To apply, in Brazil, the T-cell receptor excision circles (TRECs) quantification technique using real-time polymerase chain reaction in newborn screening for severe combined immunodeficiency and assess the feasibility of implementing it on a large scale in Brazil.
Methods8715 newborn blood samples were collected on filter paper and, after DNA elution, TRECs were quantified by real-time polymerase chain reaction. The cutoff value to determine whether a sample was abnormal was determined by ROC curve analysis, using SSPS.
ResultsThe concentration of TRECs in 8,682 samples ranged from 2 to 2,181TRECs/μL of blood, with mean and median of 324 and 259TRECs/μL, respectively. Forty-nine (0.56%) samples were below the cutoff (30TRECs/μL) and were reanalyzed. Four (0.05%) samples had abnormal results (between 16 and 29TRECs/μL). Samples from patients previously identified as having severe combined immunodeficiency or DiGeorge syndrome were used to validate the assay and all of them showed TRECs below the cutoff. Preterm infants had lower levels of TRECs than full-term neonates. The ROC curve showed a cutoff of 26TRECs/μL, with 100% sensitivity for detecting severe combined immunodeficiency. Using this value, retest and referral rates were 0.43% (37 samples) and 0.03% (3 samples), respectively.
ConclusionThe technique is reliable and can be applied on a large scale after the training of technical teams throughout Brazil.
aplicar no Brasil a técnica de quantificação de T-cell Receptor Excision Circles (TRECs) por polymerase chain reaction em tempo real para triagem neonatal de imunodeficiência combinada grave (SCID) e avaliar se é possível realizá-la em larga escala em nosso país.
Métodosforam coletadas 8.715 amostras de sangue de recém-nascidos em papel filtro e, após eluição do DNA, os TRECs foram quantificados por polymerase chain reaction em tempo real. O valor de corte para determinar se uma amostra é anormal foi determinado pela análise de curva ROC utilizando-se o programa SSPS.
Resultadosa concentração de TRECs em 8.682 amostras analisadas variou entre 2 e 2.181 TRECs/μL de sangue, com média e mediana de 324 e 259 TRECs/μL, respectivamente. 49 (0,56%) amostras ficaram abaixo do valor de corte (30 TRECs/μL) e foram requantificadas. Quatro (0,05%) mantiveram resultados anormais (entre 16 e 29 TRECs/μL). Amostras de pacientes com diagnóstico clínico prévio de imunodeficiência combinada grave e síndrome de DiGeorge foram utilizadas para validar o ensaio e todas apresentaram concentração de TRECs abaixo do valor de corte. Recém-nascidos prematuros apresentaram menores níveis de TRECs comparados aos nascidos a termo. Utilizando a curva ROC em nossos dados, chegamos ao valor de corte de 26 TRECs/μL, com sensibilidade de 100% para detecção de imunodeficiência combinada grave. Utilizando este valor, as taxas de repetição e encaminhamento, ficaram em 0,43% (37 amostras) e 0,03% (3 amostras), respectivamente.
ConclusãoA técnica é factível e pode ser implantada em larga escala, após treinamento técnico das equipes envolvidas.
Severe combined immunodeficiencies (SCID) comprises a heterogeneous group of diseases characterized by severe defects in the development and function of T, B, and NK lymphocytes. They are recognized as pediatric emergencies, as they lead to severe and recurrent infections and are fatal in the first two years of life, if not diagnosed and treated adequately.1,2
Due to the disease severity and the urgency in diagnosis and treatment, in 2005, United States researchers developed the newborn screening (NBS) for SCID, which consists of quantifying T-cell receptor excision circles (TRECs) using real-time polymerase chain reaction (PCR) (qRT-PCR).3,4 TRECs are small circular pieces of DNA formed during normal thymic processing, when T-cell receptor gene rearrangement occurs. As they do not replicate during cell division, they work as markers for the number of “naïve” T-cells recently emigrated from the thymus, and they are reduced in all forms of SCID.5
In most cases, NBS is the only way to detect SCID before infections occur, as more than 80% of cases have no family history of primary immunodeficiency.6
A significant altered finding observed after the development of neonatal screening was the disease incidence. As many children die before the diagnosis is attained, the incidence of SCID was estimated at 0.10/10,000; after the American experience, this figure is currently close to 0.17/10,000 (1:58,000), or almost two-fold higher.6,7
In Brazil, there is no neonatal screening test for primary immunodeficiencies applied during medical routine assessment and the diagnosis of these diseases falls short of what is required.8–10 For this reason, the aim of this study was to apply the TRECs quantification technique by qRT-PCR in the NBS for severe combined immunodeficiencies and assess whether it is possible to do so on a large scale in Brazil.
