To describe the characteristics of opportunistic infections in pediatrics regarding their clinical aspects, as well as the diagnostic strategy and treatment.
Source of dataNon-systematic review of literature studies in the PubMed database.
Synthesis of dataOpportunistic infections caused by non-tuberculous mycobacteria, fungi, Herpesvirae, and infections affecting individuals using immunobiological agents are analyzed. Because these are severe diseases with a rapid evolution, diagnostic suspicion should be early, associated with the patient's clinical assessment and history pointing to opportunistic infections. Whenever possible, samples of secretions, blood, and other fluids and tissues should be collected, with early therapy implementation.
ConclusionsDespite the improved diagnosis of opportunistic infections in recent years, they remain a challenge for pediatricians who are not used to these infections. They should raise the suspicion and start treating the case, but should also resort to specialists in the management of these infections to provide a better outcome for these patients, who still have high mortality.
Descrever as características das infecções oportunistas em pediatria em seus aspectos clínicos, bem como a estratégia diagnóstica e o tratamento.
Fonte dos dadosRevisão de trabalhos de literatura de forma não sistemática na base de dados Pubmed.
Síntese dos dadosSão apresentadas as infecções oportunistas causadas por micobactérias não tuberculosas, fungos, herpervírus e as infecções que acometem indivíduos em uso de imunobiológicos. Por se tratar de doenças graves e de evolução rápida, a suspeita diagnóstica deve ser precoce, associada à clínica do paciente e aos dados de história que apontam para infecções oportunistas. Sempre que possível, amostras de secreções, sangue e outros fluidos e de tecidos devem ser coletadas, com instituição precoce de terapia.
ConclusõesApesar da melhoria do diagnóstico de infecções oportunistas nos últimos anos, elas ainda são um desafio para o pediatra pouco habituado a essas infecções. Ele deve fazer a suspeita e iniciar a condução do caso, mas recorrer a especialistas com prática no manejo dessas infecções de modo a propiciar um melhor desfecho para esses pacientes que ainda apresentam alta mortalidade.
Opportunistic infections are those caused by pathogens (bacteria, viruses, fungi, or protozoa) that benefit from a host with a weakened immune system, an altered microbiota, or the breach of skin barriers.
The condition characterized by a balance between the several species that comprise the microbiota is called eubiosis. Any disturbances in eubiosis, known under the broad name of dysbiosis, can trigger infectious and noninfectious diseases. Opportunistic infections occur in situations of dysbiosis, predisposing the individual to exogenous and endogenous infections. They occur in the context of autoimmunity or show reactions of varying intensity, both increased (in allergic reactions and conditions of chronic inflammation) and decreased (in cases of immunodeficiency and cancer).1
The presentation of the infection varies according to the patient's comorbidity, which in turn is associated with aspects of the immune system that are not fully functional.
Comorbidities or situations that predispose to opportunistic infections are increasingly present in pediatric practice. Human immunodeficiency virus (HIV) infection, innate immunity errors (formerly called primary immunodeficiencies), neoplasms, autoimmune conditions, and use of chemotherapy, radiotherapy, or immunomodulatory drugs of the immune system are some examples of these comorbidities.
This review describes the opportunistic infections grouped according to the different classes of pathogens. Diagnostic and therapeutic aspects of mycobacterial, fungal, herpes virus infections, and those affecting individuals using immunobiological agents will be discussed.
Non-tuberculous mycobacteriaNon-tuberculous mycobacteria are largely disseminated in the environment and can cause diseases known as mycobacterial infections. Most disseminated infections are associated with impaired cellular immunity, such as patients with innate immunity errors affecting the interferon-gamma (IFN)/interleukin (IL)-12/IL-23 axis (IL-12deficiency, IFN-gamma deficiency, NF-kappa-B essential modulator [NEMO] mutations,and IFN-gamma and IL-12 receptor defects), hematopoietic stem cell transplant recipients, or individuals with advanced HIV2 infection.
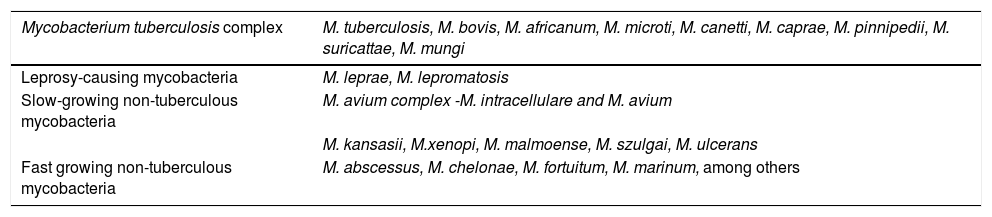
They present as respiratory infections, usually in patients with previous pulmonary pathologies (cystic fibrosis, chronic obstructive pulmonary disease, bronchiectasis), and those with skin and soft tissue infections, including lymphadenitis and surgical wound infections, whether or not associated with device implantation. They may also manifest as disseminated infections in immunocompromised patients. They are classified into two groups according to their phenotypic characteristics: slow-growing and fast-growing mycobacteria (Table 1).
Distribution of species of the genus Mycobacterium spp.
| Mycobacterium tuberculosis complex | M. tuberculosis, M. bovis, M. africanum, M. microti, M. canetti, M. caprae, M. pinnipedii, M. suricattae, M. mungi |
|---|---|
| Leprosy-causing mycobacteria | M. leprae, M. lepromatosis |
| Slow-growing non-tuberculous mycobacteria | M. avium complex -M. intracellulare and M. avium |
| M. kansasii, M.xenopi, M. malmoense, M. szulgai, M. ulcerans | |
| Fast growing non-tuberculous mycobacteria | M. abscessus, M. chelonae, M. fortuitum, M. marinum, among others |
Source: Esteban and Navas, 2018 (modified).3
Among the slow growing mycobacteria, those of the Mycobacterium avium complex are the most frequent cause of pulmonary infection and also the main cause of lymphadenitis in children under 5 years. They affect patients living with HIV with cluster of differentiation 4 (CD4) T lymphocytes below 50/mm3 or those with innate immunity errors, causing extrapulmonary and disseminated clinical pictures.3M. kansasii, another non-tuberculous mycobacterium, causes pulmonary infection with tuberculosis-like fibrocavitary pattern and, less frequently, focal or disseminated infections in patients with HIV or other immunosuppression conditions.
