To review the principles of neonatal-pediatric extracorporeal membrane oxygenation therapy, prognosis, and its establishment in limited resource-limited countries in Latino America.
SourcesThe PubMed database was explored from 1985 up to the present, selecting from highly-indexed and leading Latin American journals, and Extracorporeal Life Support Organization reports.
Summary of the findingsExtracorporeal membrane oxygenation provides “time” for pulmonary and cardiac rest and for recovery. It is used in the neonatal-pediatric field as a rescue therapy for more than 1300 patients with respiratory failure and around 1000 patients with cardiac diseases per year. The best results in short- and long-term survival are among patients with isolated respiratory diseases, currently established as a standard therapy in referral centers for high-risk patients. The first neonatal/pediatric extracorporeal membrane oxygenation Program in Latin America was established in Chile in 2003, which was also the first program in Latin America to affiliate with the Extracorporeal Life Support Organization. New extracorporeal membrane oxygenation programs have been developed in recent years in referral centers in Argentina, Colombia, Brazil, Mexico, Perú, Costa Rica, and Chile, which are currently funding the Latin American Extracorporeal Life Support Organization chapter.
ConclusionsThe best results in short- and long-term survival are in patients with isolated respiratory diseases. Today extracorporeal membrane oxygenation therapy is a standard therapy in some Latin American referral centers. It is hoped that these new extracorporeal membrane oxygenation centers will have a positive impact on the survival of newborns and children with respiratory or cardiac failure, and that they will be available for an increasing number of patients from this region in the near future.
Analisar os fundamentos, prognóstico e estabelecimento da terapia de oxigenação por membrana extracorpórea ECMO neonatal-pediátrica em países da América Latina com recursos limitados.
FontesA base de dados PubMed foi explorada de 1985 até hoje, selecionando os principais periódicos da América Latina e relatos da Organização de Suporte de Vida Extracorpóreo.
Resumo dos achadosA oxigenação por membrana extracorpórea proporciona “tempo” para descanso pulmonar e cardíaco e para recuperação. Ela é usada no campo neonatal-pediátrico como terapia de resgate, com mais de 1.300 pacientes com insuficiência respiratória e cerca de 1.000 pacientes com cardiopatias por ano. Os melhores resultados de sobrevida de curto e longo prazo são de pacientes com doenças respiratórias isoladas, estabelecendo uma terapia padrão em centros de encaminhamento para pacientes de alto risco. O primeiro programa de oxigenação por membrana extracorpórea neonatal/pediátrico na América Latina foi estabelecido no Chile em 2003, que também foi o primeiro programa na América Latina a se afiliar à Organização de Suporte de Vida Extracorpóreo. Novos programas de oxigenação por membrana extracorpórea foram desenvolvidos nos últimos anos em centros de encaminhamento na Argentina, Colômbia, Brasil, México, Peru, Costa Rica e Chile, que atualmente estão fundando a seção da América Latina da Organização de Suporte de Vida Extracorpóreo.
ConclusõesOs melhores resultados de sobrevida de curto e longo prazo são de pacientes com doenças respiratórias isoladas. Atualmente, a terapia de oxigenação por membrana extracorpórea é uma terapia padrão em alguns centros de encaminhamento da América Latina. Esperamos que esses novos centros de oxigenação por membrana extracorpórea tenham um impacto positivo sobre a sobrevida de neonatos e crianças com insuficiência respiratória ou cardíaca e que estejam disponíveis para um número cada vez maior de pacientes de nossa região no futuro próximo.
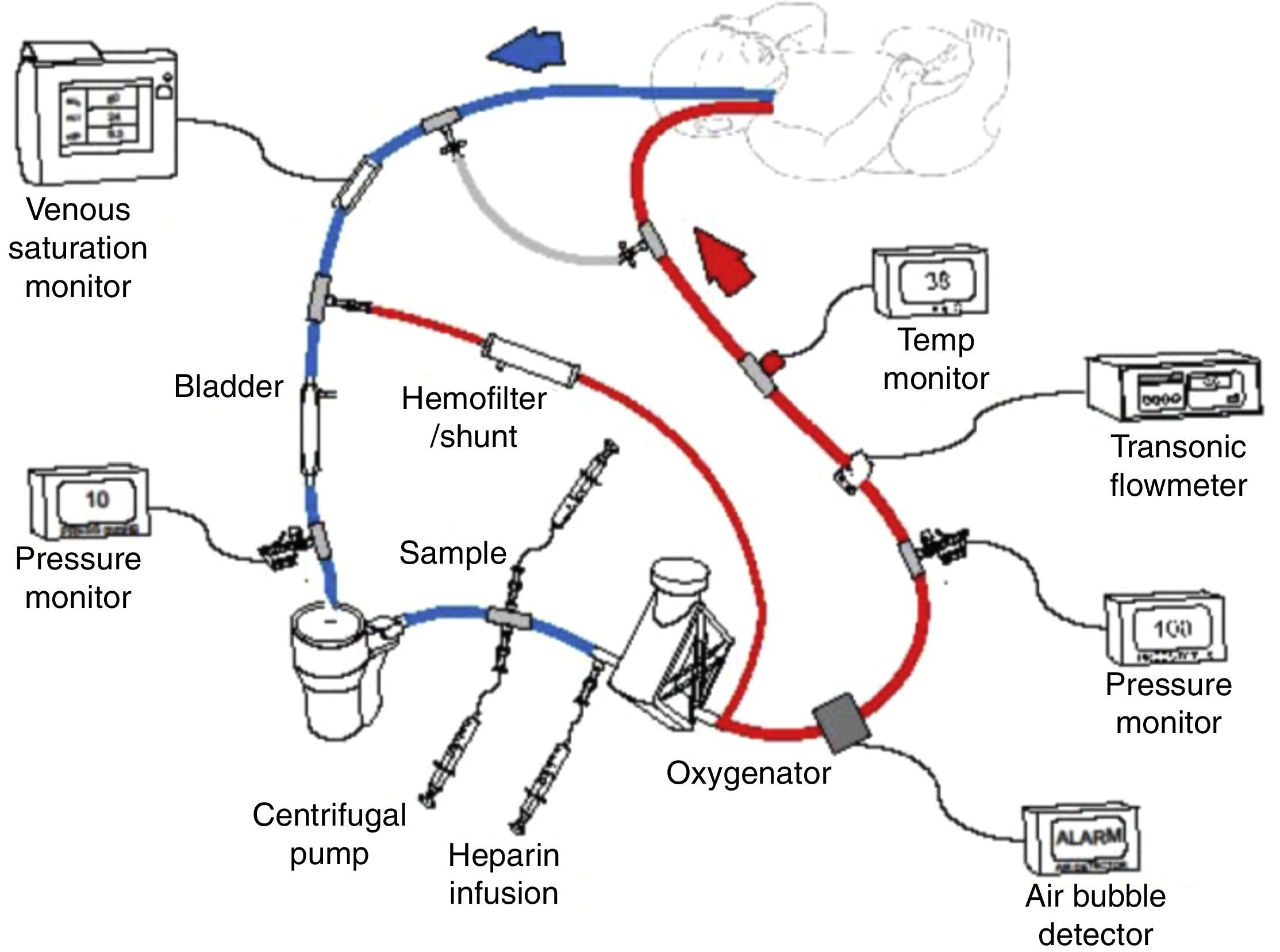
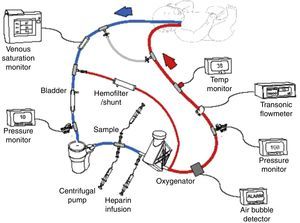
Extracorporeal membrane oxygenation (ECMO) or extracorporeal life support (ECLS) is a therapy that uses a modified partial cardiopulmonary bypass to provide pulmonary and/or cardiac support for an extended period, generally one to four weeks (Fig. 1). It is used for patients with reversible cardiopulmonary failure due to pulmonary, cardiac, or other diseases. ECMO provides time for a pulmonary and/or cardiac rest and for recovery. Given that ECMO is invasive, it involves potential risks, thus criteria have been established to select patients with a 50–100% prediction of mortality. The ideal ECMO candidate has a high prediction of mortality, but with a potentially reversible pulmonary or cardiovascular disease.1,2
Scheme of venoarterial extracorporeal membrane oxygenation (ECMO) circuit with centrifugal pump and polymethylpentene oxygenator. Venous blood is obtained from the right atrium via the right internal jugular vein, then pumped, oxygenated, heated, and returned to the aorta via the right carotid artery. Published with authorization from the ECMO Manual of the Children's National Medical Center, George Washington University, Washington D.C., 2010.