MethodsAll samples were collected following the current ethical standards of the Research Ethics Committee of ICB-USP (Opinion 967/CEP) and the parents/guardians of the newborns signed the informed consent. The concentration of TRECs was determined after blood samples were collected on 903 filter paper cards through heel puncture, which is part of the current routine for newborn screening. Samples from children born in three maternity services of hospitals and APAE de São Paulo (APAE-SP), located in the metropolitan region of São Paulo, as well as samples collected at Clínica de Medicina Preventiva do Pará (CLIMEP) were analyzed.
Disks measuring 3.2mm in diameter were cut from filter paper and were added to a 96-well polypropylene plate, where the DNA elution procedure was carried out, as previously described.3 All plates, in addition to the neonates’ samples, had a positive control (adult blood with low levels of TRECs), and a no-target control (piece of filter paper with no sample). The qRT-PCR reaction for TRECs and/or beta-actin was performed as described.3,11 All reagents were purchased from Life Technologies® (Life Technologies, USA).
Initially, a cutoff value of 30TRECs/μL of blood was arbitrarily used to determine whether a sample was normal, based on the value provided by Baker et al. of 25TRECs/μL. In cases where the result was below this value, a new piece of the same sample had its TRECs analysis repeated, accompanied by beta-actin analysis, as extraction control. After the second analysis, the samples with values <30TRECs/μL and beta-actin >8000/μL were considered abnormal, and patients were referred to a pediatric immunologist for consultation and confirmatory tests (complete blood count and measurement of T, B lymphocytes and NK cells). Those with TRECs <30/μL and beta-actin <8000/μL were classified as inconclusive result and a new sample was requested.
Data analysis was carried out using descriptive statistics. After applying the Kolmogorov–Smirnov test, the data were not normally distributed and were thus shown as median and interquartile values. Comparison of TREC concentrations between full-term and preterm newborns was established using the Mann–Whitney test, utilizing GraphPadPrism 5.0 software (GraphPadPrism, USA). The cutoff value to determine whether a sample was abnormal was determined by the ROC curve analysis (area under the curve) using SSPS software (IBM Corp. Released 2013, IBM SPSS Statistics for Windows, Version 22.0, NY, USA).
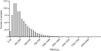
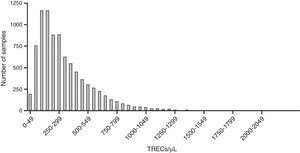
ResultsSamples were collected from 8,715 newborns, of whom 3,353 (38%) were from CLIMEP and the rest from hospitals located in the metropolitan region of São Paulo. Of the total, 33 were considered inadequate as they were inappropriately collected. Thus, 8,682 samples were analyzed for the concentration of TRECs, and the values in the first quantification varied from 2 to 2,181TRECs/μL of blood, with a mean and median value of 324 and 259TRECs/μL, respectively. Following the established protocol, samples below the cutoff value (30TRECs/uL) were reanalyzed. Thus, 49 (0.56%) samples were requantified and only four (0.05%) had abnormal results, with TRECs between 16 and 29/μL. Among these four samples, one was from a 27-week male newborn (24TRECs/μL), which could not be located for the protocol to be completed. The three remaining infants were assessed and submitted to the lymphocyte number evaluation, whose results were normal in all cases. Fig. 1 shows the distribution of samples according to the concentration of TRECs obtained at the first analysis.
To verify assay efficacy, samples from patients previously diagnosed with SCID (n=5) and DiGeorge syndrome (n=2) were analyzed and used as controls. All SCID samples showed TRECs values below 3TRECs/μL, and the DiGeorge samples showed results of 5 and 24TRECs/μL (Table 1).