Fast-growing mycobacteria also cause chronic respiratory infections in people with pre-existing pulmonary lesions, and skin and soft tissue infections, many associated with aesthetic procedures, as well as infections associated with catheters and prostheses. They can form biofilms, which makes treatment difficult, and it is necessary to remove these devices to cure the patient. Of this group, M. abscessus has special relevance, causes respiratory infections, and shows great difficulty in its therapeutic management.3
The definitive diagnosis of mycobacterial infections requires identification of the agent. If there is a clinical suspicion of non-tuberculous mycobacterial infection, the laboratory should be contacted to ensure adequate specimen handling and cultivation conditions are utilized to allow pathogen isolation. Generally, in adults, two or more specimens of sputum or one specimen of bronchoalveolar lavage should result in the isolation of non-tuberculous mycobacteria for diagnosis. In children, these criteria are yet to be established. Moreover, the isolation of non-tuberculous mycobacteria from sterile sites is evidence of infection. It is worth recalling that the tuberculin skin test in cases of mycobacterial infection is usually positive, because several antigens are common to M. tuberculosis and other mycobacteria. In the case of interferon-gamma release assays, cross-reaction may occur in case of M. kansasii, M. marinum, and M. szulgai infections.
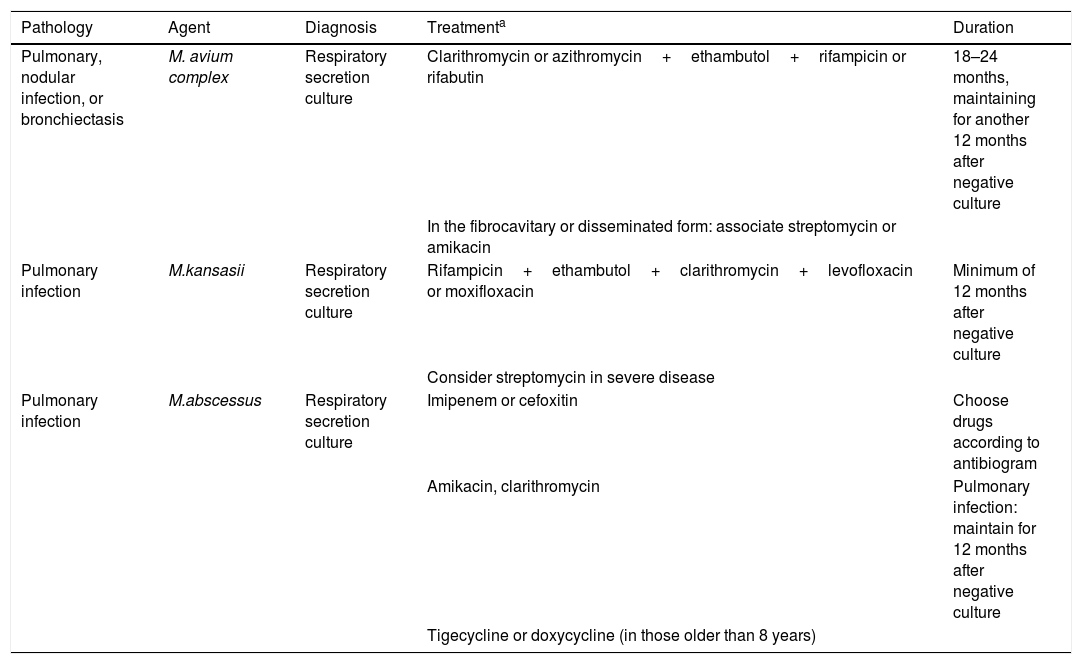
The diagnosis and treatment of mycobacterial infections are described in Table 2.
Diagnosis and treatment of the main infections caused by non-tuberculous mycobacteria.
| Pathology | Agent | Diagnosis | Treatmenta | Duration |
|---|---|---|---|---|
| Pulmonary, nodular infection, or bronchiectasis | M. avium complex | Respiratory secretion culture | Clarithromycin or azithromycin+ethambutol+rifampicin or rifabutin | 18–24 months, maintaining for another 12 months after negative culture |
| In the fibrocavitary or disseminated form: associate streptomycin or amikacin | ||||
| Pulmonary infection | M.kansasii | Respiratory secretion culture | Rifampicin+ethambutol+clarithromycin+levofloxacin or moxifloxacin | Minimum of 12 months after negative culture |
| Consider streptomycin in severe disease | ||||
| Pulmonary infection | M.abscessus | Respiratory secretion culture | Imipenem or cefoxitin | Choose drugs according to antibiogram |
| Amikacin, clarithromycin | Pulmonary infection: maintain for 12 months after negative culture | |||
| Tigecycline or doxycycline (in those older than 8 years) |
Source: Esteban and Navas, 2018 (modified).3
Opportunistic mycoses are fungal infections of low pathogenicity that specifically infect immunocompromised hosts.4 They are caused by ubiquitous fungi in the environment, such as filamentous fungi (Aspergillus spp., Fusarium spp., Mucorales, etc.), or yeasts that are part of the endogenous or exogenous fungal microbiota, such as Candida spp.
Infections by Candida spp. present as bloodstream, urinary tract, bone, skin, or surgical site infections, myocarditis, meningitis, and abscesses, with the latter being associated with catheter insertion. The most common clinical picture is the onset of fever unresponsive to antibiotics in at-risk patients.
Most cases of candidemia are believed to be endogenously acquired by pathogen translocation through the gastrointestinal tract, in which up to 70 % of the immunocompetent healthy population is colonized by Candida spp.
Factors that increase intestinal colonization by Candida (use of antibiotics, corticosteroids, paralytic ileus, intestinal occlusion) or determine intestinal mucosa atrophy or injury (prolonged fasting, total parenteral nutrition, hypotension, surgical procedure, mucositis secondary to chemotherapy, or radiotherapy) can potentiate the phenomenon of translocation from the gastrointestinal tract. Less frequent are exogenous infections due to medical procedures, contamination of solutions or prostheses, or central venous catheter colonization.