The first adult survivor of ECMO therapy was treated in 1971 by J. Donald Hill, who used a Bramson oxygenator with a polytraumatized patient.1 However, by the late 1970s the use of the therapy with adults was abandoned because of poor results in controlled studies. Years later ECMO experienced a resurgence for neonatal and pediatric patients thanks the surgeon Robert Bartlett.1 In 1975 at the Orange County Medical Center, Bartlett successfully used ECMO with an abandoned Latin newborn suffering from a respiratory distress syndrome.1 The use of ECMO with newborns increased in the late 1980s with survival of close to 80% among patients with 60–80% predictions of mortality. Owing to the increased use of ECMO with neonatal patients, a voluntary alliance, the Extracorporeal Life Support Organization (ELSO), was formed among ECMO centers in 1989.1
Newborns are the principal age group for which ECMO therapy is much superior to maximum conventional therapy, as shown in a controlled and randomized multicenter study with 185 newborns with severe respiratory insufficiency in 55 hospitals in the United Kingdom.3–5 This study showed that mortality and severe disability assessed at year 1, 4, and 7 of life significantly decreased with the use of ECMO (59% for the conventional therapy group versus 37% for the ECMO group).4–6 At 7 years follow-up, 76% of the children had normal cognitive development.5
Systematic reviews show that the use of ECMO with newborns close to term with severe but potentially reversible respiratory failure significantly improves survival without increasing severe disability, while being cost-effective compared to other intensive care therapies.7,8
With respect to the use of ECMO as a rescue therapy for newborns with congenital diaphragmatic hernia (CDH) with severe respiratory failure, controlled prospective studies indicate reductions only in early mortality.3,9 However, a meta-analysis of retrospective studies and the authors’ own experience indicate a higher rate of short and long-term CDH survival in health units that include ECMO.9–11
New therapies emerged in the 1990s to combat cardiorespiratory pathologies, such as high-frequency oscillatory ventilation (HFOV), surfactants, and inhaled nitric oxide (iNO).12 With these therapies in association with ECMO centers, the morbimortality of these pathologies has been significantly reduced in more developed countries.5
According to the ELSO, in the last decade ECMO has been used annually as a rescue therapy for close to 800 newborns who did not respond to intensive care with HFOV and/or iNO.2,13 Currently, the rate of use of ECMO in the USA is approximately one newborn for every 5000 live births.2 This therapy has clearly shown a higher rate of global survival (74% to hospital discharge) among newborns with severe respiratory insufficiency, better quality of future life, and a favorable cost-effectiveness ratio.2,6
The indications that lead to using ECMO for pediatric patients are more diverse and difficult to define than those for neonatal patients.14,15 Nevertheless, in recent years the number of respiratory cases reported to ELSO has risen to around 500 children per year, with a global survival rate of 58% to hospital discharge or transfer.2,13,14 Acute hypoxic respiratory failure (HRF) is the most common respiratory condition for accepting patients for ECMO.16 In this group, viral pneumonia is the most common cause and one of the conditions with the best survival rates, together with aspiration pneumonia and acute post-traumatic respiratory distress.15 Nowadays, patients are accepted who have immunosuppression and suspicion of sepsis, the latter often with multi-organ failure.16 The groups of pediatric patients with the poorest prognoses are those that have had bone marrow transplants or pneumonia associated with Bordetella pertussis and pulmonary hypertension, as well as patients with multi-organ failure, in contrast to the good prognosis for patients with isolated pulmonary involvement.16
In the 1970s ECMO was used to manage respiratory failure and pulmonary hypertension, and somewhat later for ventricular cardiac assistance. In 1972, Dr. Bartlett successfully provided ECMO support to a 2 year old boy following a Mustard procedure for correction of transposition of the great vessels with subsequent cardiac failure.1 Half of the patients who require cardiac ECMO have complex cyanotic congenital heart disease.2 The largest groups requiring ECMO are patients following cardiotomy by a complete AV canal (20%) and patients with single ventricle physiology (17%) or tetralogy of Fallot (14%).17 Among the main causes that lead to applying peri-operative cardiac ECMO are: hypoxia (36%), cardiac arrest (24%), and failure to wean from cardiopulmonary bypass (14%). Consequently, the use of iNO and HFOV can reduce the need for ECMO by decreasing the degree of hypoxia.17
ECMO is superior to ventricular assistance devices in cases where the predominant physiopathology mechanisms are hypoxia, pulmonary hypertension, or biventricular failure.17
Neonatal-pediatric cardiac indications have been increasing steadily over time, reaching more than 1000 cases reported to ELSO annually, constituting a valuable support therapy in high-complexity cardiac surgery centers.2,18
Until 2009, close to 80% of the more than 50,000 patients treated with ECMO and reported to the ELSO were newborns or children, with newborns treated for respiratory problems representing approximately half of all reported patients.2 In recent years, adult respiratory and cardiac ECMO has increased progressively by 1500%, and pediatric respiratory ECMO has increased by 100%, partially explained by the pandemic H1N1 influenza and new evidence from randomized trials in adults.19,20
