To evaluate the effect of oropharyngeal colostrum immunotherapy (OCI) on the mortality of preterm newborns (PTNB) with very low birth weight (VLBW).
MethodNon-randomized clinical trial, carried out with 138 mother-child pairs attended at a public maternity hospital. The treatment group used raw colostrum, dripping 4 drops (0.2 ml) into the oropharyngeal mucosa, totaling 8 administrations in 24 h, up to the 7th complete day of life (OCI). The control group was composed of newborns admitted to the same maternity hospital before the implementation of the OCI. Analyzes were performed: descriptive, bivariate, multiple logistic regression, and survival analysis, with a significance level of 5% and 95% CI.
ResultsThe treatment group had an RR of death of 0.26 (95% CI = 0.07–0.67; p = 0.00), adjusted for maternal age, marital status, gestational hypertension, type of delivery, number of prenatal visits, and birth weight. Number Needed to Treat (NNT) demonstrated that for every 5 individuals treated with OCI, one death was prevented NNT = 4.9 (95% CI = 1.84–5.20); however, for PTNB with VLBW who remained hospitalized for 50, 100 and 150 days, the NNT reduces to 4, 4 and 3, respectively.
ConclusionThe OCI proved to be a beneficial intervention, since it reduced the risk of mortality in PTNB with VLBW when compared to the control group.
Premature birth is currently configured as a public health problem in the world. It is known that preterm newborns (PTNB) have a higher risk of death, due to pathologies such as necrotizing enterocolitis, neonatal septicemia, and pneumonia associated with mechanical ventilation1 and, when they survive, they may develop long-term disabilities.
It is in this cycle of premature birth, risk of morbidities, neonatal mortality, and disabilities in the short and long term that oropharyngeal colostrum immunotherapy (OCI) has been suggested as an intervention to promote the clinical improvement of these newborns, especially those with very low birth weight (VLW).2,3 This therapy is based on the expectation of absorption of bioactive factors present in colostrum, by the oropharyngeal mucosa, with an amplified response to various devices, with systemic bacteriostatic, bactericidal, antiviral, anti-inflammatory, and immunomodulatory effects.2
It is important to highlight that PTNB-VLW present immunological immaturity and of devices and systems, especially of the gastrointestinal tract. It has been reported that the institution of OCI practice favored positive clinical outcomes, such as a reduction in the incidence of necrotizing enterocolitis, pneumonia associated with mechanical ventilation, and late septicemia, in addition to a decrease in the time for PTNB to reach full enteral feeding and hospital stay.1 Despite the biological plausibility, the evidence for OCI in reducing the risk of mortality in very low birth weight preterm is insufficient,4-12 and the present study intends to further elucidate the role of OCI through a clinical trial.
MethodsThis manuscript was written under the guidance of CONSORT 2010.13
Study designThe study consists of a non-randomized, superiority, ambispective clinical trial, with an intervention group consisting of VLBW-PT admitted to the neonatal unit of a maternity hospital who used OCI (treatment); and a historical control group, composed of VLBW-PT who were born in the same maternity hospital before the protocol implementation, i.e., without the use of this immunotherapy (Brazilian Clinical Trial Registry (ReBEC), under n. U1111-1222-0598, http://www.ensaiosclinicos.gov.br/rg/RBR-2cyp7c/). The allocation ratio was 1:1 with participants entering sequentially, and there were no deviations from the study protocol.
Historical control was defined in compliance with the opinion of the Research Ethics Committee of the Universidade Estadual de Feira de Santana (CAAE no. 93056218.0.0000.0053), considering that not offering colostrum to PTNB in the control group violates the principle of beneficence, since the positive effects of colostrum are already established in the literature.
ParticipantsVLBW-PT (≤ 1,500g), born with gestational age ≤ 37 weeks, regardless of the type of diet (zero, enteral by orogastric tube or parenteral), and clinically stable in the last three hours before starting immunotherapy were included. The beginning of the OCI protocol occurred within 72 hours of life.
