Permanent hypoparathyroidism can be presented as part of genetic disorders such as Sanjad–Sakati syndrome (also known as hypoparathyroidism—intellectual disability-dysmorphism), which is a rare autosomal recessive disorder. Our aim was to confirm the diagnosis of a group of patients with dysmorphism, poor growth, and hypoparathyroidism clinically labeled as Sanjad–Sakati syndrome and to identify for the first time the genetic variations on Iranian patients with the same ethnic origin.
MethodsIn this study, 29 cases from 23 unrelated Arab kindreds with permanent hypoparathyroidism and dysmorphism indicating Sanjad-Sakati syndrome were enrolled for 10 years in the southwest of Iran. The mutational analysis by direct sequencing of the tubulin folding cofactor E gene was performed for the patients and their families, as well as their fetuses using genomic DNA.
ResultsTwenty-eight out of 29 cases had parental consanguinity. Twenty-seven cases presented with hypocalcemia seizure and two were referred because of poor weight gain and were found to have asymptomatic hypocalcemia. The dysmorphic features, hypocalcemia in the setting of low to normal parathyroid hormone levels and high phosphorus led to the diagnosis of these cases. Sequencing analysis of the tubulin folding cofactor E gene revealed a homozygous 12-bp deletion (c.155–166del) for all patients. Following that, prenatal diagnosis was performed for eight families, and two fetuses with a homozygous 12-bp deletion were identified.
ConclusionThese results make it much easier and faster to diagnose this syndrome from other similar dysmorphisms and also help to detect carriers, as well as prenatal diagnosis of Sanjad-Sakati syndrome in high-risk families in this population.
O hipoparatireoidismo permanente pode estar presente como parte das doenças genéticas como na síndrome de Sanjad–Sakati (também chamada de síndrome de hipoparatireoidismo, retardo e dismorfismo), que é um distúrbio autossômico recessivo raro. Nosso objetivo foi confirmar o diagnóstico de um grupo de pacientes com dismorfismo, crescimento deficiente e hipoparatireoidismo clinicamente identificado como síndrome de Sanjad–Sakati e identificar as variações genéticas, pela primeira vez, em pacientes iranianos com a mesma origem étnica.
MétodosNeste estudo, foram inscritos 29 casos de 23 famílias árabes sem parentesco com hipoparatireoidismo e dismorfismo indicando síndrome de Sanjad-Sakati, durante 10 anos no sudoeste do Irã. Foi feita a análise mutacional por sequenciamento direto do gene do cofator E de dobramento da tubulina dos pacientes e de suas famílias e também de seus fetos com o DNA genômico.
ResultadosApresentaram consanguinidade parental 28 dos 29 casos. Desses, 27 casos apresentaram convulsão por hipocalcemia e dois foram encaminhados devido ao baixo ganho de peso, considerando diagnóstico de hipocalcemia assintomática. As características dismórficas, hipocalcemia na configuração de níveis de hormônio da paratireoide baixos a normais e alto nível de fósforo levaram ao diagnóstico dos casos. A análise de sequenciamento do gene do cofator E de dobramento da tubulina revelou deleção homozigótica de 12 pares de base (pb) (c.155–166del) em todos os pacientes. Após isso, foi feito o diagnóstico pré-natal em oito famílias e dois fetos foram identificados com deleção homozigótica de 12 pb.
ConclusãoEsses resultados tornam o diagnóstico dessa síndrome muito mais fácil e rápido do que outros dismorfismos semelhantes e também ajudam a detectar portadores, bem como, o diagnóstico pré-natal da síndrome de Sanjad-Sakati em famílias de alto risco nessa população.