Given the evidence of clear benefits in survival, quality of life, and cost effectiveness of this therapy, and the absence of a formal ECMO program in Chile, it was decided in 1998 to establish a neonatal-pediatric ECMO program in the neonatal intensive care unit at the Catholic University Hospital (ECMO-UC Program), in accordance with the standards of the ELSO for patients with severe but reversible cardiovascular or respiratory insufficiency refractory to maximum conventional treatment.21,22 Work began in 1999 on developing a multidisciplinary team (neonatologist, intensive care pediatricians, cardiac and pediatric surgeons, nurses, perfusionists, respiratory therapists, psychologists) with training in ECMO centers in the USA affiliated to the ELSO.21 Training was consolidated in Chile with an experimental course using sheep.21
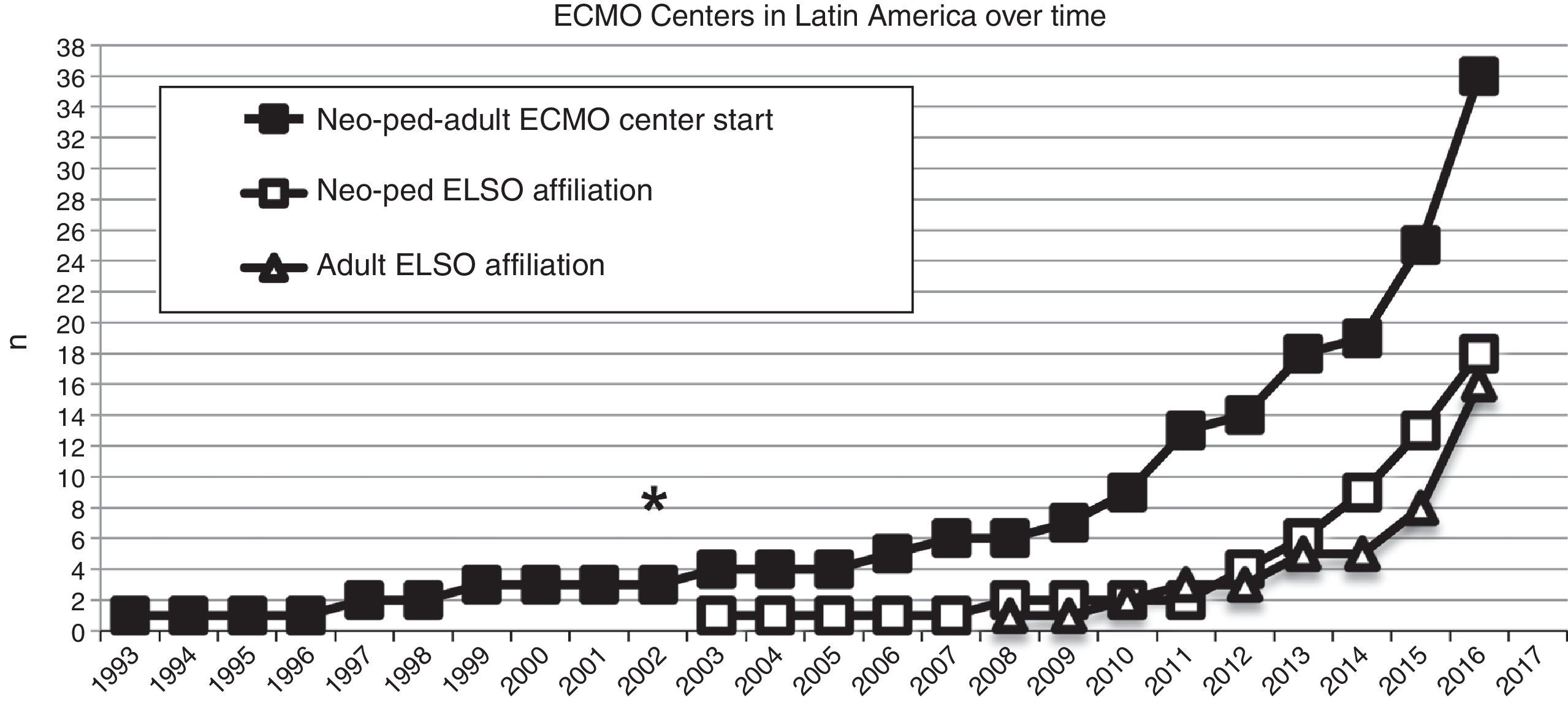
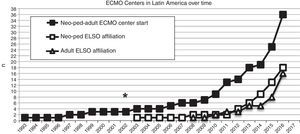
In 2003, the first neonatal-pediatric ECMO program was consolidated in Chile and became the first Latin American member of the ELSO (Fig. 2). From May 2003 to June 2016, the center treated 181 patients (155 newborns and 26 children), with both severe respiratory and cardiac pathologies.
Number of extracorporeal membrane oxygenation (ECMO) centers created in Latin America since 1993 (black squares), and the number of this ECMO centers in Latin America affiliated to the Extracorporeal Life Support Organization (ELSO) and LATAM ELSO since 2003, separated in neonatal-pediatric centers and adult centers (white squares and triangles, respectively). Asterisk (*) marks the year 2003, when the first neonatal-pediatric ECMO center started.
To determine the impact of the establishment of a neonatal ECMO program on the outcome of newborns with severe HRF in a developing country like Chile, the authors studied data of newborns (BW >2000g and GA ≥35 weeks) with HRF and oxygenation index (OI) >25 were compared before and after ECMO was available.11 ECMO was initiated in infants with refractory HRF who failed to respond to iNO/HFOV. Data from 259 infants were analyzed; 100 born in the pre-ECMO period and 159 born after the ECMO program was established. Survival significantly increased from 72% before ECMO to 89% during the ECMO period. During the ECMO period, 98/159 (62%) patients with HRF were rescued using iNO/HFOV, while 61 (38%) did not improve; 52 of these 61 neonates were placed on ECMO.11 The ECMO survival rate to hospital discharge was 85%. After adjusting for potential confounders, the severity of the pretreatment OI, late arrival to the referral center, the presence of a pneumothorax, and the diagnosis of a diaphragmatic hernia were significantly associated with the need for ECMO or death. The conclusion of this study was that the establishment of an ECMO program was associated with a significant increase in the survival of newborns ≥35 weeks with severe HRF in a developing country.11
In 2013, in an editorial in the Journal of Pediatric Critical Care Medicine, Steinhorn and Keller commented that the Chilean experience is notable for a number of reasons.23 “Chile is a resource-limited country, and ECMO is notoriously expensive and resource intensive because of the need for sophisticated equipment and well-trained nurses and technicians for constant monitoring”.23 They continue pointing out: “After beginning the ECMO program, nearly one out of every 10,000 neonates born in Chile was transferred to P. Universidad Católica de Chile for advanced respiratory care. Establishing the program and facilitating complex transports of critically ill infants across a large country is truly a remarkable achievement. In an era when the expenditure of economic resources on pediatric healthcare is being scrutinized, it is encouraging to see Chile's commitment to enhancing the survival of infants in their community, knowing that good quality survival of infants with ECMO is likely to return more over the years than the initial expenditure of resources”.23 Steinhorn and Keller concluded that the lessons from the Chilean experience will also be valuable to other resource-limited countries planning to increase their level of available care, highlighting the advantages of regionalization of specialized medical therapies such as ECMO, which accelerates the accumulation of experience of surgeons, neonatologists, and other professionals who are best positioned to treat these infants.11,23
In Bucaramanga, Colombia, a report from the Fundación Cardiovascular de Colombia in 2013 showed that in spite of reduced availability of technical and economic resources, ECMO therapy can be implemented successfully in a developing country. Their model of care is based on nurses as ECMO specialists supported by a multidisciplinary team. Their protocol with reduced circuitry and laboratory monitoring, and a simple and less expensive circuit are important for good outcomes with reduced staff.24 Recently, Miana LA from the Heart Institute, University of Sao Paulo, Brazil, demonstrated that investment in ECMO team training combined with a cost-effective investment in technology can bring significant benefits in postcardiotomy patients.25
Following the Euro-ELSO and Asia-Pacific ELSO examples, in 2012 Latin America created the local chapter of ELSO (LATAM ELSO), with the mission of contributing to the dissemination of ECMO therapy according to the recommendations of the ELSO, practical and theoretical education through courses and workshops, and encouraging collaborative work among Latin American centers. The LATAM ELSO foundation was based in Santiago, Chile, during the Latin American ECMO Symposium 2012. About 250 ECMO practitioners from Latin America attended the event, which also featured the participation of ELSO representatives (Dr. Steve Conrad, Dr. Michael Hines, Peter Rycus, and Dr. William Lynch).