Maternal exclusion criteria were reports of substance or drug abuse, psychological disorder, multiparity (triplets and more), and medical contraindications for breastfeeding (retrovirus and cytomegalovirus). With regard to newborns, they were not included when they were using vasopressor medication > 10 mcg/Kg/min, in need of immediate surgical intervention and presence of syndromes or malformations.
The study was conducted in the neonatal unit of the Hospital Inácia Pinto dos Santos (Women's Hospital) in Feira de Santana, Bahia, Brazil, a medium-sized public maternity hospital accredited by the Baby-Friendly Hospital Initiative, linked to the Unified Health System (SUS - Sistema Único de Saúde), maintained by the Fundação Hospitalar de Feira de Santana. Also noteworthy is the assistance provided by the Human Milk Bank (HMB) linked to the Brazilian Network of Human Milk Banks, the sector responsible for complying with part of this protocol, such as welcoming mothers of PTNB, extracting colostrum, and distributing of colostrum-destined for OCI.
InterventionThe OCI intervention consisted of using raw colostrum, from mother to child, initiated in the treatment group within 72 h of life of VLBW-PT, after medical prescription by the neonatal team. Treatment was offered until the 7th full day of life of newborns (totaling up to 56 syringes), with 8 (eight) daily administrations of 0.2 ml (04 drops) of dripped colostrum within 10 (ten) seconds in the oropharyngeal mucosa, with 0.1 ml (two drops) in the tissue of the right oral mucosa in the first 5 (five) seconds and the other two drops in the tissue of the left oral mucosa in the remaining seconds. Newborns’ vital conditions were monitored during the procedure. Therapy was considered complete when more than 75% of planned doses were administered for each PTNB, and clinical evolution was monitored until hospital discharge.
When the neonatal team identified changes in newborns’ clinical stability criteria, at the time of treatment or periodic follow-up, the intervention was immediately interrupted, being resumed as soon as clinical stability was restored. Complete interruption of treatment occurred upon justifiable medical prerogative, especially in severe cases of VLBW-PT.
Immunotherapy did not occur in the control group, since this group consisted of VLBW-PT admitted to the neonatal intensive and intermediate care units from 2015 to 2016, that is, the period prior to OCI implementation in the institution (historical control). For data collection, the records of the Medical Archive and Statistics Service (SAME - Serviço de Arquivo Médico e Estatística) of the institution were consulted. More details of the intervention are described in the study by da Cruz Martins et al. (2020).3
OutcomesThe main independent variable was OCI use, defined in this study as the use of colostrum for immunological and not nutritional supplementation purposes by VLBW-PT during hospitalization in the neonatal unit and with follow-up of clinical evolution. As an outcome (dependent variable), death was assessed, verified by the permanent disappearance of all signs of life, at any time after birth, with no possibility of resuscitation, as defined by the World Health Organization,14 and with a medical record and/or Death Certificate. Other variables were investigated in both groups such as: maternal sociodemographic characteristics; prenatal and childbirth variables and variables related to newborns.
Sample sizeIn the protocol, an α = 5% was defined for sample size calculation, ß = 80%, with an incidence of death in the intervention group = 12.5% and control = 25%, in a 1: 1 ratio, obtained in the study by Lee et al. (2015).7 A fixed sample was calculated for an infinite population, using the Bioestat 5.3 software, which resulted in 152 participants in each group, with an increase of 15% to cover possible losses, totaling 350 participants.3
However, as this is a finite population, without replacement and with a mean admission to the neonatal unit of 68 VLBW-PT in the last five years (136 individuals), it was possible to readjust the sample calculation through the population correction factor.15 Thus, the final sample size estimate was around 76 (seventy-six) participants. 15% were also added for possible losses, totaling 88 (eighty-eight) participants, 44 (forty-four) for each group.
