The association between diabetes mellitus and infections is very common. These infections, even when mild, interfere with blood glucose control. The aim of this review is to describe infections that occur in children and adolescents with DM, as well as to provide recommendations on glycemia management during these episodes.
Source of dataA non-systematic review was carried out in the PubMed database, using the terms “diabetes mellitus,” “infection,” “children,” and “adolescents.” The most relevant publications were selected.
Synthesis of dataIn addition to the usual community diseases, some infections may occur predominantly in diabetic patients, especially when there is inadequate glycemic control, and common infections can be more severe in these patients. Alterations caused by the disease itself and the immune response are responsible for the risk of higher frequency and severity of infections. During infections, an increase in blood glucose occurs and usually an increase in insulin dose is required.
ConclusionsPediatric patients with diabetes have some immune system disorders that, when associated with high glycemia, increase the risk of infections and their severity, and should be promptly identified and treated. The presence of an infectious condition, in turn, raises blood glucose and increases the risk of decompensation, and pediatricians should be cautioned to intensify monitoring and insulin therapy, and to avoid the risk of DKA. It should also be noted that many infections are preventable and can be avoided with adequate vaccine coverage.
A associação entre diabetes mellitus e infecções é muito frequente. Essas infecções, mesmo quando leves, interferem no controle da glicemia. O objetivo desta revisão é descrever as infecções que ocorrem em crianças e adolescentes com DM, bem como orientar o manejo glicêmico nestes episódios.
Fonte dos dadosFoi feita uma revisão não sistemática na base de dados PubMed, com os termos “diabetes mellitus’’, “infecção’’, “crianças’’ e “adolescentes”. Foram selecionadas as publicações mais relevantes.
Síntese dos dadosAlém de infecções comunitárias habituais, algumas infecções ocorrem predominantemente no paciente com diabetes, principalmente quando não há um controle glicêmico adequado, e infecções comuns podem ser mais graves nesse paciente. Alterações da própria doença e da resposta imune, em conjunto com alterações do microbioma, são responsáveis pela maior frequência e gravidade das infecções. Durante as infecções, ocorre um aumento da glicemia e habitualmente é necessário o aumento da dose de insulina.
ConclusõesO paciente pediátrico com diabetes apresenta algumas desordens imunes que, quando associadas a elevaçao da glicemia, aumentam o risco de infecção e sua gravidade. A presença da infecção, por sua vez, eleva a glicemia e aumenta o risco de descompensação. Desta forma, a monitorização da glicemia, bem como o aumento da dose de insulina, são fundamentais para evitar o risco de cetoacidose diabética. Destaca-se ainda que muitas infecções são imunopreveníveis e podem ser evitadas com uma cobertura vacinal adequada.
The association between diabetes mellitus (DM) and infections is a topic of great interest and a reason for considerable discussion in the medical literature. Several studies have assessed this association, mostly in adults, but many have also assessed the impact and incidence of infections in children and adolescents with DM. It has been reported that, in addition to the usual community-acquired infections, some infections occur preferentially in patients with diabetes, and other common infections may be more aggressive in these patients. There is evidence that adequate glycemic control improves immune function and decreases morbidity and mortality associated with severe infections in patients with DM.1–5
It is important to remember that DM is classified according to the etiopathogenesis of the disease, with the most frequent being type 2 DM (T2D), especially in adults, followed by type 1 DM (T1D), especially in children and adolescents. Studies do not always define the type of DM when assessing the risk and prevalence of infections. However, regardless of the type of diabetes, the diagnostic criteria are very clear and well-established. Currently, DM is considered when the following criteria are met: fasting blood glucose ≥ 126mg/dL; blood glucose within two hours during the oral glucose tolerance test ≥ 200mg/dL; HbA1c ≥ 6.5%; or presence of diabetes symptoms associated with random glucose levels ≥ 200mg/dL.6
T1D is caused by an autoimmune process directed against pancreatic beta cells, responsible for the production and secretion of insulin, leading to their destruction, with consequent insulin deficiency and hyperglycemia. It is the most common type in children and adolescents in Brazil, and it is mainly associated with microvascular complications (retinopathy, nephropathy, and neuropathy).6
T2D is caused by metabolic alterations that include the presence of peripheral insulin resistance associated with secondary pancreatic failure. It is the most common type in adults and is strongly associated with obesity and physical inactivity. Although most children and adolescents have T1D, there has been an increase in the onset of T2D for some years due to the increased prevalence of overweight and obesity in this age group.7 Therefore, this review assesses infections in DM in a general manner, pointing out the specific aspects when they exist.