Among the species of Candida spp., C. albicans is the most often found in clinical practice. However, several non-albic an species, such as C. tropicalis, C. parapsilosis, C. glabrata, and C. krusei are involved in the increased incidence of invasive infections, with high rates of treatment failure related to resistance to azoles and echinocandins.5,6C. glabrataranks second in deep soft tissue infections in the United States and Europe, with a frequency of multidrug resistance > 10 %. Its incidence has also increased in Brazilian hospitals.5
In a retrospective study of pediatric cancer patients with candidemia, it was observed that patients from whom Candida tropicalis was isolated had more skin lesions when compared to those with candidemia by other species.7
Until a few years ago, there were no reports of multiresistant Candida, but the current scenario comprises invasive infections by multiresistant non-albicans Candida, most of them by C. glabrata and C. auris.5
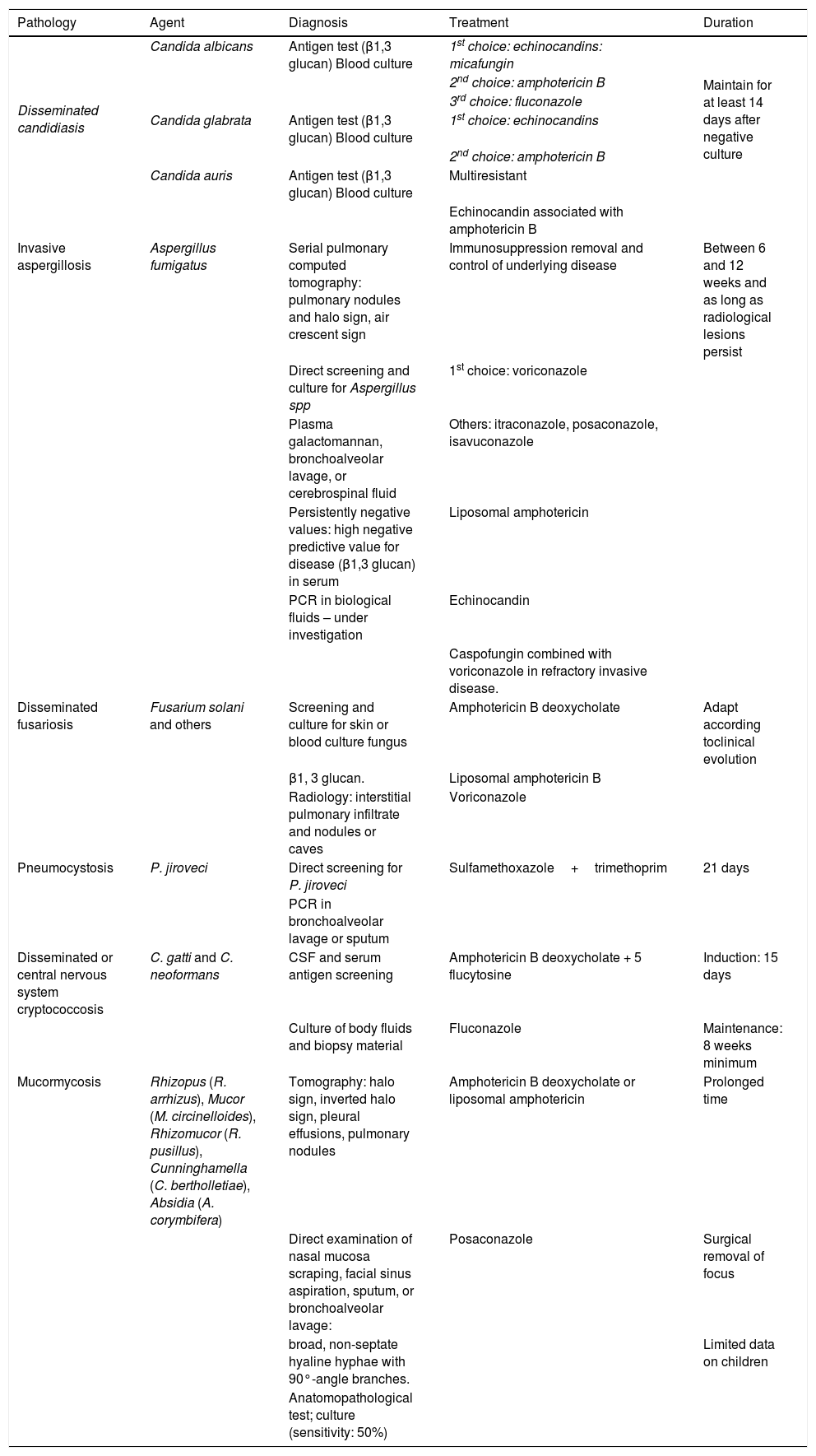
C. auris is an emerging species. Discovered in 2009, it has been described in more than 30 countries on six continents.8 It has a high antifungal resistance rate, with an estimated mortality rate of 30%–72%.9 Its isolation is difficult when the usual biochemical methods are employed, and it may be mistaken for C. haemulonii. The use of the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) allows the differentiation of C. auris from other species. As in other Candida spp. species, the presence of C. auris in non-sterile sites may represent only colonization, but its detection is very important since, besides being a difficult-to-eradicate colonization, there is a risk of horizontal transmission. Early recognition of sporadic cases, identification of reservoirs, and reporting are important measures for outbreak prevention. The definitive diagnosis of invasive candidiasis requires agent isolation from a usually sterile site or demonstration of the presence of the microorganism in a tissue sample. However, negative results do not exclude the diagnosis of invasive infection in the immunocompromised host. The sensitivity of a blood culture can be lower than 50 %. The diagnosis and treatment of systemic candidiasis are described in Table 3.