The results of this study are based on the already published protocol, whose analysis was planned to verify the occurrence of treatment damage/benefits between the compared groups, in the second year of OCI follow-up.3
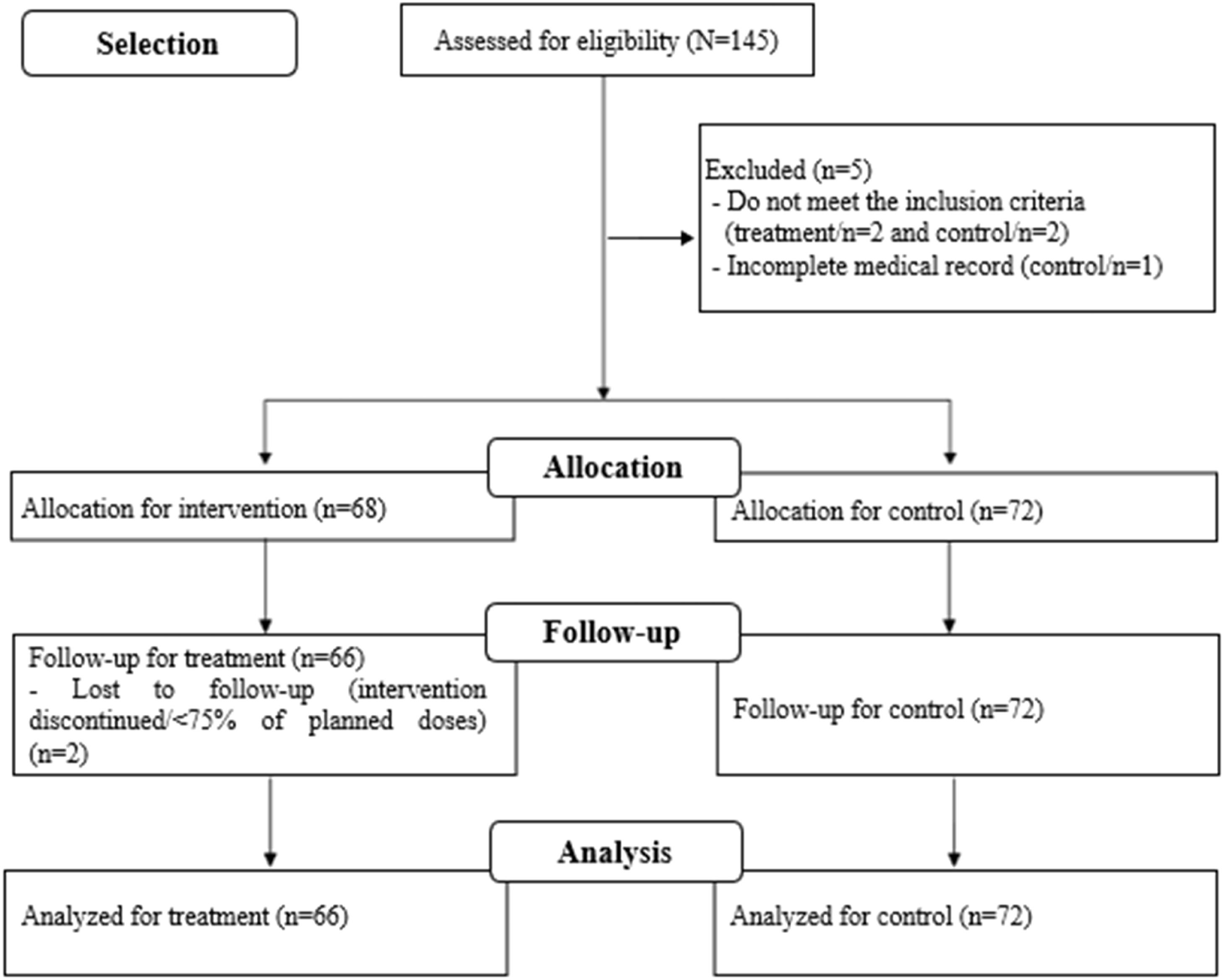
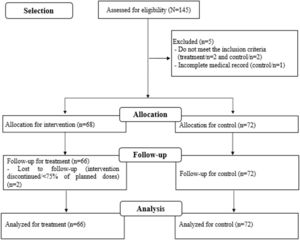
RecruitmentStudy recruitment to the intervention group was from October 2018 to August 2020; for the control group, medical records of VLBW-PT hospitalized between May 2015 and November 2016 were assessed. Losses to follow-up were monitored during recruitment (Figure 1), as described in protocol.3 The intention-to-treat analysis strategy was adopted.