Immunophenotyping of lymphocytes in patients with clinical diagnosis of primary immunodeficiencies and samples sent to pediatric immunologist for evaluation after altered neonatal screening.
| Patients with clinical diagnosis of primary immunodeficiencies | Newborn screening samples that were reanalyzed and sent to pediatric immunologist | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Sample 1 | Sample 2 | |
| Age | 2y5m months | 1y2m months | 5 months | 6 months | 3 months | 1y11m months | 8 months | 40 days | 2 months 12 days |
| TRECs/uL | 5 | 24 | 0 | 0 | 0 | 2 | 0 | 21 | 16 |
| Diagnostic hypothesis | DiGeorge S. | DiGeorge S. | SCID | SCID | SCID (RAG1) | SCID | SCID | – | – |
| Total lymphocytes | 2614 | 7682b | 6783 (57%) | 5676 (33%) | 6364 (74%) | 2710a | 5382 (60%) | 6471 | |
| CD3 | 953a | 3773 | 3615 (53.3%) | 3321 (58.5%) | 3451 (58.9%) | 3290 (51.7%) | 510 (24%)a | 2852.5 (53%) | 4051 (61%) |
| CD4 | 608a | 954 (12%) | 461.9a (6.8%) | 2390 (42.1%) | 342 (5.8%)a | 1591 (25%) | 239 (11.3%)a | 1954 (36.3%) | 2895 (44%) |
| CD4+CD45RA+CCR7+ (naïve) | 2.9a (0.6%) | 5.7 (0.24%)a | 0.8 (0.052%)a | 1260 (64.5%) | |||||
| CD4+CD45RA−CCR7− (peripheral memory) | 400.5 (86.7%)b | 1995 (83.5%)b | 1519 (95.5%)b | 216.9 (11.1%)b | |||||
| CD8 | 289a | 2675b (35%) | 2815 (41.5%)b | 257.7a (4.54%) | 2220 (37.9%)b | 1426 (22.4%) | 53 (2.5%)a | 710.4 (13.2%) | 1019 (15%) |
| CD8+CD45RA+CCR7+ (naïve) | 2.2 (0.08%)a | 0.3 (0.13%)a | 2.42 (0.17%)a | 503.7 (70.9%) | |||||
| CD8+CD45RA−CCR7− (peripheral memory) | 2550 (90.6%)b | 165.4 (64.2%) | 1176 (82.5%)b | 26.8 (3.77%) | |||||
| CD16+CD56+ | 343 | 2205 (32.5%)b | 1862 (32.8%)b | 2354.7 (37%)b | 171 (8.1%) | 904.2 (16.8%) | 709 (11%) | ||
| CD19 | 1254b | 441.6 (6.5%)a | 22.7 (0.4%)a | 1828 (31.2%) | 4.5 (0.07%)a | 1294 (61%) | 1130 (21%) | 1092 (27%) | |
TRECs, T-cell receptor excision circles; DiGeorge S., DiGeorge syndrome; SCID, severe combined immunodeficiency; RAG1, recombination activation gene 1; CD, cluster of differentiation; CCR7, chemokine (C-C motif) receptor 7.
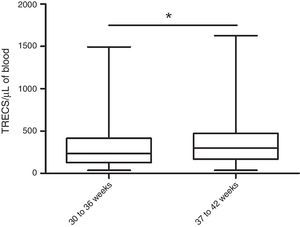
Part of the samples from CLIMEP (2,416) contained data on gestational age: 9.9% (239) of the newborns were preterm (30–36 weeks gestational age). That allowed the comparison of the TRECs concentration between the two groups, which allowed the conclusion that preterm infants have lower TRECs levels than full-term newborns (Fig. 2).
Concentration of T-cell receptor excision circles (TRECs)/μL for preterm and full-term infants. TRECs were quantified by qRT-PCR in dried blood spots of 239 preterm (30–36 weeks) and 2177 full-term newborns (37–42 weeks). Preterm newborns showed lower TRECs concentrations than the full-term ones (237TRECs/μL vs. 300TRECs/μL, respectively; *p<0.05, Mann–Whitney test). The concentration of TRECs is shown in a boxplot showing median, 25th and 75th percentiles, and minimum and maximum values.