Diagnosis and treatment of major opportunistic fungal infections.
| Pathology | Agent | Diagnosis | Treatment | Duration |
|---|---|---|---|---|
| Disseminated candidiasis | Candida albicans | Antigen test (β1,3 glucan) Blood culture | 1st choice: echinocandins: micafungin | Maintain for at least 14 days after negative culture |
| 2nd choice: amphotericin B | ||||
| 3rd choice: fluconazole | ||||
| Candida glabrata | Antigen test (β1,3 glucan) Blood culture | 1st choice: echinocandins | ||
| 2nd choice: amphotericin B | ||||
| Candida auris | Antigen test (β1,3 glucan) Blood culture | Multiresistant | ||
| Echinocandin associated with amphotericin B | ||||
| Invasive aspergillosis | Aspergillus fumigatus | Serial pulmonary computed tomography: pulmonary nodules and halo sign, air crescent sign | Immunosuppression removal and control of underlying disease | Between 6 and 12 weeks and as long as radiological lesions persist |
| Direct screening and culture for Aspergillus spp | 1st choice: voriconazole | |||
| Plasma galactomannan, bronchoalveolar lavage, or cerebrospinal fluid | Others: itraconazole, posaconazole, isavuconazole | |||
| Persistently negative values: high negative predictive value for disease (β1,3 glucan) in serum | Liposomal amphotericin | |||
| PCR in biological fluids – under investigation | Echinocandin | |||
| Caspofungin combined with voriconazole in refractory invasive disease. | ||||
| Disseminated fusariosis | Fusarium solani and others | Screening and culture for skin or blood culture fungus | Amphotericin B deoxycholate | Adapt according toclinical evolution |
| β1, 3 glucan. | Liposomal amphotericin B | |||
| Radiology: interstitial pulmonary infiltrate and nodules or caves | Voriconazole | |||
| Pneumocystosis | P. jiroveci | Direct screening for P. jiroveci | Sulfamethoxazole+trimethoprim | 21 days |
| PCR in bronchoalveolar lavage or sputum | ||||
| Disseminated or central nervous system cryptococcosis | C. gatti and C. neoformans | CSF and serum antigen screening | Amphotericin B deoxycholate + 5 flucytosine | Induction: 15 days |
| Culture of body fluids and biopsy material | Fluconazole | Maintenance: 8 weeks minimum | ||
| Mucormycosis | Rhizopus (R. arrhizus), Mucor (M. circinelloides), Rhizomucor (R. pusillus), Cunninghamella (C. bertholletiae), Absidia (A. corymbifera) | Tomography: halo sign, inverted halo sign, pleural effusions, pulmonary nodules | Amphotericin B deoxycholate or liposomal amphotericin | Prolonged time |
| Direct examination of nasal mucosa scraping, facial sinus aspiration, sputum, or bronchoalveolar lavage: | Posaconazole | Surgical removal of focus | ||
| broad, non-septate hyaline hyphae with 90°-angle branches. | Limited data on children | |||
| Anatomopathological test; culture (sensitivity: 50%) |
PCR, polymerase chain reaction; CSF, cerebrospinal fluid.
Infections by Aspergillus spp. include respiratory diseases due to hypersensitivity (allergic sinusitis and allergic bronchopulmonary aspergillosis), skin and epithelial infections, intracavitary colonization (pulmonary fungal ball), and invasive forms (invasive and chronic necrotizing pulmonary aspergillosis, sinusitis, and disseminated forms with nervous system invasion and brain abscess formation).
Invasive aspergillosis occurs in immunocompromised patients with severe and persistent neutropenia due to corticosteroid treatment or chemotherapy, stem cell transplantation, or solid organ transplantation, especially the lungs. It has a high mortality rate, with Aspergillus fumigatus, A. flavus, A. niger, A. terreus, and A. versicolor the most frequently involved species.A.fumigatusis the main agent of invasive pulmonary aspergillosis, followed by A. flavus and A. terreus.10,11 It was recently demonstrated that A. fumigatus can produce aerosols and has the potential to be transmitted to others.12
In invasive aspergillosis, the earliest manifestation is fever in a patient with prolonged neutropenia, respiratory symptoms such as cough and dyspnea, and poor lung auscultation. Patients with strong immunosuppression may progress to the disseminated forms with central nervous system involvement, leading to brain abscess and, rarely, meningitis.
Diagnosis is made through clinical suspicion, imaging tests, antigen screening (galactomannan and beta-D glucan), and isolation of the fungus through microscopy and culture.
Chest tomography is more sensitive than chest radiography, especially at the disease onset and may have two radiological characteristics: the halo sign and the air crescent sign; the latter is rare in neutropenic individuals.13
Galactomannan is a cell wall polysaccharide released by the fungus during its growth in tissues, detected in serum and other fluids. It may be present five to eight days prior to clinical manifestation and should be ordered as a screening test for patients with prolonged neutropenia and recipients of allogeneic hematopoietic stem cell transplantation who are not on antifungal prophylaxis. The measurement is made by the immunoenzymatic method and has a sensitivity and specificity of 90 % in neutropenic patients. The highest test accuracy is when two consecutive samples have a value ≥ 0.5 and the best performance is when it is performed two to three times a week to monitor at-risk patients, correlated with imaging test and clinical picture.
Screening for galactomannan in bronchoalveolar lavage is also a good test for invasive aspergillosis; the optimal cutoff ranges from 0.5 to 1.0. False positive results may occur when patients receive piperacillin/tazobactam, blood product transfusions, and in other fungal infections, such as histoplasmosis, fusariosis, and talaromycosis.10
(1 → 3)-beta-d-glucan is also a polysaccharide component of the fungal cell wall that can be released in several fungal infections: Aspergillus, Candida, Fusarium, Trichosporon, Saccharomyces, Acremonium, and Pneumocystis jiroveci. Therefore, it is not specific for Aspergillus.
Bronchoalveolar lavage and/or lung biopsy are the methods of choice for microscopy and culture. Specific fungal stainings should be employed in the histological analysis. The presence of dichotomized and septate hyaline hyphae in sterile materials constitute evidence of Aspergillus infection even without culture isolation. While its presence in the respiratory tract of immunocompetent individuals may represent only colonization, it may indicate invasive disease in immunosuppressed individuals.