Statistical methodsData were entered by previously trained health professionals, into two different databases, with subsequent comparison and validation using the EPIDATA software. Data analysis procedures were developed with IBM-SPSS (version 24.0, Chicago, IL, USA, serial number: 10101181103) and STATA 15.1 (serial number: 401506208261) software.
Descriptive analysis was performed for all variables according to comparison groups. The Kolmogorov-Smirnov test was applied and the histogram was inspected to verify the normality of continuous variables. Student's t test or Mann-Whitney U test was used, according to the normality test, using measures of central tendency and variability to verify differences between groups. Categorical covariates were assessed using chi-square or Fisher's exact tests, and a significance level of 5% was adopted.
Initially, relative risk (unadjusted and adjusted) was estimated as a measure of effect, with respective 95% confidence intervals and 5% significance levels. For this purpose, logistic regression was used with the backward strategy and, subsequently, conversion was performed using the method by Zhang and Yu (1998)16 to obtain the appropriate measure for the study design.
Potential confounders were selected in stratified analysis. A covariate was considered confounding when, after being eliminated in the saturated model, there was a variation in the effect measure greater than 10%. Moreover, the criterion of epidemiological importance was used for the selection of confounding during modeling, adopting a theoretical-conceptual model to guide said selection.5,9,17
The impact of effect magnitude was further assessed by the Number Needed to Treat (NNT) and Cohen's h index, which assessed the effect size (h) based on mortality risks in the groups studied. Cohen's h index is interpreted as follows: small (h = 0.20), medium (h = 0.50), and large (h = 0.80) effect).18
Finally, a survival analysis was performed to estimate the effect measure as a function of time (unadjusted and adjusted hazard ratio and their respective confidence intervals). For this purpose, the Kaplan-Meier method was used in order to assess the size size on survival over time between the comparison groups. The crude survival effect was estimated at the beginning of the curve by the logrank test (Mantel-Cox) and from the middle to the end by the Breslow test (generalized wilcoxon), with a 5% significance level. The Cox regression model estimated the hazard ratio, duly adjusted for confounding variables selected based on the theoretical-conceptual model adopted to assess the effect of therapy on the study.
ResultsParticipant flowThe study participant flow is described in the flow diagram (Figure 1) as well as the total number of exclusions and losses in each stage of follow-up, with the respective justifications.
Baseline characteristics and auxiliary analysesA total of 138 (one hundred and thirty-eight) mothers/children were assessed, 66 (sixty-six) in the treatment group and 72 (seventy-two) in the control group. The total number of doses administered was 2735 (two thousand, seven hundred and thirty-five) syringes containing 0.2 ml of colostrum each, 32 (48.5%) of VLBW-PT started treatment within 24 hours of life and 42 (63.6%) VLBW-PT received > 90% of planned treatment, demonstrating adherence and compliance with the protocol in the unit.