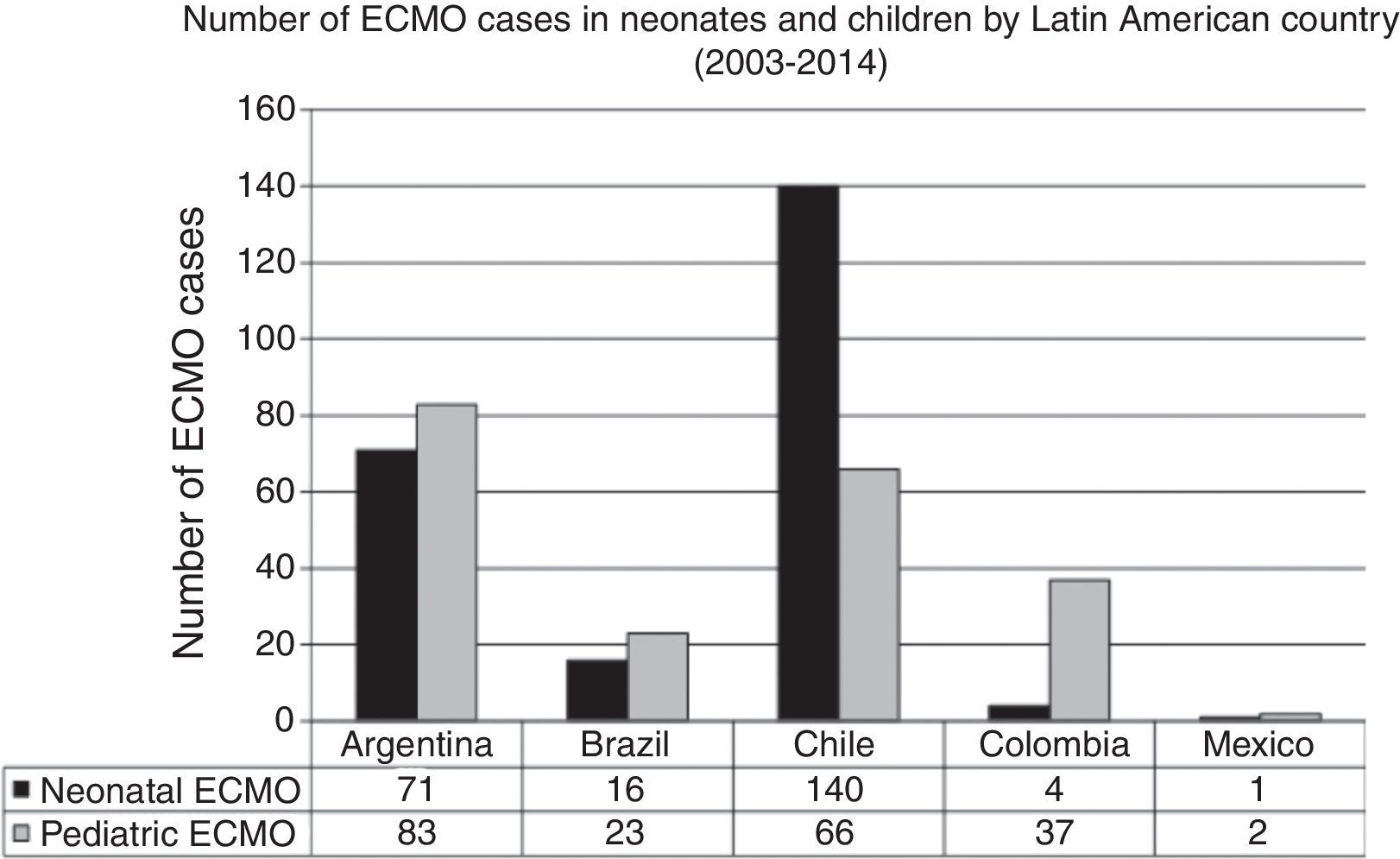
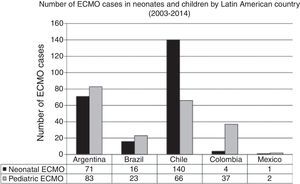
In the last decade, new neonatal-pediatric ECMO programs have been established in high-complexity and high-volume centers in several Latin American countries, such as Argentina, Colombia, Brazil, Mexico, Perú, and Costa Rica.21,26–28 The majority of these centers have progressively entered the ELSO and the LATAM ELSO chapter. After the LATAM ELSO chapter was created, a notable increase of ECMO centers were seen in the region (Fig. 2). There are currently 33 ELSO centers in seven countries, 16 of them neonatal and/or pediatric centers, with more than 270 newborns and 220 children reported to ELSO up to July 2016 (Fig. 2).2 As seen in Fig. 3, the proportion of neonatal or pediatric ECMO patients differs in each country, with more neonatal cases in Chile compared to pediatric cases, but more pediatric cases in Colombia, Argentina, and Brazil, compared to neonatal cases (Fig. 3).
Number of ECMO cases in neonates and children by Latin American country in main extracorporeal membrane oxygenation (ECMO) centers between 2003 and 2014. Data obtained by LATAM Extracorporeal Life Support Organization (ELSO) Survey 2014, presented at the 25th ELSO Meeting in Ann Harbor, Michigan, 2014.
