (16%) subjects were taking high doses of ICS; and seven (16%) subjects underwent SABA. Additionally, 26 (60%) subjects were classified as GINA steps 1, 2 (mild asthma: low dose of ICS and leukotriene receptor antagonists or chromones), and 3 (moderate asthma: low dose of ICS/LABA), and 17 (40%) were classified as GINA steps 4 and 5 (severe asthma: high dose of ICS/LABA).
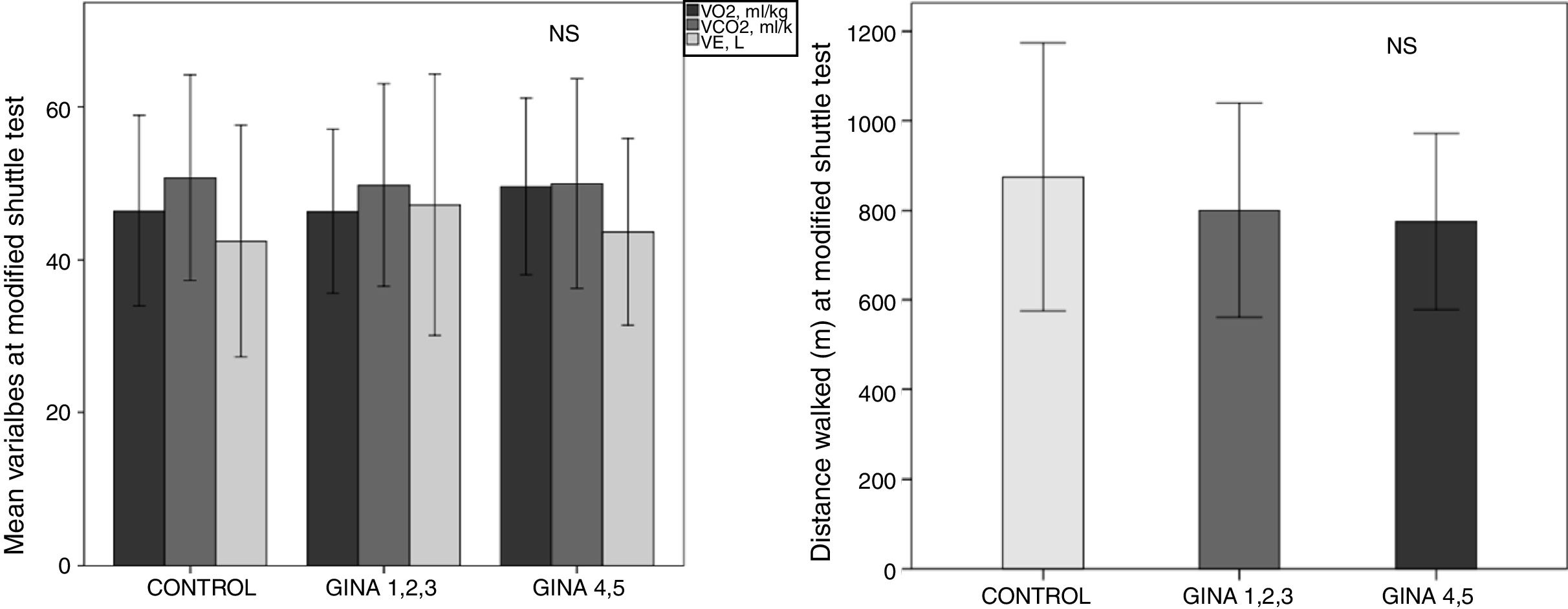
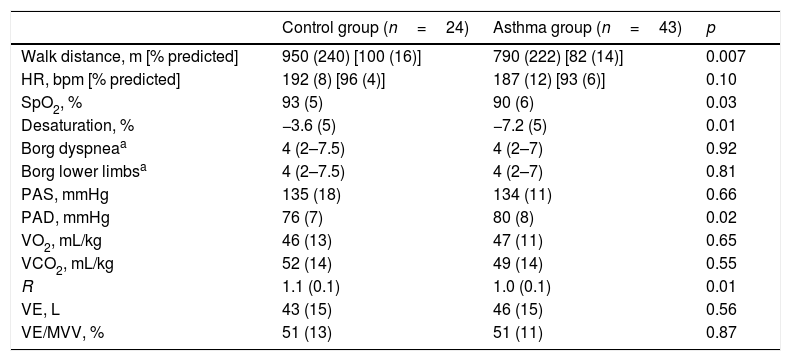
Functional capacity, evaluated based on the distance walked (DW), and SpO2 in the AG were lower than that in the CG (Table 2). There were no differences in VO2 peak, VCO2 peak, VE, and DW according to asthma severity (p>0.05, Fig. 1).