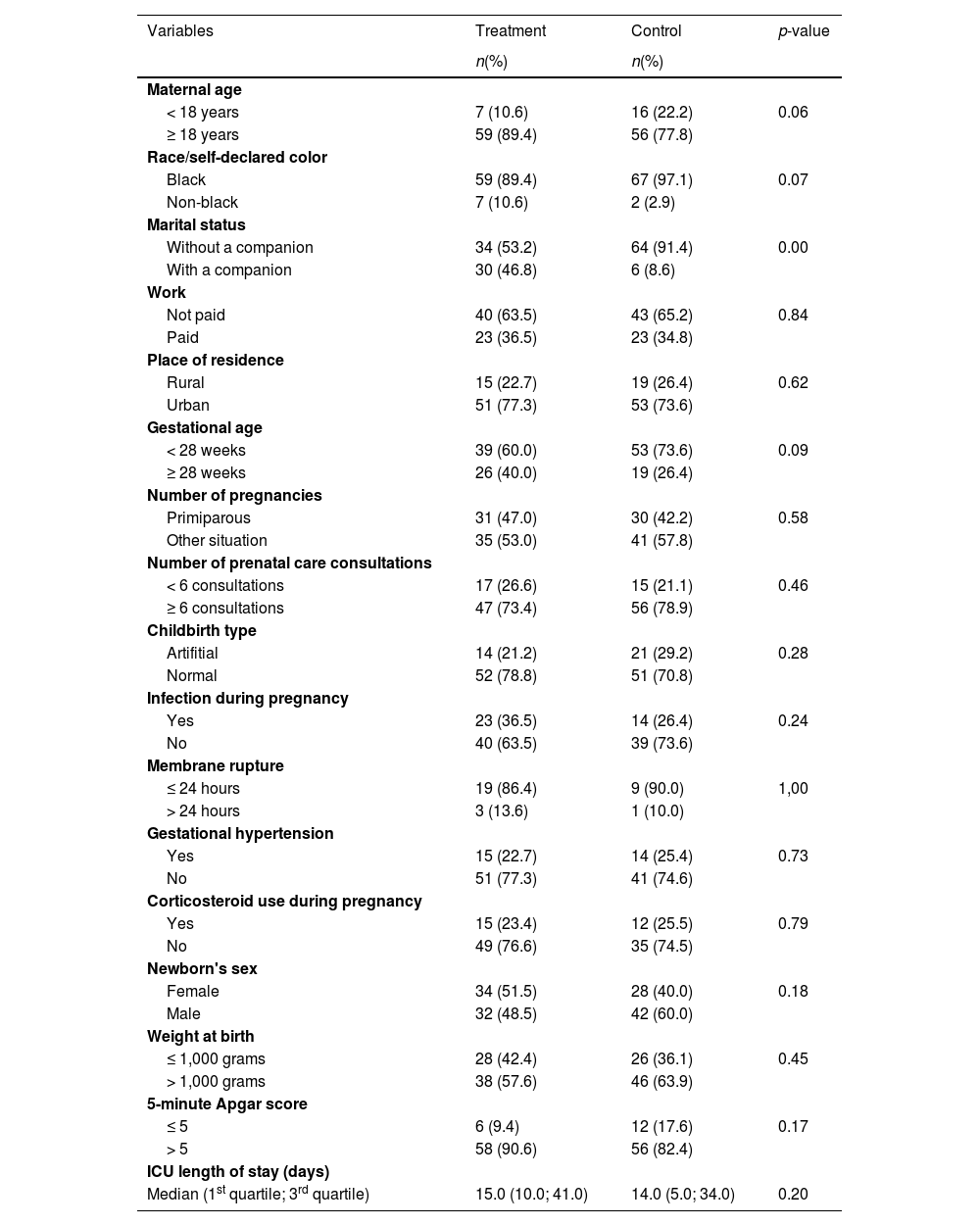
Maternal and neonatal characteristics associated with treatment are described in Table 1 and demonstrated similarities between the groups, except for the marital status covariate, which was demonstrated by stratified analysis as a possible confounder and inserted in the final analysis model, along with other variables justified by the literature.
Maternal characteristics and very low birth weight preterm newborns according to colostrum oropharyngeal immunotherapy (n = 138).
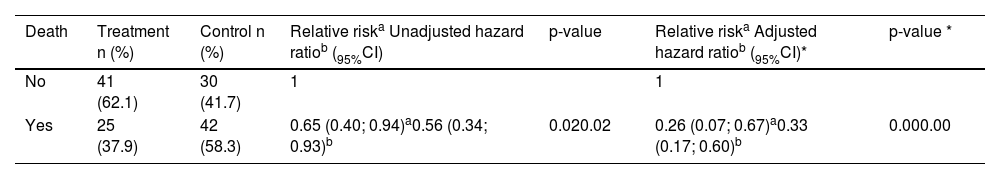
The magnitude of the intervention effect was calculated with the data presented in Table 2, both unadjusted and adjusted, the latter by the following confounders: maternal age, marital status, gestational hypertension, childbirth type, number of prenatal consultations, and birth weight.