This review addresses the following aspects regarding patients with diabetes: (i) changes in the immune system; (ii) main diabetes-associated infections, (iii) how to manage insulin therapy in the presence of an infectious disease, (iv) vaccines for the patient with diabetes.
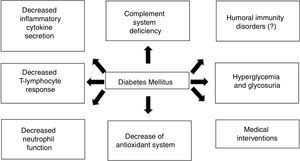
The DM patient’s immune systemOverall, the alterations caused by the disease itself and the immune response are probably responsible for the increased risk of infections in the diabetic patient, especially decreased T-cell response and neutrophil, monocyte/macrophage, dendritic cell, and natural killer (NK) cell function, together with microbiome alterations.1–4Fig. 1 schematically summarizes the interaction between DM and the immune response.
More specifically, the immune response alterations that can be found in patients with DM are discussed below:
Complement systemThe complement system has, as its main functions, participation in the inflammatory response, opsonization, and phagocytosis by macrophages and neutrophils, as well as microorganism lysis. Some studies show that patients with diabetes have C4 component deficiency, which may be associated with polymorphonuclear leukocyte dysfunction and decreased cytokine response.3,8
Inflammatory cytokinesPatients with DM have lower interleukin (IL)-1, IL-6, IL-10, and IL-22 secretion. There is a decrease in the amount of interferon-gamma (IFN-γ) released by T cells and NK cells, and of tumor necrosis factor (TNF) released by T cells and macrophages. Moreover, there is less expression of the major class I histocompatibility complex, impairing cellular immunity.3,8
Polymorphonuclear and mononuclear leukocytesDuring hyperglycemia and/or acidosis, decreased polymorphonuclear leukocyte (PMN) mobilization, chemotaxis, and phagocytic activity can occur. Antimicrobial function blockade takes place, with increased PMN apoptosis and decreased endothelial transmigration. The antioxidant system involved in bactericidal activity may also be compromised.3,5,8
The mononuclear cells and monocytes of DM patients release lower amounts of IL-1 and IL-6 in response to lipopolysaccharide stimulation.3,8
AntibodiesClinical data on humoral immunity are limited, but the antibody response after vaccination and common infections is known to be normal in diabetic patients.3 Several studies have shown an adequate and even increased response to some vaccines, such as the influenza vaccine. This could be explained by the chronic hyperactivation that occurs in the disease.1
The microbiome of the DM patientPatients with DM can show several alterations in their microbiome: skin flora alterations, including increased colonization by Staphylococcus aureus, skin lesions favored by chronic hyperglycemia, and alterations in the intestinal microbiome caused by the disease and/or treatment.1,2
Main infections associated with DMTable 1 shows the main infections associated with DM.1–3
Main diabetes mellitus-associated infections.
| Infections occurring most frequently in patients with DM: |
|
| Infections strongly associated with diabetes: |
|
Considering the topics of interest of the article, the most common infections in the pediatric age range and those most frequently associated with diabetes in general are discussed below.
Most common infections in children and adolescents with diabetesRespiratory infectionsRespiratory infections are the leading cause of infection in children, including diabetic patients. The main etiological agents of lower respiratory tract infections are Streptococcus pneumoniae and respiratory viruses, including influenza viruses.