The culture has low sensitivity and has a positivity in 63 % in the bronchoalveolar lavage of patients with infection. When it is not possible to collect the lavage, the culture can be tried in three sputum samples.10
The diagnosis and treatment of invasive aspergillosis are described in Table 3.
Infections by Fusarium spp., another opportunistic fungus, may manifest clinically as persistent fever non-responsive to broad-spectrum antibiotic therapy in neutropenic patients with T-cell immunodeficiency or in acute leukemia patients. Immunosuppressed individuals may have skin lesions characterized by painful erythematous macules or papules that develop into necrotic ulcers, known as ecthyma gangrenosum, which is more common in the extremities and rapidly disseminate. The main entryway for Fusarium spp. is the respiratory tract, followed by damaged or burned skin. Catheter infection can lead to fungemia and its removal, associated with the antifungal, is crucial for treatment.14 Fungal isolation can be performed on skin biopsy or blood culture. Mortality in the disseminated forms is high15 but seems to be lower when voriconazole and liposomal amphotericin are used, compared to amphotericin deoxycholate.16 The diagnosis and treatment of disseminated fusariosis are described in Table 3.
Pneumocystis jiroveci is known as an opportunistic pneumonia agent in individuals infected with HIV. The incidence has decreased in this group due to combined antiretroviral therapy and the use of prophylaxis for pneumocystosis. On the other hand, there is currently an increased incidence of infection in those receiving immunosuppressants for oncological or autoimmune disease, in hematopoietic stem cells or solid-organ transplantation recipients. There are few data on P. jiroveci infection in children. A recent study evaluating adults and children shows that in the latter, oncohematologic diseases, post-transplantation period, and innate immunity errors were the most common comorbidities. The main clinical manifestation was pneumonia with fever, cough, dyspnea, and oxygen desaturation, developing into respiratory failure in adults and children. The radiological examination showed bilateral consolidation or bilateral interstitial infiltrate. Mortality was 25 %.17
The diagnosis of P. jiroveci infection is based on the visualization of the agent with specific staining in lung tissue or specimens of respiratory secretions, such as bronchoalveolar lavage, induced sputum, or endotracheal aspiration in intubated patients.
The treatment of choice is sulfamethoxazole trimethoprim (SMZ-TMP) administered intravenously for 21 days. Intravenous pentamidine can be an alternative for those who cannot tolerate SMT-TMP or who have not responded after four to eight days of SMZ-TMP therapy. In patients with partial O2 pressure below 70mmHg in ambient air, oral prednisone for 21 days is recommended (Table 3).
Cryptococcus, an encapsulated yeast that causes cryptococcosis, is part of the yeast group, and is found predominantly in patients with hematological malignancies, those using high-dose corticosteroids, solid-organ transplant recipients, and those infected with HIV and cell immunodepression. In these children, hematogenous dissemination may occur to the central nervous system, bones, and skin. The most common form is cryptococcal meningitis, which has an indolent course, with fever, headache, and behavioral changes.Intracranial hypertension and inflammatory immune reconstitution syndrome are common complications.18 The diagnosis may be attained by CSF or serum antigen screening, but the definitive diagnosis depends on the agent isolation in culture of body fluids or biopsy material. The differentiation between the two species, C. neoformans and C. gatti, depends on the use of selective culture media.19 The diagnosis and treatment of cryptococcosis are described in Table 3.
Another deep mycosis, mucormycosis, should be a differential diagnosis of invasive aspergillosis. Both have similarities in the affected patients (oncologic and diabetic patients, in the case of mucormycosis), in the risk factors (prolonged neutropenia), and in clinical and radiological signs. A recent study comparing invasive aspergillosis with mucormycosis in oncologic patients showed that mucormycosis was more frequent in children and adolescents than in adults and in patients with acute leukemia and graft-versus-host disease, whereas aspergillosis was more frequent in patients with lymphoma. Pulmonary involvement was lower; however, the involvement of the paranasal sinuses, central nervous system, and infection in more than two sites was more frequent in mucormycosis.20 The diagnosis and treatment of mucormycosis are described in Table 3.
Herpesvirus infectionsHerpes simplex 1 (HHV-1) and 2 (HHV-2) are among the nine herpesviruses that infect humans. With the exception of the neonatal period, HHV-1 and HHV-2are usually localized. In immunocompromised patients, localized severe intense lesions and, less commonly, disseminated HHV-1 and HHV-2 infection may occur, with generalized vesicles on the skin and/or visceral involvement.
Reactivation of HHV-1 and HHV-2 after the primary infection occurs more often in the immunosuppressed individual and is more prolonged. Reactivations may be preceded by a burning or itching sensation at the site of recurrence, which may be helpful in the early onset of the antiviral therapy.
HSV (herpes simplex virus) encephalitis occurs after primary or recurrent infection. It is characterized by fever, altered state of consciousness, personality alteration, seizures, and focal neurological signs. It usually has an acute onset, with fulminant evolution, progressing to coma and death if early therapy is not instituted. Magnetic resonance imaging is the most sensitive imaging exam and typically shows temporal lobe involvement. Different mutations have been associated with predisposition to the development of encephalitis.21
Meningitis may also occur. The diagnosis of HHV-1 and HHV-2 can be made by inoculation of vesicle secretion or cerebrospinal fluid in cell cultures, with the cytopathic effect being observed in one to three days after inoculation, and it can be confirmed by direct immunofluorescence of the culture material. Polymerase chain reaction (PCR) can be performed in a cerebrospinal fluid (CSF) sample. If both tests (culture and PCR) are repeatedly negative, histopathological analysis and viral culture of brain tissue biopsy are the most reliable tests to confirm the diagnosis of encephalitis caused by HHV-1 and HHV-2.
The treatment in immunosuppressed patients should be carried out using intravenous acyclovir for mucocutaneous HSV. In cases of acyclovir-resistant HSV, parenteral foscarnet is recommended. For encephalitis, parenteral acyclovir is recommended for 21 days.21
Infections caused by the varicella-zoster virus (VZV) (herpesvirus 3, HHV-3) in immunosuppressed individuals may show the presence of lesions that appear successively, sometimes with a hemorrhagic aspect, accompanied by high fever. Visceralization of the infection may occur, with encephalitis, hepatitis, and pneumonia. Severe cases of varicella have been observed in children using corticosteroids at immunosuppressive doses, as well as in individuals with inborn errors of immunity with T-cell impairment and in those infected with HIV. Children and adolescents with underlying lung or skin alterations are also prone to have severe varicella.