As Caneo from Sao Paulo, Brazil recently commented: “The good news is that with the support of experts from the USA, Europe, and Canada, the results in Latin America's ELSO centers are improving by following its guidelines for training, and using a standard educational process”.29
ECMO physiologyDuring extracorporeal support the blood is drained from the patient to an external pump (roller or centrifuge), which pumps the blood through an exchange membrane (silicon or polymethylpentene oxygenator) for oxygenation and CO2 removal, and a heater for returning the blood to the patient's circulation (Fig. 1). This therapy requires anticoagulation of the circuit and use of heparin administered to the ECMO circuit, with the aim of avoiding activating the coagulation cascade. As well, several pressure, flow, bubble, and temperature monitors are used. Continuous coagulation monitoring is essential, with hourly measurements of activated clotting time (ACT), anti-factor Xa level, platelet count, fibrinogen levels, and in some patients, anti-thrombin III level and thromboelastography.30
There are essentially two forms of ECMO:
- (a)
Veno-arterial (VA): in which the blood is drained from the right atrium with a cannula inserted in the right internal jugular vein, femoral vein, or directly in the right atrium, and is returned to the thoracic aorta through a right, femoral, or aortic carotid cannula (Fig. 1). VA-ECMO provides cardiac and pulmonary support. Transthoracic cannulas (right auricular and aortic cannula) are often used with postoperative cardiac patients.30
- (b)
Veno-venous (VV): in which the blood is drained from the right atrium through the posterior and lower orifices of a double-lumen cannula inserted in the right jugular and returned to the same right atrium through the anterior orifices of the same cannula, which is directed toward the tricuspid valve. One of the limits of this method is the recirculation of already oxygenated blood through the double-lumen cannula, which has been corrected with new VV cannula designs. VV-ECMO is also performed on older children with the use of two cannulas, removing blood from the jugular vein and returning it through the femoral vein. VV-ECMO requires a well-functioning heart. This ECMO modality avoids cannulation of the carotid or femoral artery, thus decreasing complications arising from cannulation or from ligation of these arteries and from the entry of air in the ECMO circuit. The use of this mode has increased in recent years; it is now used in around 40 and 50% of neonatal and pediatric respiratory cases, respectively.30
With both forms of ECMO, the ventilator and FiO2 parameters are kept low to allow for the lungs to recover, but generally positive end-expiratory pressure (PEEP) remains high (6–8cm H2O) to avoid atelectasis.
Oxygen is delivered during ECMO by the combination of blood oxygenation through membranes, blood flowing through the extracorporeal circuit, oxygenation through the native lung, and native heart output.30 In turn, oxygenation in the ECMO membrane is a function of the membrane's geometry, material composition and thickness, blood and FiO2 laminar thicknesses, the time red cells remain in the exchange area, hemoglobin concentration, and O2 saturation.30
CO2 removal by ECMO is a function of the geometry, material and surface area of the membrane, blood pCO2 and to a lesser degree blood and gas flows through the membrane.30
The bypass in VA-ECMO generates an essentially non-pulsatile blood flow. As the flow of blood to the extracorporeal circuit increases, the pulse wave decreases, and with 100% bypass ceases completely, except for occasional waves. However, normally VA-ECMO involves an 80% bypass, leaving 20% or more of blood circulated by the heart and lungs, resulting in a highly reduced but visible pulse.30 The kidney is undoubtedly the most affected organ by the absence of pulsatility, resulting in an anti-diuretic effect owing to juxtaglomerular stimulation. As well, non-pulsatile flow has been related to stimulation of the pressure receptors of the carotid sinus, provoking a major release of catecholamine, with deleterious effects on microcirculation.30
Selection criteria for applying ECMOThe criteria differ for neonatal or pediatric patients and depend on whether the cause is primarily cardiac or respiratory. The criteria are general and should be individualized for each patient, evaluating the risks and benefits of applying ECMO.16
- •
Gestational age ≥34 weeks
- •
Weight at birth ≥2kg
- •
Unresponsive to maximum medical treatment (HFOV, iNO, surfactant)
- •
Reversible cardiopulmonary condition
- •
Mechanical ventilation ≤10–14 days
- •