Unadjusted and adjusted association of the effect of oropharyngeal colostrum immunotherapy on death risk in very low birth weight preterm newborns, with respective 95% confidence intervals (95%CI).
The adjusted relative risk (RR) was 0.26 (95% CI = 0.07–0.67; p = 0.00), relative risk reduction (RRR) of 35% (95% CI = 0.33–0.93), the absolute risk for the treatment group of 37.9% and control group of 58.3%, and absolute risk reduction (ARR) of 20.4% (95% CI = 0.19–0.54). Calculation of NNT showed a probability of survival throughout the study of 4.9 (95% CI = 1.84–5.20), with an effect size estimated by Cohen's h index of 0.41, which indicates a medium effect. At 50, 100, and 150 days the NNT was 4.0, 4.0 and 3.0, respectively.
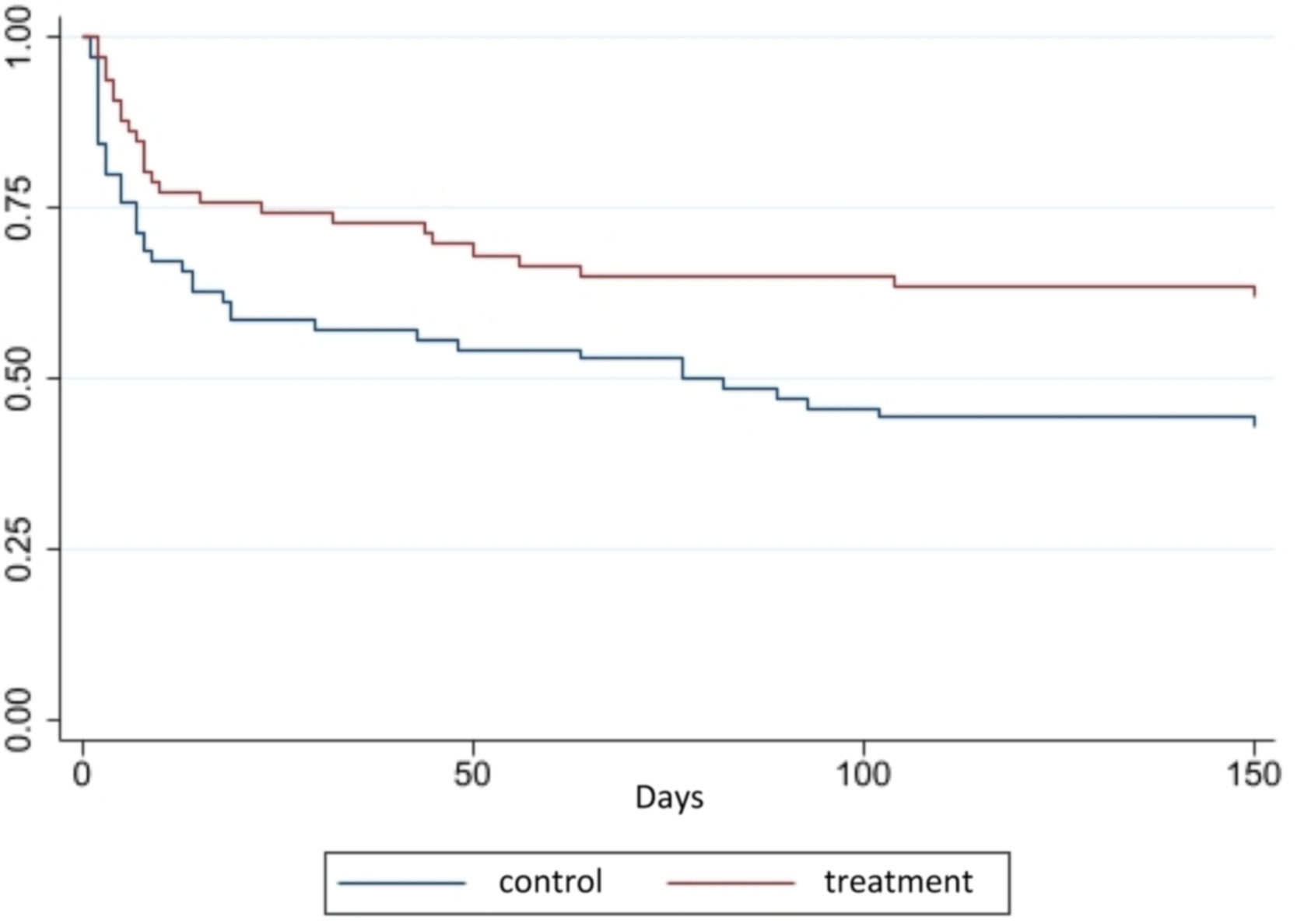
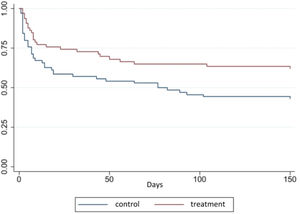
Figure 2 shows the layout of the survival curves over time for the treatment and control groups. Equality logrank (Mantel-Cox) and Breslow tests (Generalized Wilcoxon) between the survival functions showed, respectively, X2 = 6.81; gl = 1; p = 0.02 and X2 = 6.98; gl = 1; p = 0.02. The survival rate was higher in the treatment group along the curve. For instance, the point referring to the 50 (fifty) days of follow-up survival in the treatment group was 68.2% (45 VLBW-PT) compared to the control, which was 52.7% (38 VLBW-PT).
The Cox regression model showed that the hazard ratio (HR) of death cumulative incidence in the treatment group compared to the control group was 0.33 (95% CI 0.17–0.60; p 0.00), after adjusting for maternal age, marital status, gestational hypertension, childbirth type, number of prenatal consultations and birth weight (Table 2), i.e., OCI reduced, on average, 67% the risk of VLBW-PT dying during the study period when compared to the control group, adjusted for the covariates.
With regard to damage, no VLBW-PT had an adverse effect on treatment, either during or after the administration of therapy doses, according to records in hospital records.
DiscussionOCI proved to be beneficial in reducing the risk of death in VLBW-PT in this study when adjusted for maternal age, marital status, gestational hypertension, childbirth type, number of prenatal consultations, and birth weight. The RR of death of patients in the intervention group in relation to the control group was 0.26, i.e., there was a 74% reduction in the mortality of patients who received OCI. The ARR calculation showed that VLBW-PT in the intervention group had a 20.4% reduction in the probability of death.