The infection by the VZV can also manifest as herpes zoster, which occurs after reactivation of the virus that was latent in the dorsal root of the medulla, cranial nerves, or enteric autonomic nerves. In immunosuppressed individuals, the lesions tend not to be restricted to one or two dermatomes and may disseminate. Most commonly, zoster lesions appear with skin manifestation, but may also present as isolated aseptic meningitis, encephalitis, infarction, or gastrointestinal tract involvement.
In a retrospective study comparing zoster episodes in adolescents infected with vertically transmitted-HIV and adolescents with juvenile systemic lupus erythematosus, those infected with HIV were more likely to have recurrent zoster when compared to those with lupus.22
The diagnosis of choice for VZV infections is PCR of vesicle or scab material. Direct immunofluorescence for virus detection and viral culture can also detect the virus, but they are less sensitive than PCR and, unlike PCR, do not differentiate the vaccinal from the wild virus. The serological diagnosis of varicella is not recommended when immunodeficiency is suspected, due to its low sensitivity. Cases of varicella in immunocompromised individuals with varicella antibodies have also been reported.23
Therapy with intravenous acyclovir is recommended for immunocompromised individuals. The treatment should be started early, preferably within 24h of the onset of exanthema. Valacyclovir has been recommended by some to treat children aged 2–17 years for cases of immunocompromised patients at lower risk of developing severe varicella, such as HIV-infected patients with higher levels of CD4 T-lymphocytes and some children with leukemia, under close medical supervision. In rare cases of acyclovir-resistant varicella, intravenous foscarnet can be used.24
Immunocompromised individuals exposed to varicella should receive prophylaxis with intramuscular VZV-specific immunoglobulin or intravenous immunoglobulin as soon as possible after the exposure.
Chemoprophylaxis after VZV exposure of immunocompromised individuals has been recommended by some, although there is little evidence demonstrating its benefit in the literature. Acyclovir or valacyclovir is used (starting seven to ten days after the exposure and lasting seven days.
Epstein-Barr virus (EBV) infections (Herpesvirus 4, HHV-4) can cause a wide variety of clinical manifestations. They range from asymptomatic infections in infants to infectious mononucleosis, which is more common in schoolchildren and adolescents, which can become severe conditions when they affect the immunosuppressed individual. In these cases, lymphoproliferative disorders such as hemophagocytic syndrome, X-linked lymphoproliferative syndrome, post-transplantation lymphoproliferative disorders, Burkitt's lymphoma, nasopharyngeal carcinoma, and undifferentiated T- and B-cell lymphomas are observed. Special attention should be given to transplant recipients and to those individuals infected with HIV.
While in immunocompetent individuals the diagnosis of EBV infection can be made through serology, in immunosuppressed individuals, the diagnosis may require the use of PCR for virus detection in serum, plasma, and tissue, and of real-time PCR in lymphoid cells, tissues, and body fluids.25
The use of corticosteroids (prednisone at a dose of 1mg/kg/day orally, maximum 20mg/day) for seven days may be recommended for EBV infections with tonsillar hypertrophy at risk of airway obstruction, significant splenomegaly, myocarditis, hemolytic anemia, and hemophagocytic syndrome. In cases of hemophagocytic syndrome, it may be necessary to use other cytotoxic and immunomodulatory agents, such as etoposide orcyclosporine. For EBV patients with post-transplant lymphoproliferative disorders, reduced immunosuppressive therapy may be required. When cytomegalovirus infections (Herpesvirus 5, HHV-5) affect immunosuppressed patients, they can trigger pneumonia, colitis, retinitis, meningoencephalitis, and transverse myelitis. Moreover, a syndrome characterized by fever, thrombocytopenia, leukopenia, and mild hepatitis may occur.
Cytomegalovirus (CMV) can be acquired by vertical transmission, by person-to-person transmission through contact with contaminated secretions (saliva, urine, or genital secretions), via blood component transfusion, and also via solid organ or hematopoietic stem cell transplantation. Additionally, the virus persists in the body, replicates, and can be transmitted intermittently, particularly in immunosuppressive situations.
Individuals especially at risk for these manifestations are those undergoing cancer treatment, those infected with HIV, and those receiving immunosuppressive therapy for hematopoietic stem cell and solid organ transplantation. Patients on biological agents may show retinitis and hepatitis, although more rarely.
Isolation of the CMV from the infection target organ is the best evidence that the disease is caused by this virus. Traditional cell line culture can take up to 28 days; fast shell-vial culture with viral detection by immunofluorescence gives results in 24–72h. CMV antigenemia (pp65 antigen detection) is another way of screening for CMV, but it is technically more difficult than the PCR. Moreover, the several body fluids can be evaluated for the presence of CMV through PCR.26
Intravenous ganciclovir is approved for the treatment of CMV retinitis in immunocompromised adults, including those infected with HIV, and for the prophylaxis and treatment of CMV disease in transplant recipients. Valganciclovir is also approved for the prevention of CMV disease in kidney transplant recipients older than 4 months and in pediatric heart transplant recipients older than one month.
Secondary prophylaxis in children should be maintained in HIV-infected patients older than 6 years until reaching CD4 counts > 100/mm3 for six consecutive months; for those under 6 years of age, they should maintain CD4 levels above 15 % for the same period before discontinuing the prophylaxis. In children with innate immunity error who have had retinitis, prophylaxis withdrawal should be evaluated on a case-by-case basis, with ophthalmologist monitoring at least every three to six months.
The reactivation of herpesvirus 6B infection (HHV-6B) is associated with disease in solid-organ and hematopoietic stem cell recipients, with fever, exanthema, hepatitis, spinal cord suppression, graft rejection, pneumonia, and encephalitis.27 Herpesvirus 6A (HHV-6A) and herpesvirus 7 (HHV-7) infection are much rarer in immunosuppressed individuals.