High pulmonary mortality (50–100%). One of the following:
- -
OI >40 for 4h (iNO, HFOV)
- -
PaO2 <40–50mmHg for 4h (100% O2)
- -
Gradient A/aDO2 >600mmHg for 4h
- -
OI ≥25 after 72h with HFOV-iNO31
- -
- •
Unmanageable metabolic acidosis (pH<7.15 for 2h)
- •
Reduced cardiac output with reversible etiology
- •
Impossibility to wean from cardiopulmonary bypass
- •
As a bridge for cardiac transplant32
- •
Without post-cardiac surgery lesions
- •
Absence of major intracranial hemorrhage
- •
Absence of uncontrollable hemorrhage
- •
Without evidence of massive cerebral damage
- •
Without malformations or syndromes with fatal prognosis
The fundamental criteria are similar for pediatric patients with respiratory failure, with particular emphasis on whether the patient faces a serious pulmonary risk with high risk of death, but possible reversibility of the condition through respiratory, gasometrical, and hemodynamic repose. Among the pediatric criteria are14:
- •
OI >40 for 6h in invasive mechanical ventilation (IMV) and/or HFOV
- •
OI >35 for >12h
- •
Adverse effects of mechanical ventilation
- •
Mechanical ventilation ≤10–14 days
- •
Hypercapnia with pH<7.1 for 4h
- •
Acute deterioration with optimal therapy
Specific contraindications for ECMO for cardiac patients are the presence of residual post-surgical lesions and contraindications for cardiac transplant. However, each case should be analyzed individually given that the contraindications can be relative or can change over time.17,33 Among the cardiac or hemodynamic indications for pediatric patients are:
- •
Severe but potentially reversible cardiovascular failure that does not respond to vasoactive, vasodilatation, or anti-arrhythmic drugs
- •
Persistent SVO2<60%; pH<7.15
- •
Failure to wean from cardiopulmonary bypass after surgery
- •
Severe arrhythmia with poor perfusion
- •
Rapid ventricular deterioration or severe dysfunction
The initial parameters point to achieving a bypass of 50% or more of cardiac output (estimated at 200mL/kg/min) and are adjusted to maintain adequate pressure and an acid-base state. When cardiac function is conserved and the main pathology is pulmonary, VV-ECMO can be used to assist oxygenation and ventilation. Meticulous attention to all aspects of the patient is essential. Frequent checks are required of blood gases, the ECMO circuit, clotting, and renal function, as well as cerebral ultrasound assessment in search of intracranial hemorrhage and cerebral infarction. Patients are sedated, but generally not paralyzed, which facilitates neurological assessment. To the degree that the patient improves, ECMO support is gradually reduced. Patients are decannulated when they cannot tolerate a minimal ECMO support (10% of bypasses in VA-ECMO) with low-to-moderate mechanical ventilation parameters. ECMO treatment generally lasts between five and ten days for neonatal patients with respiratory diseases, and longer in cases of CDH (10–12 days on average).2
ComplicationsThe ECMO procedure has several risks of complications from the use of anticoagulants and changes in blood flows as a consequence of the seriousness of the patient's condition upon entering the ECMO. Among the most common complications are hemorrhage (surgical site 6%, pulmonary 4%, gastrointestinal 2%), infarction or cerebral hemorrhage (9% and 5% respectively), convulsions (11%), cardiac dysfunction (myocardial stunning 6%, arrhythmia 4%), kidney failure (4%), sepsis (6%), hyperbilirubinemia (9%), arterial hypertension (12%), and hemolysis (13%).2 By far the most common complication with cardiac-based ECMO is the need for vasoactive drugs during extracorporeal support, followed by surgical site bleeding.34
Intracranial hemorrhage is the primary cause of death during ECMO, and the appearance of convulsions is sign of a poor prognosis. Additionally, there are complications arising from circuit failures of the oxygenator or of other ECMO equipment.2
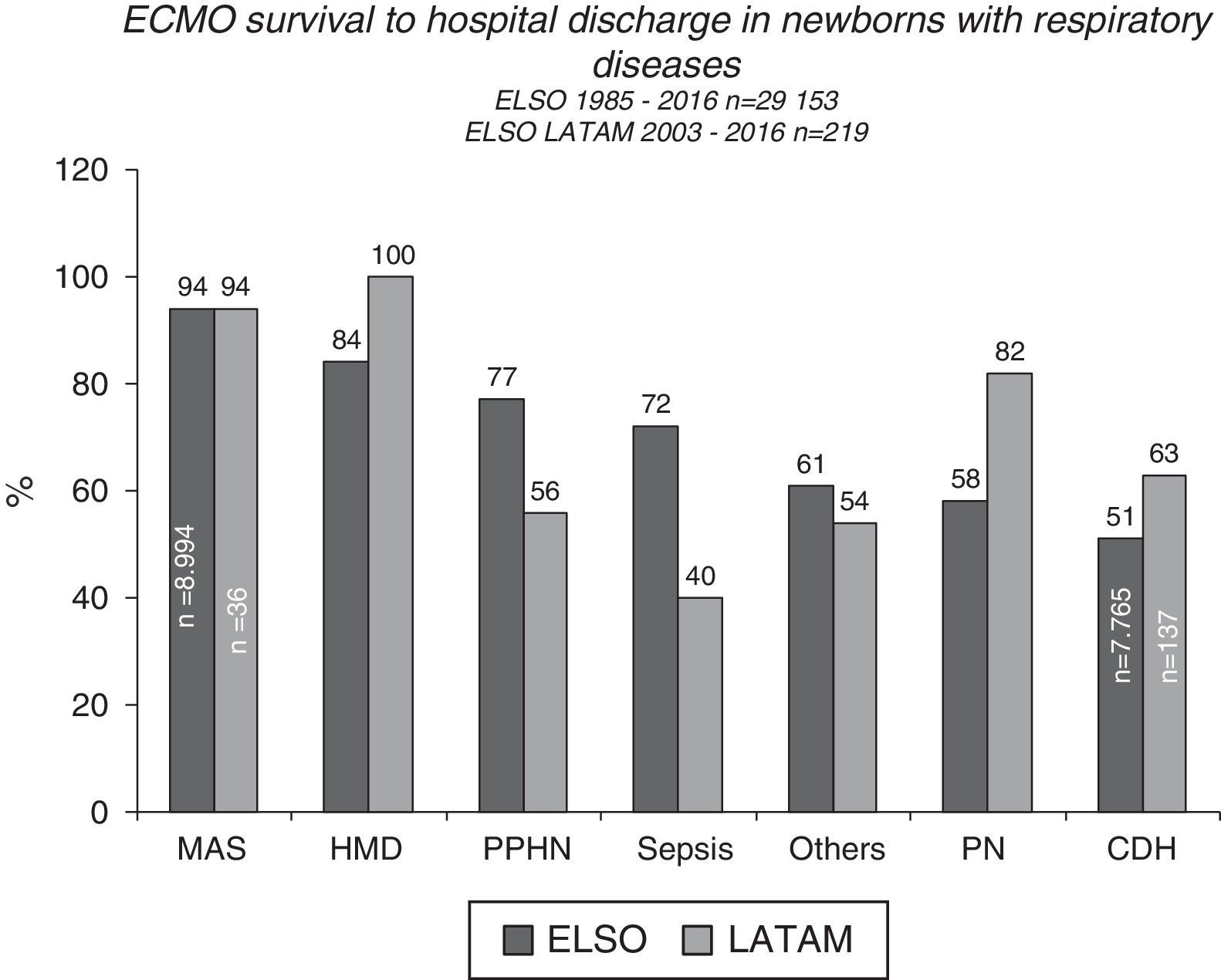
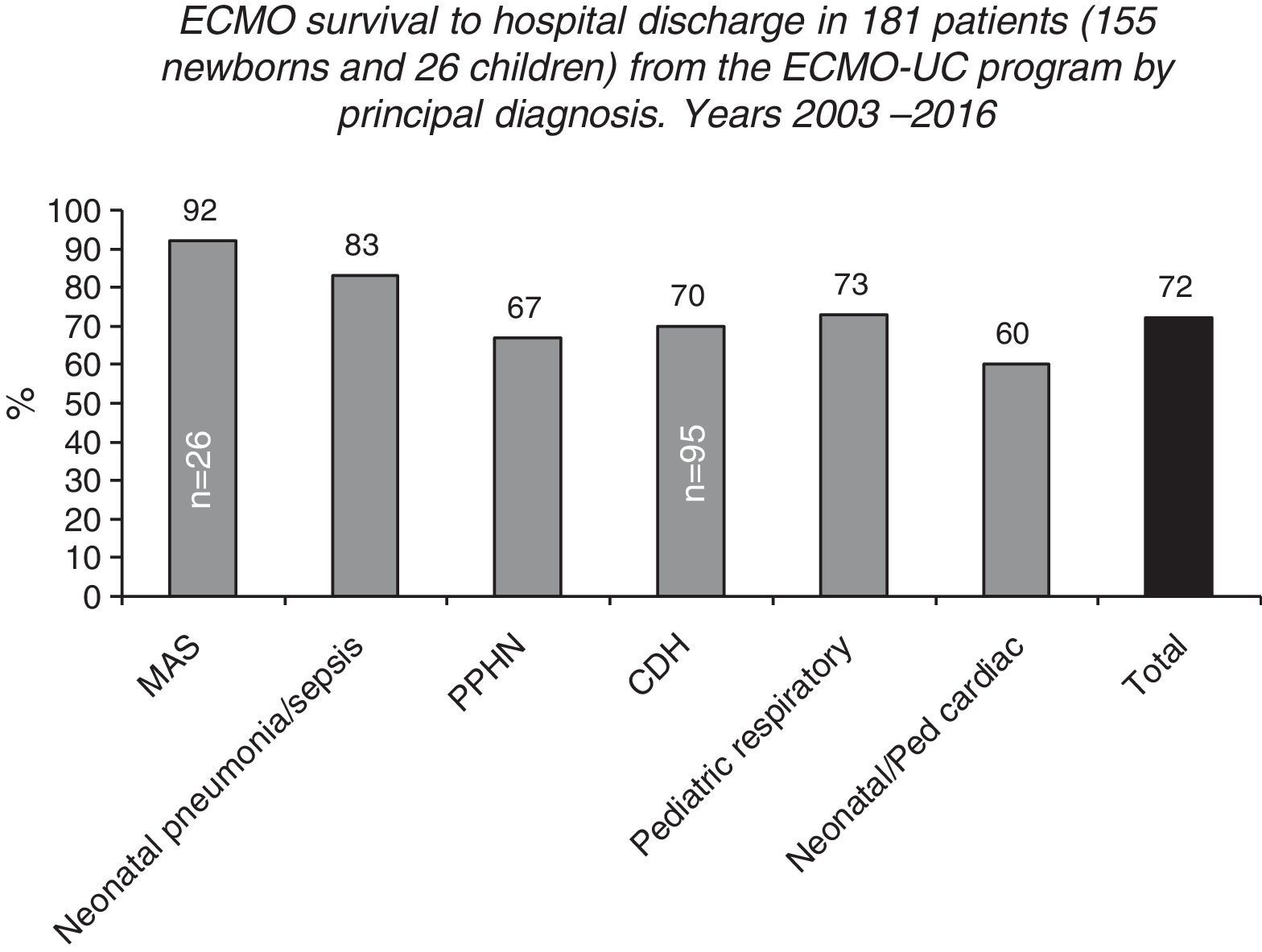
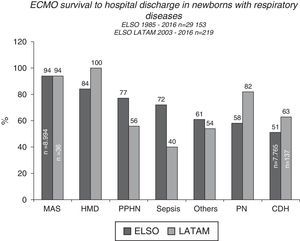
ECMO prognosis and follow upPost-ECMO survival among neonatal patients varies according to the underlying disease, with cases with respiratory causes having the best results, demonstrating close to 70% survival to hospital discharge, according to ELSO and LATAM ELSO reports2 (Figs. 4 and 5). Among all the respiratory causes, neonates with meconium aspiration syndrome (MAS) have the highest survival rate: 94% to hospital discharge2,3 (Figs. 4 and 5). VV ECMO is generally used for SAM, which is associated with a lower rate of risks and complications such as cerebral infarctions and convulsions, and minor changes in blood flow patterns.