Although the prevalence of Streptococcus pneumoniae in the nasopharynx of children with diabetes is not increased in relation to healthy children or those with other chronic diseases,9 some studies have shown that diabetic patients are more susceptible to pneumococcal infections and also at higher risk of bacteremia, worse prognosis, and higher mortality in the presence of these infections.10 There are few data in the literature on children, but overall, diabetic patients are more likely to be hospitalized and to develop complications than non-diabetic patients during the influenza season.11
Candida spp. infectionsDiabetes is a well-known risk factor for candidiasis. The most common location is the vulvovaginal area, and the risk is higher depending on the type of DM, severity, and degree of glycemic control. Type 1 DM patients with elevated glycated hemoglobin and inadequate glycemic control are more likely to have Candidaspp colonization. The hyperglycemia impairs neutrophil function, including phagocytosis, while acting as a nutrient for Candida. Patients with poorlycontrolled DM have higher acid proteinase activity, which are enzymes that facilitate the adhesion of the fungus to the epithelial cells.12,13
Several studies show a higher prevalence of asymptomatic vaginal colonization and symptomatic infection by Candida spp. in diabetic women. Vaginal colonization in prepubertal diabetic girls is uncommon, at around 12.5%, as the low estrogen levels result in rich anaerobic flora, which inhibit the growth of Candida. Colonization increases with age, reaching 55%. The most commonly found species is Candida albicans, followed by Candida glabrata.12,13
The clinical diagnosis in patients with vulvovaginal candidiasis is based on typical clinical signs such as thick, whitish vaginal discharge, itching, pain, burning, erythema, and edema. Diabetic patients may have recurrent conditions, with up to four episodes per year. When recurrence occurs, vaginal secretion culture should be performed to identify the Candida species.14
Treatment may be topical with clotrimazole or ketoconazole, or systemic with a single dose of fluconazole. In cases of recurrent candidiasis, the treatment should be directed to the isolated species; it may be topical with clotrimazole cream or systemic with fluconazole for ten to 14 days. In these cases, a maintenance schedule with fluconazole is recommended once a week for at least six months.14
Periodontal infectionsChildren and adolescents with T1D, especially poorly controlled patients, have a two- to three-fold higher prevalence of periodontal infection. The predisposing factor is the alteration of gingival vascularization, in addition to immune impairment.15 Periodontitis can lead to ligament loss, tooth mobility, and need for extraction. Dental abscesses and bacteremia may occur.
Urinary tract infectionsUrinary tract infections (UTIs) are more prevalent in individuals with DM, and severe complications and manifestations may occur.16 Some factors contribute to a higher risk of UTI: compromised host immune response, incomplete bladder emptying due to autonomic neuropathy (in T2D), and altered metabolic control, as elevated urine glucose also favors colonization by pathogenic microorganisms. The most common agents are the same as those in the general population: Escherichia coli and other enterobacteria.16
The most common infections are asymptomatic bacteriuria, lower UTI (cystitis), upper UTI (pyelonephritis), and urosepsis. Complications may also occur, such as renal papillary necrosis, emphysematous cystitis, and renal abscess.16
Much is discussed about asymptomatic bacteriuria in patients with diabetes. Although more prevalent,17 a randomized controlled trial of antimicrobial treatment in asymptomatic bacteriuria showed no difference in UTI development, time until symptom onset, risk of pyelonephritis, or need for hospitalization.18 Therefore, although controversial, there seems to be no need for screening or treatment of asymptomatic bacteriuria in these patients.
Skin and soft tissue infectionsSkin and soft tissue infections are common in individuals with diabetes, particularly when glycemic control is inadequate. Candidiasis, bacterial infections, dermatomycosis, and onychomycosis may occur. In bacterial infections (furunculosis, cellulitis) the main agents are the Streptococcus pyogenes and Staphylococcus aureus.