Most clinical trials conducted with the same objective as the current study found no association between OCI and reduced risk of neonatal mortality.4-12,19-21 However, two meta-analyses reported a trend towards a positive effect of OCI in reducing mortality in the treatment group,22,23 although others did not find an association.1,24-26 Meta-analysis was recently published that reported a positive association between OCI and reduced mortality.27
It is expected that OCI reduces mortality risk in PTNB, especially in those with VLW at birth, since morbidities related to prematurity, which are frequent causes of neonatal death, can be prevented by this intervention.1 In this aspect, the literature has documented a lower risk of ventilator-associated pneumonia,5 necrotizing enterocolitis,28,29 septicemia,7,19,21,28,29 intraventricular hemorrhage,29 extrauterine growth restriction,28 reduction in antibiotic use duration7 and reduction in length of hospitalization.5,10,20
In addition to infections related to immune system immaturity, premature infants’ nutrition is another challenge, due to gastrointestinal tract immaturity that delays the start of enteral feeding, a characteristic that contributes to mortality, since long periods of absence of enteral feeding favor morbidity appearance that leads to death. Studies show that OCI has beneficial effects in reducing the time to start enteral nutrition,1,9 the time to reach full enteral nutrition,5,8,20,21,28,29 the clinical manifestations of food intolerance21 and the time to reach oral feeding,5 in addition to providing better average weight gain1,5,20 and accelerating the growth rate of VLW PTNB.28
The originality of the results found in the current study is likely to be related to some characteristics that strengthen OCI's internal validity and biological plausibility such as protocol use in the form of an algorithm, with systematization of care provided to the VLBW-PT and the puerperal women, through standardized clinical practice; maintenance of treatment until the seventh full day of life of VLBW-PT, a conduct that may have allowed greater absorption and systemic effects of bioactive factors present in colostrum, such as serum IgA,30 important immunoglobulin that rises between the fourth and fifth day after childbirth; in addition to the HMB support in the uninterrupted supply of colostrum for 24 hours.