Sanjad–Sakati syndrome (SSS), or hypoparathyroidism—intellectual disability-dysmorphism, is a rare autosomal, recessive, permanent, congenital hypoparathyroidism disorder.1 At first, Sanjad and his colleagues (1991) described this syndrome in the children of consanguineous parents from the Middle East that was characterized by hypocalcemia and hyper-phosphatemia in the setting of low parathormone (PTH), indicating hypoparathyroidism.2,3 Hypoparathyroidism in neonates is relatively common. It usually appears as a temporary situation associated with maternal diabetes, prematurity, and prenatal asphyxiation.4 Permanent congenital hypoparathyroidism is a serious condition that can exist as an isolated disorder or in combination with facial dysmorphic features, intrauterine and (extreme) postnatal growth failure, and developmental delay.1,5–7 The SSS has been reported exclusively in children born to consanguineous parents in Arab populations of the Middle East. It is caused by defects in the parathyroid hormone (PTH) secretion, the main factor in calcium homeostasis, that is released by the parathyroid glands.7–9 It can be related to the mutations in the tubulin folding cofactor E (TBCE) gene on chromosome 1q42-43, which encodes the tubulin-specific chaperone E.10 Parvari et al.10 studied 52 SSS patients from nearby Middle Eastern countries and demonstrated a 12-bp deletion (c.155–166del) within the TBCE gene, while the deletion was not present in more than 350 control chromosomes from Arab persons. The TBCE gene encodes one of the five chaperone proteins that are necessary for the suitable folding of α-tubulin subunits and the constitution of α–β-tubulin heterodimers.11 Padidela et al.12 suggested that TBCE may play a role in the development of the parathyroid glands. After that, Padidela et al.12 reported six children from four unrelated Middle Eastern families with SSS confirmed by genetic analysis. All were homozygous for the 12-bp deletion in the TBCE gene. Moreover, other mutations in the TBCE gene have been reported in some other studies.10,11
Until now, the characterization of TBCE gene mutations has been reported in Kuwait and Jordan Arab populations, as well as in a few number of studied patients in other studies with a limitation.1,13 Finding a molecular defect as the cause for a collection of clinical and biochemical abnormalities is mandatory for diagnosis confirmation and management, especially in a very rare disease like SSS. Based on the published data, this is the first report on the clinical study and TBCE gene mutation characterization in Iranian SSS patients, and it also presented one of the highest numbers of screening of SSS patients during the last 10 years. In this study, we evaluated 29 cases with permanent hypoparathyroidism clinically diagnosed as SSS.
Materials and methodsSubjectsWe studied 29 SSS patients from 23 Iranian Arab kindreds who were selected from the pediatric endocrinology clinic of the Abuzar Children's Hospital in Ahvaz, located in the southwest of Iran, from 2007 to 2017. This hospital is affiliated to the Ahvaz Jundishapur University of Medical Sciences and it is the main tertiary referral hospital for children in the region. Informed consent was obtained from each patient and patient's family, and the ethics committee of the Ahvaz Jundishapur University of Medical Sciences has approved this study (IR.AJUMS.REC.1397.181). A detailed questionnaire was completed by the physicians caring for the patients with SSS. Clinical and laboratory data were collected from the patients from their birth until September 2017, or until their death if they died before this date.
Sequencing analysis of the TBCE geneGenomic DNA was extracted from peripheral blood samples of each patient and their parents using the salting out method. In those receiving prenatal testing, genomic DNA was obtained from 30mg of chorionic villi at 13 weeks of pregnancy using a DNA isolation kit for cells and tissues (Roche). In order to identify the mutation in 29 SSS patients, polymerase chain reaction (PCR) was performed for exon 3 of the TBCE gene using primers and conditions that were previously described.1 After that, PCR products were subjected to Sanger sequencing using the ABI Prism 3700 apparatus (BigDye Terminator sequencing kit, Applied Biosystems), and sequencing data were compared with the reference sequence (NM_001287801). If a mutation was detected, parents of that patient were analyzed for the validation of segregation, and prenatal testing was carried out for eight families by also using Sanger sequencing.