Deep soft tissue infections also occur more frequently in diabetic individuals, such as pyomyositis, necrotizing fasciitis, and Fournier's gangrene.3
Infections strongly associated with diabetes, but less frequent in the pediatric populationNecrotizing fasciitisNecrotizing fasciitis is a rare and severe infection with high mortality (50–70%). DM is the predisposing condition most commonly associated with this infection, which affects subcutaneous cell tissue and muscle fascia, causing extensive necrosis. Suggestive clinical signs are: skin lesion with failure to respond to initial antibiotic therapy, hardened subcutaneous tissue extending beyond the involved skin area, toxemia, bullous lesions, skin with areas of necrosis and bruising, and presence of crepitus. The clinical picture includes toxemia, fever, lethargy, cellulitis (90% of cases), edema (80%), change in skin color or gangrene (70%), and anesthesia of the involved skin area. Computed tomography (CT) or magnetic resonance imaging (MRI) may show edema extending beyond the fascial plane.3 The most affected sites are the extremities, abdominal wall, and perineum. When it affects the perineum, penis, or scrotum, it is called Fournier's gangrene.3
Surgical intervention is the main treatment, with maintenance of antibiotic therapy until the surgical procedures are no longer necessary, the patient shows clinical improvement, and has been without fever for more than 48h. The infection is usually polymicrobial and the first antimicrobial option is third-generation cephalosporin, associated with metronidazole or clindamycin.3
TuberculosisAccording to data from the World Health Organization, in 2017, 790,000 tuberculosis (TB) patients had diabetes, and the absolute number of TB cases associated with DM is already similar to that of TB-HIV (acquired immunodeficiency virus) co-infection.19 In Brazil, the Ministry of Health assessed the historical TB data from 2009 to 2017 and observed that the percentage of individuals with TB who have DM increased from 5.5% to 7.7%, which represents an average annual increase of 3.9%.20
Although published data associating DM and TB mostly refer to adult T2D, studies with patients with T1D show reduced IL-1B production in response to Mycobacterium tuberculosis.21 There is also a higher risk of TB in patients with T1D, which in some populations is three- to four-fold higher than in non-diabetic controls, both in adolescents and adults.22
Patients with DM are more easily infected and may progress more quickly to tuberculosis. DM can also negatively affect the course of tuberculosis, delaying the microbiological response, reducing the chance of cure, and increasing the chance of recurrence.20 Thus, it is important to screen for TB symptoms and identify respiratory symptoms in patients with DM.20 During TB treatment in individuals with established DM, special attention should be given to blood glucose control.20
Malignant otitis externaMalignant otitis externa is an invasive infection of the external ear canal and skull base that occurs in the elderly with diabetes. More than 98% of cases are caused by Pseudomonas aeruginosa. It can rarely be caused by Aspergillus spp. or other fungi. The predisposing factor is auditory canal microangiopathy.3 The clinical picture comprises severe headache, otalgia, otorrhea, and deafness, which can last months to years. Cellulitis may occur at the site, together with focal neurological signs, cranial nerve paralysis, and osteomyelitis of the skull base bones and the temporomandibular joint. Otoscopy shows granular tissue in the ear canal, without involvement of the tympanic membrane. Treatment should be systemic with anti-Pseudomonas antibiotic agents, with quinolones being the most frequently used, for six to eight weeks; and surgical debridement, if necessary.3
Surgical site infectionsThe association between DM and surgical site infections is well known and is attributed to postoperative hyperglycemia, showing the importance of adequate glycemic control.
MucormycosisMucormycosis is a rare type of infection caused by fungi of the Rhizopus and Mucor species, which cause infections in immunocompromised individuals, invading blood vessels. Ketone reductase production by Rhizopus spp. allows its growth in conditions of hyperglycemia and acidosis.3
Rhinocerebral, pulmonary, gastrointestinal, cutaneous, and disseminated forms have been described. The rhinocerebral form is the most frequent in patients with DM, besides being the most severe, with 80% lethality. It presents with facial or eye pain, nasal obstruction, fatigue, and fever. There may be darkened intra-nasal lesions. The diagnosis is made through the biopsy of the lesions. Treatment is carried out with amphotericin B or voriconazole.3
Management of the DM patient with infectionThe effects of disease on diabetesAchieving good glycemic control in children and adolescents with diabetes is crucial and may prevent the increase in risk of infection. However, in this age group it is very difficult to achieve adequate metabolic control, currently considered as a glycated hemoglobin level below 7.0%.23
The largest multicenter study carried out in Brazil, the Brazilian Type 1 Diabetes Study Group (BrazDiab1SG) analyzed data from 1,692 young individuals (<18 years of age) diagnosed with T1d and showed inadequate glycemic control in the vast majority (87.1%). Mean HbA1c was 9.4%, differing according to social class: high income=8.6±1.9%; middle income=9.1±2.1; lower income=9.4±2.4%, and very low income=9.8±2.7% (p<0.001). Female patients had higher mean HbA1c (9.6±2.5%) when compared to male patients (9.2±2.4%); whereas adolescents (13–18 years old) had higher HbA1c (9.9±2.2%) compared to children under 13 years (8.8±1.9%), with more children reaching the HbA1c target (29.2%) when compared to adolescents (17.9%).24 Even in developed countries, the achievement of good glycemic control is inadequate, and some studies suggest that the percentage of patients outside the glycemic target is even increasing.25
The association in Brazil between worse metabolic control and patients from lower socioeconomic classes favors the hyperglycemia–infection–hyperglycemia cycle. The finding of low IgG concentrations and the reduction of complement protein 4, variant B (C4B), related to poor glycemic control in children with DM, is probably part of this cycle.26
It is unquestionable that infections, even typical childhood diseases, significantly interfere with glycemic control. There is an increase in counter-regulatory hormones, cortisol, epinephrine, and growth hormone (GH) caused by the infectious process, leading to increased neoglycogenesis, glycogenolysis, and increased insulin resistance. This increase in insulin resistance requires adjustments in the patient’s usual insulin dose, both basal and bolus. There is usually a period of hours or days before this need is perceived and the increase in the dose may take time to be performed, which can result in hyperglycemia during this period.