Regarding the variables used in the regression model adjustment, such as maternal age < 18 years, marital status without the presence of a companion, gestational hypertension, artificial childbirth type, and a number of prenatal consultations < 6 consultations, are characterized as biological and social determinations for prematurity and/or neonatal death since women exposed to these conditions are at greater risk of the clinical worsening of PTNB after birth.17 A fact also observed with the birth weight variable, which is inversely proportional to better clinical prognoses presented by newborns, i.e., the lower the birth weight, the greater the risk of morbidities and mortality.
It is necessary to comment on the magnitude of the association between OCI and VLBW-PT death, seen by calculating the NNT at 50 and 100 days, which showed that it was necessary to treat 4 (four) newborns to avoid the death of one of them. At 150 days, for every 3 (three) individuals treated, one death was prevented. Still on the magnitude of the association, despite the h-index of 0.41 indicating a mean effect size of the intervention on the treatment group compared to the control, from a clinical point of view, this finding is extremely important, as the assessed outcome means a reduction in risk of death. Thus, we can conclude that NNT and the calculated effect size reinforce the conduct of using OCI as a measure to prevent mortality risk in VLBW-PT.
The main limitation of this study was the historical control design, as it could impact the quality of the data collected and prevent their comparability due to information bias. This limitation was minimized, as the variables selected to integrate the database were taken from the form applied to pregnant women in the maternity hospital's medical routine. Another limitation concerns changes in characteristics between the groups over time, but we consider that the short period between hospitalization in treatment and control groups (two years) did not cause significant changes in the determinants. Furthermore, the researched outcome (death) was well defined, based on parameters that do not depend on changes in methods and diagnoses for its assessment. Another precaution was the survival analysis adjusted for multiple variables.
Generalizability (external validity) of the current study's clinical findings to other populations is permitted, since the association's biological plausibility applies to similar populations likely to receive the intervention, and the death outcome is easy to measure, in addition to the statistical care instituted with the aim of guaranteeing internal validity. Added to this is the fact that OCI is a strategy that is easy to apply, cheap, viable, safe and well tolerated by PTNB, which can be implemented in neonatal units, through a protocol adapted to different realities of public or private maternity hospitals, especially those that can count on the HMB support.3
Thus, the institution of OCI in clinical practice, a modifiable variable, associated with other measures, such as access improvement and quality of health care provided in prenatal, childbirth, and puerperium and efficient and immediate clinical care to the VLBW-PT can contribute to achieving the goal set by the United Nations Sustainable Development Goals (SDGs) of reducing mortality incidence and complications arising from prematurity by 2030.
Source of financingBahia State Research Support Foundation (FAPESB - Fundação de Amparo à Pesquisa do Estado da Bahia), in Public Notice 003/2017 – Research Program for SUS: Shared Management in Health – PPSUS/BA – FAPESB/SESAB/CNPQ/MS (< grant number 4996/2017 [to VIEIRA, G.O.]), regarding the acquisition of permanent materials and consumables for the protocol implementation, playing an indirect role in data collection. The institution is under public law and only the right to incorporate the name of the funding agency on all research products is required.
Dean of Research and Graduate Studies at the Universidade Estadual de Feira de Santana (PPPG/UEFS) through the Graduate Support Program (Proap) of the Higher Education Personnel Improvement Coordination (CAPES - Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), provided funding for manuscript translation.
The authors would like to thank the mothers of premature newborns for their consent to carry out this study; FAPESB for funding the research, through Public Notice 003/2017 - Research Program for the SUS: Shared Health Management - PPSUS/BA - FAPESB/SESAB/CNPQ/MS; and Hospital Inácia Pinto dos Santos (Hospital da Mulher) for their support and collaboration in the development of this research.