The diagnosis of herpesvirus 6B infection is difficult, being available only in reference laboratories. Even when using the tests of these labs, it can be difficult to differentiate infection from disease.
The use of ganciclovir (and valganciclovir) or foscarnet may be beneficial in immunocompromised patients with HHV-6B encephalitis.27
Herpesvirus 8 (HHV-8) infections are associated with Kaposi's sarcoma, multicenter Castleman's disease, and inflammatory cytokine syndrome associated Kaposi's sarcoma herpesvirus. In Brazil, HHV-8 is associated with cases of Kaposi's sarcoma in HIV-infected adult individuals without antiretroviral medication. Cases are very rare in Brazilian children. There is no antiviral medication approved for treatment of HHV-8. HHV-8-associated neoplasms are usually treated with radio- and chemotherapy.28
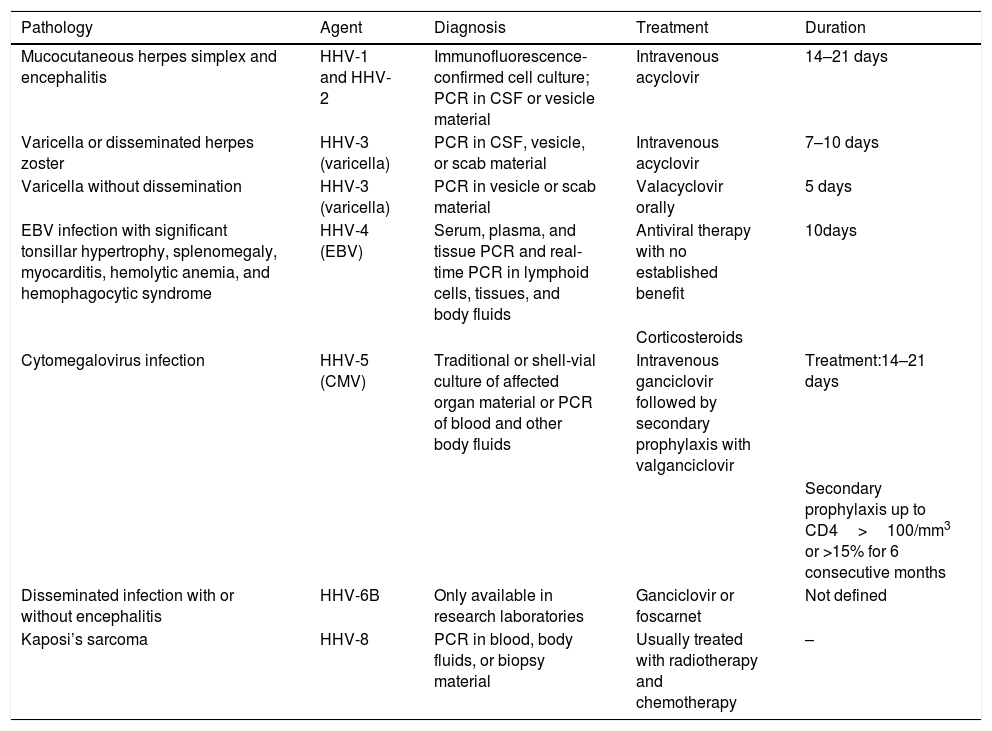
The diagnosis and treatment of herpesvirus infections29 are summarized in Table 4.
Diagnosis and treatment of herpesvirus infections in immunosuppressed individuals.
| Pathology | Agent | Diagnosis | Treatment | Duration |
|---|---|---|---|---|
| Mucocutaneous herpes simplex and encephalitis | HHV-1 and HHV-2 | Immunofluorescence-confirmed cell culture; PCR in CSF or vesicle material | Intravenous acyclovir | 14–21 days |
| Varicella or disseminated herpes zoster | HHV-3 (varicella) | PCR in CSF, vesicle, or scab material | Intravenous acyclovir | 7–10 days |
| Varicella without dissemination | HHV-3 (varicella) | PCR in vesicle or scab material | Valacyclovir orally | 5 days |
| EBV infection with significant tonsillar hypertrophy, splenomegaly, myocarditis, hemolytic anemia, and hemophagocytic syndrome | HHV-4 (EBV) | Serum, plasma, and tissue PCR and real-time PCR in lymphoid cells, tissues, and body fluids | Antiviral therapy with no established benefit | 10days |
| Corticosteroids | ||||
| Cytomegalovirus infection | HHV-5 (CMV) | Traditional or shell-vial culture of affected organ material or PCR of blood and other body fluids | Intravenous ganciclovir followed by secondary prophylaxis with valganciclovir | Treatment:14–21 days |
| Secondary prophylaxis up to CD4>100/mm3 or >15% for 6 consecutive months | ||||
| Disseminated infection with or without encephalitis | HHV-6B | Only available in research laboratories | Ganciclovir or foscarnet | Not defined |
| Kaposi’s sarcoma | HHV-8 | PCR in blood, body fluids, or biopsy material | Usually treated with radiotherapy and chemotherapy | – |
Source: American Academy of Pediatrics (2018).29
CSF, cerebrospinal fluid; PCR, polymerase chain reaction; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV, herpes simplex virus; CD4, cluster of differentiation 4.