Sustained hyperglycemia may lead to increased production of ketone bodies. The evolution of this condition may reach diabetic ketoacidosis (DKA), the main cause of death in young T1D patients.27
Special care with infected patientsAiming to avoid metabolic worsening and minimizing the risk of DKA, some practical precautions should be taken when there is an infectious condition in a child or adolescent with diabetes:
- More frequent glycemic monitoring.
- Ketone monitoring (serum or urinary) - It is important to remember that there are three types of ketone bodies: acetone, b-hydroxybutyrate (BHB), and acetoacetate (AcAc). In situations of decompensation, the BHB: AcAc ratio changes from the normal 1:1 to up to 10:1. Most urine ketone detection strips, as well as laboratory measurements, measure only AcAc and may mask the presence of ketones in the blood, limiting the perception of metabolic worsening. For this reason, the ideal in these situations of infection is to use reagent strips that measure BHB in the blood.
- -
Adjust insulin dose - most frequently there is a need for an insulin dose increase of 10%–30%.
- -
Never stop taking insulin completely, even in situations of lack of food intake, as insulin is necessary for basal metabolism, which may even be increased in stressful situations.
- -
Maintain hydration with sufficient amounts of sodium and water.
- -
Treat the triggering factor.
Included in the routine of infected patients who require special attention and blood glucose monitoring, it is important to highlight patients with cystic fibrosis-related diabetes (CFRD).28 Cystic fibrosis (CF) is the most common autosomal dominant disease among Caucasians, identified mainly by its clinical picture of recurrent infections and progressive loss of pulmonary function. The diagnosis and survival of these patients have been increasing, and it has been included in the newborn bloodspot screening test in several states of Brazil. As a result, the appearance of its comorbidities has been observed, with diabetes being the earliest and most prevalent, affecting 50% of the population with CF around the age of 30 years, with a peak incidence starting at puberty (10%).29 CFRD is usually easy to control, but requires insulin therapy as a treatment, and pediatricians should be aware to intervene more intensely during hospitalization due to pulmonary exacerbations, when there is a need to increase insulin dose.
Impact of DM-associated infections on the healthcare systemPatients with DM often seek medical attention and need to be hospitalized for infections, which has a major financial impact on the healthcare system.30,31 National data show that the average cost of hospitalization for adults with DM is 19% higher than hospitalization in individuals without DM.32
A recent study assessed the number of children and adolescents with type 1 and type 2 DM who sought medical care for infection treatment, as well as the socioeconomic impact, between 2008 and 2014. Infections accounted for 30% of the cost (US$255 million/year) of medical consultations and hospitalizations, while accounting for only 14% of consultations. The most frequent causes were respiratory infections, followed by skin and soft tissue infections.30
Vaccination in the DM patientVaccination of DM patients is a crucial strategy for reducing morbidity and mortality from infectious diseases. Infections are also responsible for triggering diabetes complications, such as hypoglycemia and DKA.4
Some immune-preventable diseases are more frequent and more severe in patients with DM. In addition to pneumococcal and influenza infections, these patients are also potentially exposed to hepatitis B virus infection, as a result of procedures related to the treatment and control of the disease, particularly capillary blood glucose monitoring. One study showed that adult patients with DM are 1.5-to two-fold more likely to have hepatitis B compared to those without the disease in the same age group.33
In addition to vaccines that are part of the Brazilian Ministry of Health's National Vaccination Calendar, the influenza, pneumococcal (23-valent polysaccharide), and Haemophilus influenzae type b vaccines are available through the Special Immunobiological Reference Centers.34
Hepatitis B vaccineThe usual regimen for immunocompetent individuals consists of three doses, with intervals of one month between the first and second dose and six months between the first and third dose (zero, 1, and 6 months of life). Brazil currently uses the basic four-dose vaccination schedule, with the first being the monovalent hepatitis B vaccine at birth, as early as possible within the first 24h, preferably within the first 12h after birth, and the others such as the pentavalent vaccine: DTP (Diptheria+Pertussis+Tetanus), Hib (Haemophilus influenzae type b), HB (Hepatitis B) at 2, 4, and 6 months of life. For the other ages, the three-dose schedule, at zero, 1, and 6 months of life is used. The hepatitis B vaccine can be given simultaneously or at any interval with the other vaccines of the Brazilian National Immunization Program for all age groups.