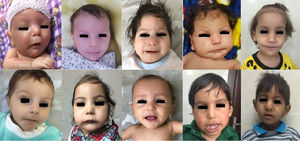
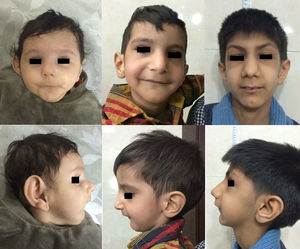
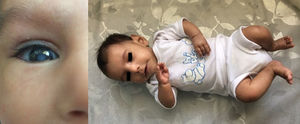
ResultsDescription of patients and kindredsWe studied 29 patients (17 males and 12 females) from 27 families and 23 kindreds with SSS. There are different ethnicities in the southwest of Iran, and all studied families belong to the Arab ethnic group. The parents were consanguineous in 26 out of the 27 families. There were double first cousins (3 families), first-degree cousins (12 families), and second-degree cousins (11 families). The parents of one patient were not consanguineous; however, they came from the same village. Twenty seven out of 29 cases were diagnosed with seizure related to low calcium between the ages of 14 days and 9 months, whereas 2 female infants were referred for clinical evaluation in the first 3 months of life because of poor growth before developing symptomatic hypocalcemia on clinical examination. All of these children had characteristic dysmorphic features including small, deep-set eyes, a bulbous or beaked nose with/without a depressed nasal bridge, long philtrum with thin upper lip, low-set ears with large floppy earlobes, micrognathia, delayed dentition and early decay, as well as small hands and feet. All of them were born with intrauterine growth retardation (1500–2500g) and had a severe symmetric growth failure (Figs. 1–3). Meanwhile, all patients had low levels of calcium in the setting of low to normal PTH levels as well as high phosphorus. At presentation, serum calcium ranged from 5 to 7mg/dL (8.5–11mg/dL), and for phosphorus the range was 6.4–13mg/dL (4–6.5mg/dL). Alkaline phosphatase was normal for age (<700U/L) in all patients except for one (1350U/L) who had an extremely low serum value of vitamin D (4.2ng/mL; reference range: 30–50ng/mL). PTH values available for nearly half the subjects were <0.4–7.5 (mean 2.18; reference range: 15–65pg/mL). Interestingly, the case with the lowest value for PTH also presented congenital cataract (Fig. 3), thus indicating severe, long-standing hypocalcemia.
Treatment was started for all 29 patients with calcium supplements and an active form of vitamin D. Definite diagnosis was made on hypoparathyroidism, dysmorphic features, and the presence of pathogenic mutation.
TBCE gene mutationThe mutational analysis by direct sequencing of the TBCE gene revealed that all 29 affected subjects studied were homozygous for a 12-bp deletion (c.155–166del) within exon 3. Screening for detected deletion in the parents of affected individuals revealed that both parents of all the patients were heterozygous carriers for this deletion. Following that, we performed prenatal diagnosis for eight families. Two out of eight fetuses showed homozygous deletion of the 12-bp within the exon 3 of the TBCE gene and were considered SSS patients (abortion performed). Among the other fetuses, three of them were heterozygote carriers, while in three fetuses we did not detect this pathogenic mutation in the TBCE gene.
DiscussionWe studied 29 SSS patients with Arab ethnicity from the southwest of Iran, where the highest Arab population of Iran is located. All patients were homozygote for c.155–166 deletion, which resulted in p.Ser52-Gly55 deletion in the CAPGly domain of the TBCE transcript.
The detected mutation in our studied cases is the same as the one commonly reported in SSS patients of Arab origin from the Middle East.1,9,10,14,15 Available genetic data, including same mutation in all Arab patients from Iran and other Arab populations in the Middle East, is consistent with a founder effect mutation which spread to the Middle Eastern area.10 Thus, until now—except for the 12-bp deletion—different mutations in the TBCE gene have been reported in other studies. Sferra et al., using haplotype analysis, identified a founder effect for I155N mutation in the TBCE gene as well as significantly reduced amounts of mutant TBCE protein in five patients from three unrelated Italian families.11 Parvari et al.10 showed compound heterozygosity for 66delAG in the first coding exon and 1113T-A transversion in exon 12 in the TBCE gene of a Belgian patient with SSS. Meanwhile, Courtens et al.16 reported a girl with SSS and normal TBCE gene and protein, but a de novo microduplication on chromosome 4q35 detected that a second gene locus for the disorder seems probable.