Variables at the peak of modified shuttle test (MST).
| Control group (n=24) | Asthma group (n=43) | p | |
|---|---|---|---|
| Walk distance, m [% predicted] | 950 (240) [100 (16)] | 790 (222) [82 (14)] | 0.007 |
| HR, bpm [% predicted] | 192 (8) [96 (4)] | 187 (12) [93 (6)] | 0.10 |
| SpO2, % | 93 (5) | 90 (6) | 0.03 |
| Desaturation, % | −3.6 (5) | −7.2 (5) | 0.01 |
| Borg dyspneaa | 4 (2–7.5) | 4 (2–7) | 0.92 |
| Borg lower limbsa | 4 (2–7.5) | 4 (2–7) | 0.81 |
| PAS, mmHg | 135 (18) | 134 (11) | 0.66 |
| PAD, mmHg | 76 (7) | 80 (8) | 0.02 |
| VO2, mL/kg | 46 (13) | 47 (11) | 0.65 |
| VCO2, mL/kg | 52 (14) | 49 (14) | 0.55 |
| R | 1.1 (0.1) | 1.0 (0.1) | 0.01 |
| VE, L | 43 (15) | 46 (15) | 0.56 |
| VE/MVV, % | 51 (13) | 51 (11) | 0.87 |
The data is represented as mean (SD).
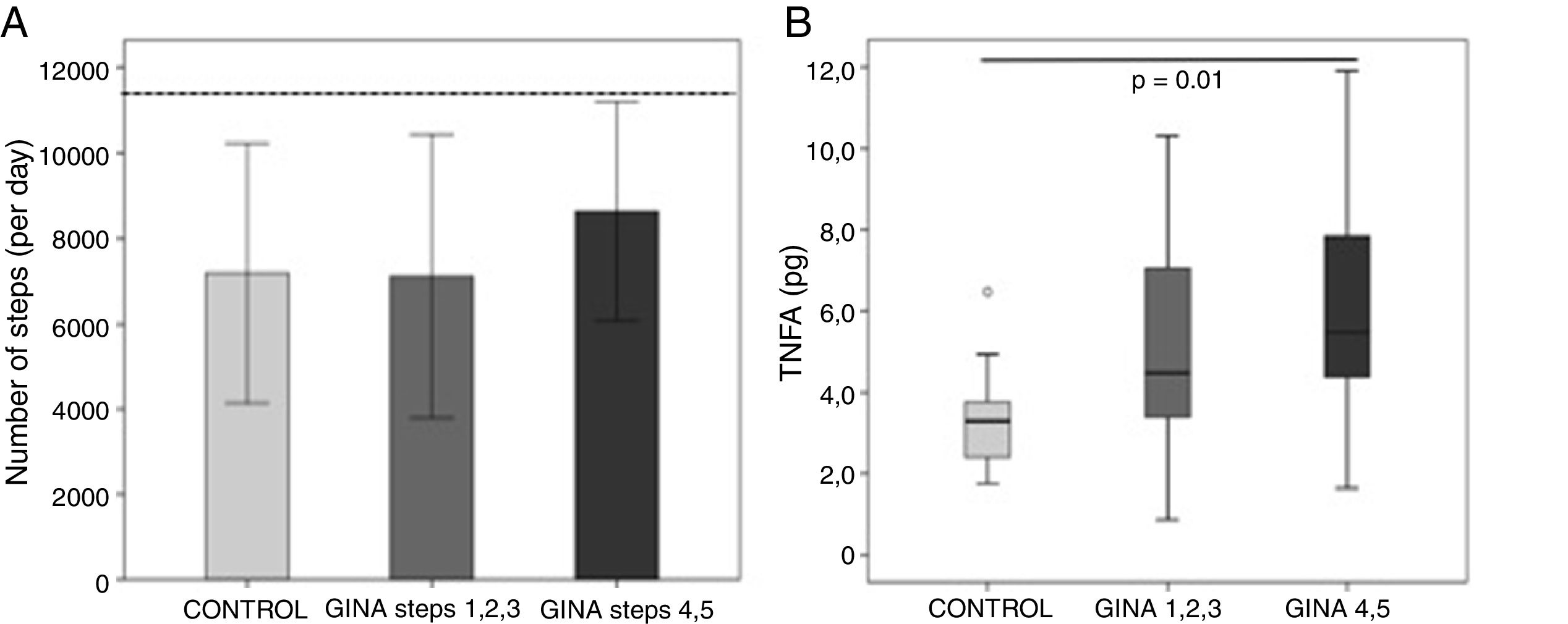
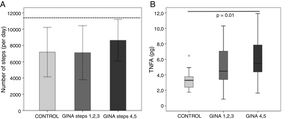
There were no differences in biceps (9.7±3.6kg vs. 8.8±2.0kg, p=0.30) and quadriceps (41.2±30.7kg vs. 39.9±21.2kg, p=0.28) muscle strength between the AG and CG, respectively, and asthma severity did not influence these variables (data not shown). The 36 subjects of the AG and 23 subjects of the CG correctly used the accelerometer, and the number of steps per day was similar between groups (CG: 7181 [3040] steps vs. AG: 7743 [3075] steps, p=0.41). Except for two subjects in the AG, all subjects performed the minimal number of steps per day to be considered to have an active daily life. The number of steps taken by subjects with severe asthma (GINA steps 4 and 5) did not differ from that taken by subjects with less severe asthma (GINA steps 1, 2, and 3) or those in the CG (p=0.37; Fig. 2A).