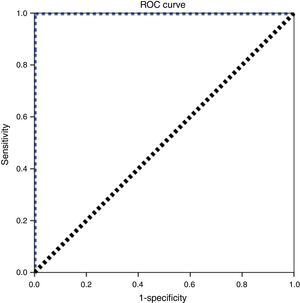
Arbitrarily using 30TRECs/μL as the initial cutoff value, a repetition rate of 0.56% was found (49 samples). After the second measurement, only 4 samples remained with abnormal values, which resulted in a referral rate to a specialized pediatric service of 0.05%. When analyzing the ROC curve for these conditions (Fig. 3), the cutoff value of 26TRECs/μL was obtained, with an area under the curve of 1.00 and 100% sensitivity for detecting SCID. Using this value, the retesting and referral rates were recalculated, which resulted in 0.43% (37 samples) and 0.03% (3 samples), respectively.
DiscussionSCID results from a variety of molecular defects, and the defects in both cellular and humoral immunity lead to similar clinical presentations. In most cases, symptoms start between the second and third months of life, and infectious complications are the predominant characteristic of the disease, with a preponderance of opportunistic infections (e.g., by P. jirovecii). Clinical signs are nonspecific and include respiratory tract infections, mucocutaneous candidiasis resistant to topical therapy, eczematous dermatitis, and local and systemic bacterial infections (otitis, mastoiditis, purulent rhinitis and conjunctivitis, septic disease, meningitis, arthritis, and local abscesses). Very often, the first sign is a reaction to the BCG vaccine, which may present as a localized or disseminated reaction.12 Severe and recurrent infections lead to inadequate growth. Additionally, intracellular parasites (Listeria, Legionella) as well as viruses (Epstein–Barr virus and cytomegalovirus) can cause fatal complications.13
One multicenter study disclosed the severe problem of underdiagnosis of SCID in Brazil. In 15 years (1996–2010), only 70 cases were diagnosed in 23 centers contacted in the country. Considering the probable incidence of 0.17 cases of SCID per 10,000 live births (1:58,000) and the mean of 2,900,000 live births/year from 1996 to 2010, 750 cases of SCID were expected in this period. As only 70 were documented, more than 90% of cases occurred without adequate diagnosis.6,10
These numbers clearly show the difficulty in attaining the diagnosis of SCID in Brazil. As previously mentioned, the signs and symptoms are nonspecific, ranging from common infections in the neonatal stage to growth difficulties. Children born to families with previous cases of SCID can benefit from the positive history and have the possibility of attaining an early diagnosis. However, 80% of cases have no previous history6 and diagnosis often occurs only when the recurrent infections have already resulted in sequelae.
Due to the urgency in diagnosis and treatment, this study aimed to validate the TRECs quantification technique by qRT-PCR for NBS of SCID and to apply the technique in Brazil.
The results showed that after analyzing samples from 8,682 newborns, a cutoff value of 26TRECs/μL was reached, which involved the retesting of 0.43% of the samples. The technique accuracy was validated through the quantification of TRECs in five previously diagnosed cases of SCID, with results varying from 0 to 2TRECs/μL (Table 1). The retest rate of 0.43% is within the range reported in the literature (0.20–3.26%) and can be considered an appropriate value, important for the implementation of a full-scale test, which prevents increased costs and unnecessary expectations and stress for the families.14 The percentage of samples to be retested is directly related to the cutoff value used, ranging from 7 to 252TRECs/μL in the studies performed to date. This is due to the different methodologies used for both DNA extraction and for the PCR reaction.14
The cutoff value obtained for the samples (26TRECs/μL) is very close to the value used by Baker et al. (25TRECs/μL), on which this study was based to validate the technique in the present laboratory.3 It is noteworthy that this cutoff value had 100% sensitivity for detecting SCID, but recent studies have reported that several other T-lymphopenias could also be detected by this methodology: DiGeorge syndrome, trisomy 21, ataxia-telangiectasia, trisomy 18, CHARGE syndrome, and cardiac abnormalities, among others.6
The lower value of TRECs/μL found in preterm infants has been reported in other studies, which also showed that the result tends to normalize when the child reaches the adjusted gestational age of 37 weeks.7,15 Thus, these results are consistent with the international literature and were validated by positive disease controls.