Survival to hospital discharge of 29,153 and 219 newborns treated with extracorporeal membrane oxygenation (ECMO), reported to the international Extracorporeal Life Support Organization (ELSO) and LATAM ELSO, respectively, according to the respiratory cause. MAS, meconium aspiration syndrome; HMD, hyaline membrane disease; PPHN, persistent pulmonary hypertension of the newborn; PTX, pneumothorax; PN, pneumonia; CDH, congenital diaphragmatic hernia.
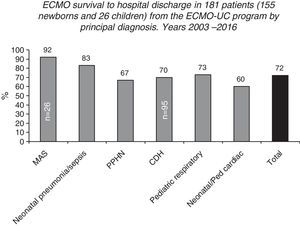
Survival to hospital discharge of 155 neonatal and 26 pediatric patients treated in the Neonatal-Pediatric extracorporeal membrane oxygenation (ECMO) Program at the Catholic University Hospital in Santiago, Chile (ECMO-UC Program) 2003–2016, reported to the Extracorporeal Life Support Organization (ELSO) according to the main diagnosis.
Conversely, patients treated with ECMO for cardiac causes have a lower survival rate, close to 45%.2,13,33 Nevertheless, for well-selected patients ECMO is a useful tool that should be available in highly complex cardiology centers.17 Among the patients treated with ECMO for cardiac causes are notably those with cardiomyopathy and myocarditis, with survival rates to hospital discharge of 61 and 51%, respectively.2,13,33 In recent years ECMO has been used as a post-cardiopulmonary resuscitation tool with variable results, with survival rates of close to 40%.2,13
The survival and neurological prognosis at five years among patients treated with ECMO for non-cardiac causes is in general very good, but worsens with a lower gestational age, a lower birth weight, and a higher pre-ECMO OI.35 The poorest survival and neurological evolution results are with patients with diagnoses of septic shock and CDH. Nevertheless, pre-existing factors and the severity of the newborns upon entering ECMO appear to be the major determinants of the long-term prognosis.10,35,36
The long-term respiratory prognosis depends on the base etiology, the degree of barotrauma, and the duration of exposure to oxygen. Between 10 and 30% of patients with CDH have episodes of wheezing by the age of 10 and close to 50% have hyperinsufflation and episodes of airway obstruction.37,38
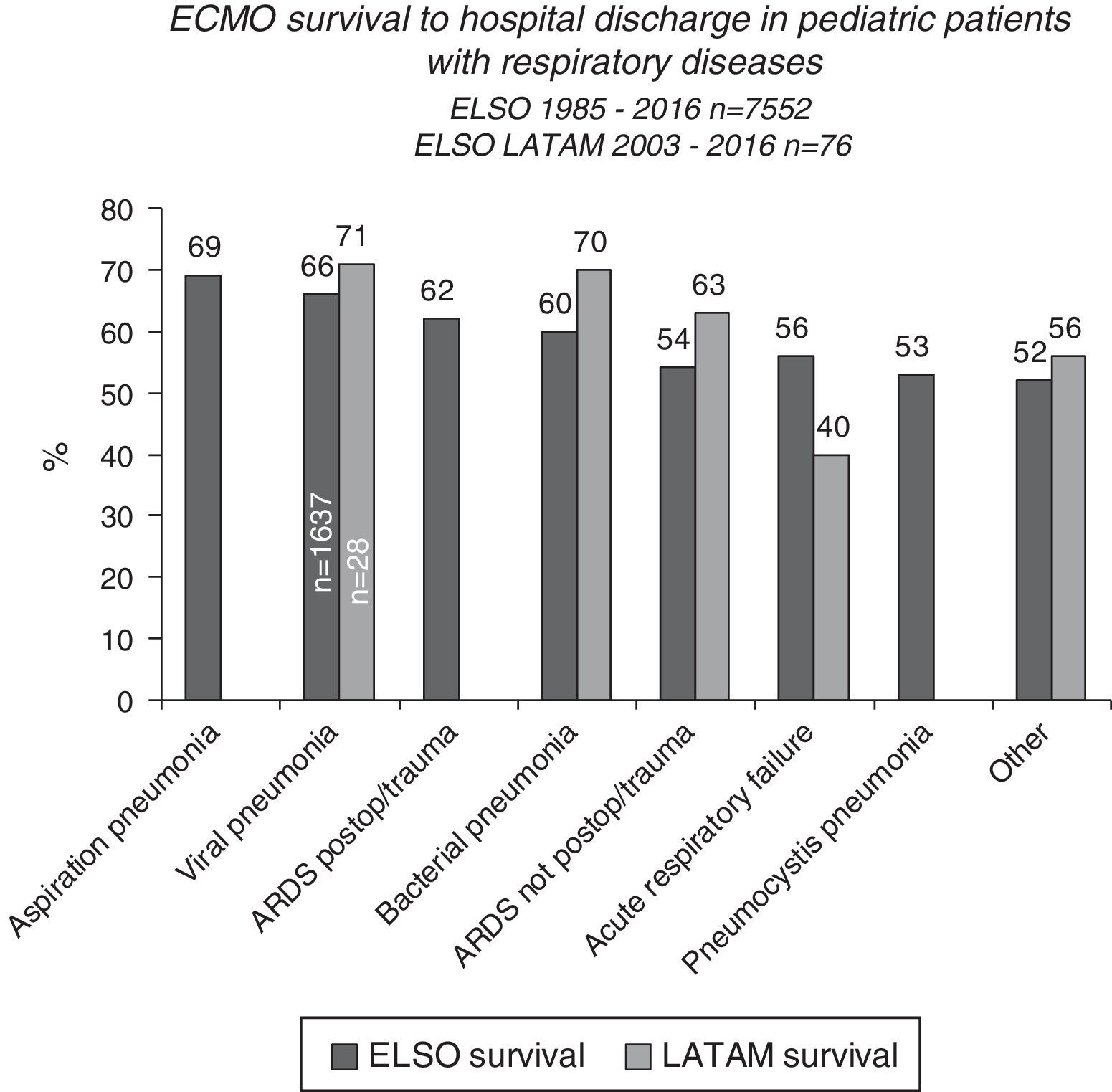
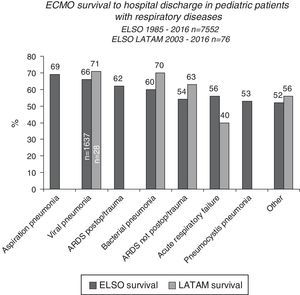
Post-ECMO survival is lower for pediatric than neonatal patients, although there is a better prognosis in the group with respiratory failure, especially patients with aspiration pneumonia, viral pneumonia, and acute post-operative or post-traumatic respiratory distress syndrome (Fig. 6).15,39 Viral pneumonia is the most common condition leading to pediatric ECMO; among its etiologies, respiratory syncytial virus has the highest post-ECMO survival rate at 70%.15 Patients with pneumonia caused by other viruses and by Bordetella pertussis have lower survival rates of 56% and 39%, respectively.15,39
The pediatric patients who receive ECMO due to cardiac causes have a somewhat higher survival rate than their neonatal counterparts2,13 (55% survival to hospital discharge), highlighting the survival rates to hospital discharge of 72% and 61% for myocarditis and cardiomyopathy, respectively.2,13
After 13 years, from May 2003 to July 2016, the ECMO-UC center treated 181 patients (155 newborns and 26 children, ranging from 0 to 11 years of age), with both severe respiratory and cardiac pathologies (Fig. 6). 72% of these newborns and children survived to hospital discharge. The 51 patients that died had as base diseases: CDH (n=29), congenital heart disease operated with failure to wean from cardiopulmonary bypass or arrhythmias (n=11), persistent pulmonary hypertension secondary to sepsis, pneumonia, MAS, SP-B or ABCA3 deficiency, or without defined cause (n=10), and pneumonia due to Bordetella pertussis (n=1).2 Among patients treated with ECMO, there are notably newborns with CDH, with a survival rate of 70% to hospital discharge (66/95).2
All the survivors in the ECMO-UC program in Chile are currently in a special ECMO follow-up program.38 Among the neurological follow-up exams, the Bayley II tests at 12–18 months showed that over 90% of the children had normal or slightly altered mental development indices (MDI) and more than 70% had normal or slightly altered psychomotor development indices (PDI).38 As well, no patient presented disabling visual or auditory alterations. In the CDH patient follow-up, over 80% had a normal MDI from the Bayley II tests at 12–18 months, and similar to the entire group, over 70% had a normal or slightly altered PDI.