Notably, the existence of a same trinucleotide GGG on each breakpoint side of the deleted region might lead to replication slipping and deletion in this location, in the primary ancestor.17 Also, since the TBCE chaperone is an essential factor for the constitution of α–β-tubulin heterodimers and the development of the parathyroid glands, it may require a function of the microtubule cytoskeleton, such as migration, or cell morphology; thereby, any change on TBCE that makes a chaperon E protein with low activity could cause illness.12,18–20
In this study, consanguinity was found in 28 out of 29 patients, which is caused by the high rate of consanguineous marriages among the Iranian Arab population—it may reach up to 50% of all marriages—due to sociocultural factors. Specifically, marriage among cousins is allowed and often encouraged in this population, thus resulting in retention and increased rate of homozygotes of autosomal recessive genetic disorders such as SSS in this population. Meanwhile, known dominant disorders are more numerous worldwide than known recessive disorders.21,22
The previous reports have indicated a significant prenatal and postnatal growth impairment.1,3,9,13 As mentioned, our cases were born with moderate intrauterine growth retardation and almost all of the cases had impaired growth indices at least 4–5 Z-score below the mean for their age and sex. Interestingly, our patients were quite similar in phenotype to what was reported in the original paper by Sanjad3 as well as by our Omani colleagues9 regarding the shape of eyes, ears, lips, and limbs, except for micrognathia, that we have found to be a common significant finding (Fig. 2) compared to what was reported for both Omani9 and Kuwaiti1 patients; for them, there is only mention of a narrow face, which is evident in their photos.
In accordance with all previous reports we also found different degrees of intellectual disability and developmental delay but no cardiac or genital abnormalities. Isolated microphallus in males seems to be part of a symmetric, generalized small size of the whole body rather than a distinct structural or endocrinologic abnormality.
Unfortunately, hypoparathyroidism requiring treatment was universal in our group of patients; however, some could be discovered before developing tetany. Almost one-fourth of our patients needed anticonvulsant to control seizures unrelated to hypocalcemia. Elhassanien and Alghaiaty7 reported seizure in all patients, but by eliminating calcium-dependent tetanies seizure alone does not seem to be a common problem.
Similar to other reports we are also losing some patients to pneumonia or sepsis like an attack, but no immunological defect was found in previous reports. More detailed investigation on this issue might open a window to other undiagnosed abnormalities in these patients. Finally, the existence of permanent congenital hypoparathyroidism from the intrauterine onset, severe postnatal growth failure, and facial dysmorphism with any degree of developmental delay strongly suggests SSS.
In conclusion, the detected mutation and presenting clinical manifestation of this rare disease in Iranian patients makes it much easier and faster to diagnose and differentiate this syndrome from other similar dysmorphisms; additionally, it helps the discovery of carriers and prospective counseling, as well as prenatal diagnosis of SSS in high-risk families. The availability of genetic testing enables accurate diagnosis of affected children and differentiation from other syndromes. Finally, detecting a unique mutation in this population shows the same origin of mutation and was one of the principal aims of this study.
Conflicts of interestThe authors declare no conflicts of interest.
We thank the patients and their families for their cooperation and trust.
Please cite this article as: Aminzadeh M, Galehdari H, Shariati G, Malekpour N, Ghandil P. Clinical features and tubulin folding cofactor E gene analysis in Iranian patients with Sanjad–Sakati syndrome. J Pediatr (Rio J). 2020;96:60–5.