Blood samples could not be collected from 13 subjects in the AG and seven subjects in the CG. Except for tumor necrosis factor-α levels, no other cytokine levels were different between groups (data not shown). The TNFα for AG was 4.5 (3.3–7.0)pg/mL vs. 3.2 (2.3–3.7) pg/mL for CG (p=0.007). The more severe asthmatic subjects (GINA steps 4 and 5) had higher levels of TNFα compared to the CG subjects (p=0.02; Fig. 2B).
There was a significant correlation between the quadriceps muscle strength and the DW (r=0.62, p<0.001) and between the PADL and TNFα level (r=−0.54, p=0.005) in the AG (Supplementary Material Fig. S1). However, no significant correlations were observed among quadriceps muscle strength, PADL, and TNFα level.
There were also no differences between DW and muscle strength according to the ACT or C-ACT scores (score >20 and those with a score ≤20; data not shown). The authors did not observe any adverse effects during the performance of the MST.
DiscussionTo the best of the authors’ knowledge, this is the first study to perform a global and integrated assessment of the functional capacity, peripheral muscle strength, PADL, and inflammatory profiles of a pediatric population with asthma undergoing regular treatment. Functional capacity was lower in the AG; PADL was similar between subjects with asthma and their healthy peers and both groups did not perform the minimal requirements to be considered active; there was an association between the quadriceps muscle strength and the distance walked, and between the PADL and TNFα.
The functional capacity assessed by the MST was different between the AG and the CG; 23 (53%) subjects in the AG walked less than 80% of the predicted distance during this test. On average, AG subjects walked 160m less than the CG subjects, which is an important difference because it is greater than the minimal clinically reported difference for DW (20–76m).29
Both groups presented similar Borg and ventilation demand; however, the AG walked shorter distance than the CG. In contrast to these results, Augusto et al.13 detected no difference in an incremental shuttle walk test of teenagers with asthma compared to a control group, but their volunteers did not run, which may result in a ceiling effect. To the best of the authors’ knowledge, no study of asthmatic subjects has used the MST, as described by Bradley et al.,10 which provides a better evaluation of functional capacity.
Peripheral muscle strength is an important factor influencing physical activity capacity; however, it is rarely studied in children and adolescents with asthma. Villa et al.17 reported reduced muscle endurance, but not strength, in the quadriceps of children with severe asthma. No differences in muscle strength were observed between groups, despite the fact that the present evaluation was performed using a more detailed evaluation (i.e., load cell assessment). This difference between the studies could be due to differences in regular treatment and the lower asthma severity among the patients in this study compared to Villa et al.’s study.
Interestingly, PADL level in the children and adolescents with asthma did not differ from the CG, and both groups were considered sedentary, because they walked less than 11,500 steps/day.27,28 A sedentary lifestyle was assumed for the AG due to exercise restrictions imposed by the disease.30 Normal PADL was reported in eutrophic asthmatic subjects31; however, different results have been reported previously.16 For PADL measurements (i.e., questionnaires or objective evaluation using an accelerometer), the severity of the disease and the regular treatment can all influence results.
The association between DW and quadriceps muscle strength for asthma patients was also observed. This was an expected correlation. Skeletal muscle dysfunction has been suggested as a factor contributing to reduce exercise tolerance in patients with cystic fibroses32,33 caused by systemic inflammation, oxidative stress, and inactivity.34 Additionally, skeletal muscle weakness has been associated with the functional capacity of patients with chronic diseases, such as cystic fibrosis35 and COPD.36 Another correlation observed was between the PADL and TNFα: the higher the inflammatory level, the lower the NS walked per day. Al-Sahir et al. proposed a correlation between TNFα and physical activity among COPD patients that was similar to our results among pediatric patients.37 Although these correlations could be theoretically expected, to the best of the authors’ knowledge, it has been demonstrated for the first time, here, among a pediatric population with asthma.
A limitation of this study was that the number of subjects classified as GINA steps 4 and 5 was lower than that for those classified as GINA steps 1, 2, and 3. This smaller number reflects the expected distribution of asthma severity. Additionally, it was not possible to evaluate PADL in all subjects due to equipment evaluability; however, most subjects were able to perform this evaluation.
The practical applications of these results are the better understanding of low exercise capacities and sedentary behavior among asthmatic patients undergoing regular treatment, which can provide better guidance for treatment and exercise prescriptions during pulmonary rehabilitation.
In conclusion, this multidimensional assessment of children and adolescents with asthma undergoing regular treatment revealed reduced functional capacity among the study population and sedentary behavior. Additionally, subjects with lower quadriceps muscle strength had lower functional capacity, and the most sedentary asthmatic subjects presented higher TNFα levels.
FundingThis study was supported by São Paulo Research Foundation (FAPESP), grant no. 2014/12040-0. RSS was supported by São Paulo Research Foundation (FAPESP), grant no. 2016/17553-0.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Reimberg MM, Pachi JP, Scalco RS, Serra AJ, Fernandes L, Politti F, et al. Patients with asthma have reduced functional capacity and sedentary behavior. J Pediatr (Rio J). 2020;96:53–9.