In patients with DM, serology (anti-Hbs) is recommended 30–60 days after the last dose. Those who do not respond with an appropriate antibody level should be revaccinated with three additional doses of vaccine. Those who remain anti-Hbs negative after two full three-dose regimens should be considered unresponsive and susceptible in case of exposure.
Influenza vaccineIt must be applied annually starting at 6 months of age. Children under 9 years of age receiving the vaccine for the first time require two doses at four to six week intervals. One annual dose is sufficient for subsequent vaccinations.
Pneumococcal vaccine
The decavalent pneumococcal vaccine (PCV10) is offered from 2 months up to 59 months of age (Table 1). The 23-valent pneumococcal vaccine (pneumovax 23, Merck Sharp & Dohme, NY, USA) can be given from 2 years of age in all age groups, and is given in two doses, with a five-year interval (Table 2).
Pneumococcal vaccination schedule, according to age.
| Age range at the start | Primary schedule | Booster doses | |
|---|---|---|---|
| PCV10 | PCV10 | Pneumovax 23 | |
| 2 to 6 months | Three doses (zero/2/4 months) | From 12 to 15 months of age | Before 2 years of age |
| 7 to 11 months | Two doses (zero/2 months) | From 12 to 15 months of age | First dose, at least 6 to 8 weeks after the last dose of PCV10 |
| 12 to 59 months | Two doses (zero/2 months) | None | Second dose, five years after the first dose of Pneumovax 23 |
PCV10, decavalent pneumococcal vaccine.
Haemophilus influenzae type b vaccine is offered from 2 months of age up to 19 years (Table 3). The Brazilian Society of Immunization recommends that patients with diabetes should be immunized with hepatitis B, influenza, pneumococcus (13-valent conjugate and polysaccharide 23V), Haemophilus influenzae type b, varicella, and herpes zoster vaccines.35
Haemophilus influenzae type b vaccination schedule.
| Age | Primary schedule |
|---|---|
| 2 to 6 months | Three doses (with a 60-day interval) |
| 7 to 11 months | Two doses (with a 4 to 8-week interval) |
| 12 to 59 months | Two doses (with a 4 to 8-week interval) if immunosuppressed. |
| Single dose, if immunocompetent. | |
| 5 years to 19 years | Two doses (with a 4 to 8-week interval) if immunosuppressed. |
| Single dose, if immunocompetent. |
Despite the increased risk of vaccine-preventable diseases and international vaccination recommendations, several national studies have shown that DM patients’ vaccination coverage regarding the vaccines recommended by the National Immunization Program is below optimal, showing the vulnerability of this group to a considerable range of preventable diseases, as well as the need to increase immunization strategies for these patients.36–40 This reduction in vaccination rates seems to be a global phenomenon, including in the pediatric age group. Studies have shown that the vaccination rate in children with DM is lower than that in healthy children and even in children with other chronic diseases, with disinformation being the main cause of the non-vaccination.41
ConclusionsPediatric patients with diabetes have some immune system disorders that, when associated with high glycemia, increase the risk of infections and their severity, and should be promptly identified and treated. The presence of an infectious condition, in turn, raises blood glucose and increases the risk of decompensation, and pediatricians should be cautioned to intensify monitoring and insulin therapy, and to avoid the risk of DKA. Vaccination has often not been performed in full in patients with diabetes. Vaccines are of vital importance for these patients, greatly assisting in reducing the risk of infections and the consequent lack of blood glucose control.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Calliari LE, Almeida FJ, Noronha RM. Infections in children with diabetes. J Pediatr (Rio J). 2020;96(S1):39–46.