TRECs quantification by RT-PCR was applied on a small scale and had an estimated cost of US$ 4 per sample in this study. This value, although it only considers the costs of reagents, can be compared to the cost of NBS for congenital adrenal hyperplasia (CAH), calculated according to a pilot study conducted in the state of Goiás, of US$ 7.16 Considering the number of live births in Brazil in 2013 of nearly 3 million children,17 the total cost would be approximately US$ 12 million, less than the NBS for CAH, a pathology recently included in the National Neonatal Screening Program.18 It is noteworthy that it is necessary to add additional costs (active search and confirmatory tests) to this value and on a large scale, this value tends to decrease.
Among the T-lymphopenias with the possibility of being diagnosed through the quantification of TRECs, SCID are undoubtedly the most severe. Most children with SCID are not diagnosed until several severe infections have manifested, or the child shows failure to thrive and, unless there is a family history of SCID, the diagnosis is often delayed and chances of a cure are diminished. Moreover, an irrefutable argument for the inclusion of SCID in neonatal screening is the fact that SCID are the only conditions identified through neonatal screening that can be cured by treatment when the diagnosis is made at an early stage.19
Hematopoietic stem cell transplantation (HSCT) is curative and is available in Brazil.5 When carried out before 3.5 months of life, it results in a 95% survival rate; after that age, long-term survival decreases to 60–70%, and the incidence of infectious complications and sequelae increases dramatically.20 Even worse consequences occur when HSCT is carried out with active infections during the procedure: the survival rate decreases to 50%, again showing that early diagnosis substantially improves the outcome.14,20
Regarding the economic aspects, it is also necessary to consider the costs of HSCT and of complications from several infections to which patients with SCID are subject. Several studies have compared the costs of early vs. late HSCT: the full early treatment, performed up to 3.5 months of age (before the infections occur) costs up to four times less than when performed after that age (US$ 365,785 vs. US$ 1.43 million).21 When considering only the cost of the transplantation, the difference was US$ 120,000 vs. US$ 360,000 when HSCT was performed before and after 3.5 months of age, respectively.22 These values, calculated for the United States, show the great benefit of neonatal screening for SCID to the detriment of high costs resulting from late diagnosis and the complications that this delay may cause.21
When analyzing HSCT values before and after the age of 3.5 months, there is an approximate cost reduction of US$ 240,000 when the transplantation is performed early. Considering the cost obtained for this study of US$ 4, it would be possible to screen approximately 60,000 children. Thus, the introduction of this test on a large scale would practically pay for itself, as the cost reduction generated with the diagnosis of a child with SCID referred to earlier HSCT would cover the costs to perform the tests required to diagnose other child (when considering the incidence of 0.17/10,000).
It should be noted that the data presented herein reflect the economic costs in the US and it is necessary for cost-benefit studies to be conducted in Brazil, considering the Brazilian public health system.
The implementation of the newborn screening test for SCID is increasing worldwide. Several countries have included SCID in their routine newborn screening or have ongoing pilot studies (United States, Canada, Iran, Taiwan, Israel, Spain, France, Sweden, and the United Kingdom).19,23 The implementation as a routine in Brazil could significantly improve the aforementioned tragic scenario, without the need for a new platform.
The application of quantification of TRECs in the neonatal screening of 8,682 newborns resulted in a cutoff value of 26TRECs/μL, with a retest rate similar to that in international studies. This study showed that the technique is feasible and can be implemented on a large scale after technical training of the involved teams.
FundingThis work was funded by FAPESP, CNPq, and Baxter Bioscience.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the volunteers, patients, and their families who agreed to participate in the study. They also thank Dr. John Routes and Dr. Mei Baker for the training, Dr. Daniel Douek for the donated plasmids, the employees of the participating hospitals and clinics, and APAE-SP employees for helping with the necessary data.
Please cite this article as: Kanegae MP, Barreiros LA, Mazzucchelli JT, Hadachi SM, Guilhoto LM, Acquesta AL, et al. Neonatal screening for severe combined immunodeficiency in Brazil. J Pediatr (Rio J). 2016;92:374–80.
This study was carried out at the Department of Immunology, Instituto de Ciências Biomédicas, Universidade de São Paulo (USP), São Paulo, SP, Brazil.