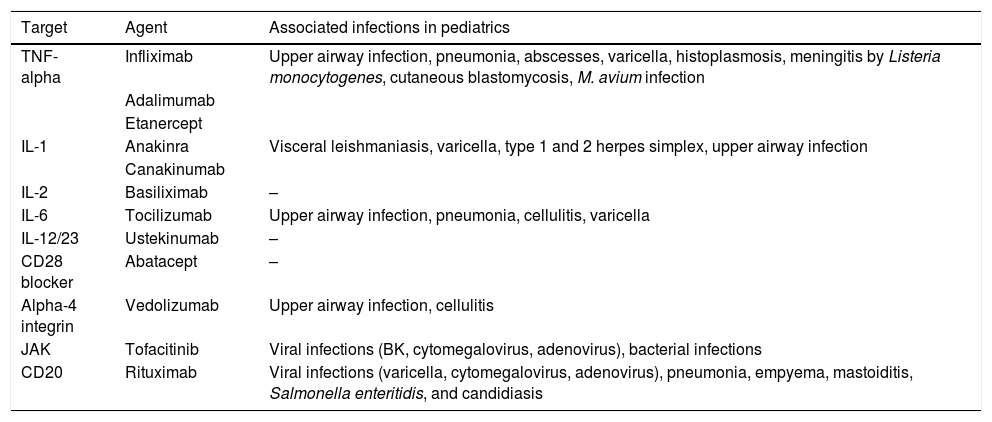
Biologicals are part of a wide range of products that include vaccines, blood components, allergens, somatic cells, gene therapy, tissues, and proteins. In recent years, biological agents have been used in pediatrics to treat rheumatologic diseases, inflammatory bowel diseases, and neoplasms (Table 5). The purpose of these drugs is to interfere with the immune alteration that leads to the clinical disease, reducing or increasing the immune response. However, alterations in the immune response pathways lead to selective deficiencies that potentiate the risk of infection.30
Biological agents and associated infectious events.
| Target | Agent | Associated infections in pediatrics |
|---|---|---|
| TNF-alpha | Infliximab | Upper airway infection, pneumonia, abscesses, varicella, histoplasmosis, meningitis by Listeria monocytogenes, cutaneous blastomycosis, M. avium infection |
| Adalimumab | ||
| Etanercept | ||
| IL-1 | Anakinra | Visceral leishmaniasis, varicella, type 1 and 2 herpes simplex, upper airway infection |
| Canakinumab | ||
| IL-2 | Basiliximab | – |
| IL-6 | Tocilizumab | Upper airway infection, pneumonia, cellulitis, varicella |
| IL-12/23 | Ustekinumab | – |
| CD28 blocker | Abatacept | – |
| Alpha-4 integrin | Vedolizumab | Upper airway infection, cellulitis |
| JAK | Tofacitinib | Viral infections (BK, cytomegalovirus, adenovirus), bacterial infections |
| CD20 | Rituximab | Viral infections (varicella, cytomegalovirus, adenovirus), pneumonia, empyema, mastoiditis, Salmonella enteritidis, and candidiasis |
Source: Danziger-Isakov.30
TNF-alpha, tumor necrosis factor-alpha; IL, interleukin; CD, cluster of differentiation.
TNF-alpha inhibitors block the immune response and reduce acute inflammation in patients with rheumatoid arthritis, juvenile idiopathic arthritis, psoriasis, and inflammatory bowel disease such as Crohn's disease and ulcerative colitis. Infliximab, adalimumab, and etanercept have an approved indication in pediatrics. The use of anti-TNF-alpha reduces the immune response to mycobacteria and predisposes to tuberculosis reactivation. In addition, severe bacterial infections by Streptococcus pyogenes, Listeria meningitis, systemic Mycobacterium avium complex infections, and endemic fungal infections such as histoplasmosis and blastomycosis have been reported in pediatric patients. Reactivation of VZV and EBV have also been described. What is not yet clear in the pediatric age group is how the use of anti-TNF-alpha effectively increases the risk of certain opportunistic infections when compared to children with autoimmune disorders using other immunosuppressants, such as corticosteroids.30
Interleukin inhibitorsDifferent interleukin (IL) inhibitors have been used aiming to interfere with the inflammatory cascade of patients with autoimmune diseases. This therapy has been associated with the development of infections.
IL-1 inhibitors. IL-1 mediates macrophage and T-cell activation, triggering fever. IL-1 inhibitors have been used in neonatal onset multisystemic inflammatory disease and cryopyrin-associated periodic syndromes, in addition to its off-label use in systemic juvenile idiopathic arthritis.
Infections in the pediatric age group have been reported with the use of anakinra: visceral leishmaniasis, varicella, HHV-1, and upper airway infections. Canakinumab, used to treat juvenile idiopathic arthritis, showed no increase in the number of infections.
IL-6 inhibitors. IL-6 modulates T- and B-lymphocyte growth and differentiation, stimulating the production of acute phase proteins. Tocilizumab is an IL-6 blocker used to treat systemic and polyarticular juvenile idiopathic arthritis. There is little experience with the use of this drug in the pediatric age group.
IL-2 inhibitors. IL-2 promotes T-cell proliferation, and IL-2 blockers have been used in solid organ transplantation to stop T-cell proliferation after transplantation. Basiliximab does not seem to be associated with an increased number of infections.
IL-12, IL-17, and IL-23 inhibitors. IL-12 is produced by macrophages and dendritic cells and promotes both NK-cell activation and T-cell differentiation. IL-23 stimulates Th17 cell proliferation, and IL-17 produced by these cells stimulates the production of proinflammatory cytokines, with an effect on the endothelium, epithelium, and fibroblasts. The use of these biologicals is still restricted in pediatrics. Few infections have been reported with their use.
Other targets different than interleukinsThe use of CD28 inhibitory drugs such as abatacept is not associated with infections. However, anti-CD20 drugs, which act on B cells, lead to hypogammaglobulinemia and are a risk factor for different infections for which the defense mechanism depends on the humoral immune response. One example of anti-CD20 is rituximab, used in cases of post-transplant lymphoproliferative disease, EBV-related hemophagocytic lymph histiocytosis, glomerular diseases, inflammatory diseases of the central nervous system, and Burkitt's lymphoma. The infections associated with the use of rituximab are bacterial sepsis, cytomegalovirus infection, varicella, acute pyelonephritis, BK nephropathy, and salmonella enteritis. The risk of infection ranges from 1 % to 10 %.
ConclusionsDespite the improved diagnosis of opportunistic infections in recent years, they remain a challenge for pediatricians who are not used to these infections. They must raise the suspicion and start managing the case, but also should resort to specialists with practice in the management of these infections to provide a better outcome for these patients, who still have high mortality.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: de Moraes-Pinto MI, Ferrarini MA. Opportunistic infections in pediatrics: when to suspect and how to approach. J Pediatr (Rio J). 2020;96(S1):47–57.