With respect to respiratory follow-up, 83% of the patients had a normal or slightly altered clinical bronchopulmonary, and 27% had moderate bronchial hyperreactivity in the evaluation at 3 years of age.38
The establishment of an ECMO program in Chile was associated with a significant increase in the survival of near-term newborns with severe respiratory insufficiency. ECMO therapy was successful and did not provoke disabling sequelae in the majority of the patients.
Conclusions and future considerationsECMO therapy, now termed more broadly as ECLS, is a standard therapy in neonatology and pediatrics, with demonstrated short- and long-term benefits. It can be incorporated into intensive therapy with good results in developing countries, but requires highly complex neonatal and pediatric centers with trained and committed staff.
Future patients who are treated with ECLS will be progressively more complicated, therefore new and simpler automated ECLS modalities will be required, with less need for anticoagulants, with the aim of minimizing the associated risks and making their extended use possible. Thus newborns and children with severe conditions can be submitted to ECLS for heart and lung transplant, or as bridge to ventricular assistance devices.18,32,33 Even premature newborns with severe cardiopulmonary failure can in the future benefit from umbilical ECLS, or low bypass using lung assist devices (LAD).40 New low-resistance micropore oxygenators can make pumps unnecessary, using the umbilical artery or vein as an arteriovenous shunt.41,42 Moreover, newborns with CDH can be admitted for early ECLS treatment to minimize pulmonary damage and to favor lung growth using growth factors and/or liquid ventilation with perfluorocarbon associated with ECLS. Some centers, like the Boston Children's Hospital, have been using ex-utero intrapartum treatment (EXIT to ECMO) for patients with CDH and prenatal markers of poor prognosis, or to ensure effective ventilation for newborns who do not have safe airways or who are expected to experience severe respiratory failure upon birth (CDH, cervical teratoma, airway pathologies, large pulmonary masses, bronchial cysts, etc.).43
The authors expect that ECLS will allow them to continue assisting pulmonary and cardiac functioning more rationally through the repair of severe but reversible cardiopulmonary processes.
It is hoped that the new ECMO programs in Latin America, as benchmark centers, will have a positive impact on the survival of newborns and children with respiratory or cardiac insufficiency, and that this treatment will be available to a greater number of patients in this region in the near future.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to acknowledge the following institutions for their contributions to this ECMO program: Extracorporeal Life Support Organization (ELSO); Latin American ELSO Chapter (LATAM ELSO), Children's National Medical Center, George Washington University, Washington D.C.; W. H. Tooley NICU, University of California, San Francisco; Child Health Foundation, University of Alabama, Birmingham; Egleston Children's Hospital, Emory University, Atlanta; Washington University, St. Louis; Ministry of Public Health of Chile, and the Neonatal Division, Department of Pediatrics and the Department of Cardiovascular Diseases and Anesthesia, Pontificia Universidad Católica de Chile.
The authors are very grateful to many Latin American ECMO Centers who contributed their ECMO patient data: Argentina: Hospital Prof. Dr. Juan Pedro Garrahan, Buenos Aires; Hospital Universidad Austral, Pilar, Buenos Aires; Hospital Sor María Ludovica, La Plata, Buenos Aires; Hospital Italiano, Buenos Aires; Clínica Bazterrica, Buenos Aires; Hospital Universitario Fundación Favaloro, Buenos Aires. Brazil: Heart Institute InCor, Sao Paulo; Hospital de Clínicas, Universidad de Sao Paulo, Campinas, Sao Paulo; Hospital da Bahia, Salvador da Bahia. Chile: Clínica Las Condes, Santiago; Clínica Alemana, Santiago; Clínica Santa María, Santiago; Hospital Roberto del Rio, Santiago. Colombia: Fundación Cardiovascular de Colombia, Bucaramanga; Fundación Clínica Shaio, Bogotá; Clínica CardioVID, Medellín. Mexico: Hospital Christus Muguerza, Monterrey. Paraguay: Hospital Pediátrico Niños de Acosta Ñu, San Lorenzo. Peru: and Hospital Nacional Edgardo Rebagliati Martins, Lima.
Please cite this article as: Kattan J, González Á, Castillo A, Caneo LF. Neonatal and pediatric extracorporeal membrane oxygenation in developing Latin American countries. J Pediatr (Rio J). 2017;93:120–9.