To investigate associations of maternal and cord blood cytokine patterns with newborn size and body composition.
MethodsThis cross-sectional study involved 70 pregnant women and their healthy newborns selected from the “Araraquara Cohort Study”. Newborn anthropometric measurements were recorded at birth. Body composition was evaluated by air displacement plethysmography. Maternal blood samples were collected from pregnant women between 30 and 36 weeks of gestation, and umbilical cord blood samples were collected immediately after placenta discharge. The concentrations of the cytokines were determined in plasma by ELISA. Multiple linear regression models were used to assess associations between maternal and cord blood cytokine concentrations and newborn anthropometry and body composition measurements.
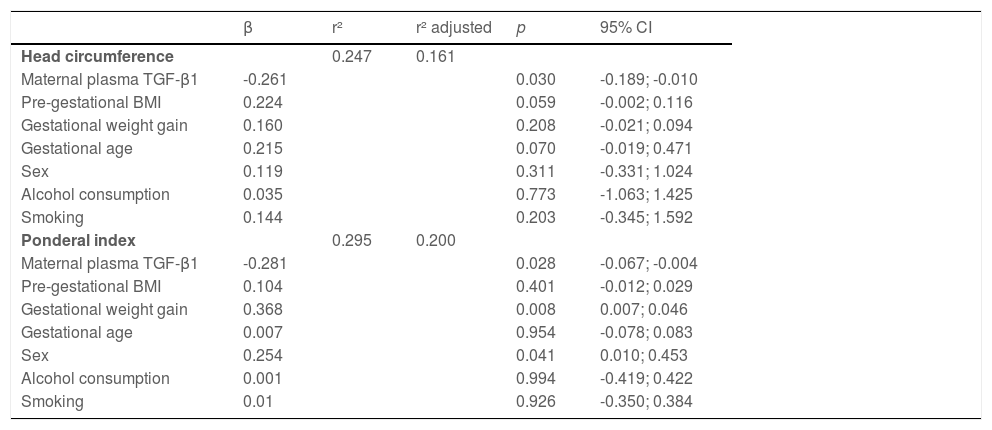
ResultsMaternal plasma TGF-β1 concentration was inversely associated with newborn weight (β = -43.0; p = 0.012), length (β = -0.16, p = 0.028), head circumference (β = -0.13, p = 0.004), ponderal index (β = -0.32, p = 0.011) and fat-free mass (β = -0.05, p = 0.005). However, the association persisted just for head circumference (β = -0.26; p = 0.030) and ponderal index (β = - 0.28; p = 0.028), after adjusting for pre-gestational BMI, gestational weight gain, gestational age, hours after delivery, newborn sex, smoking and alcohol consumption.
ConclusionsMaternal plasma TGF-β1 concentration may be involved in the regulation of newborn size, mainly head circumference and ponderal index. Further cohort studies are necessary to investigate the role of TGF-β1 in different trimesters of pregnancy and its effect during the early stages of fetal development.
The maternal environment has an important impact on fetal development. The changes that occur during the intrauterine period can program the physiology and functions of the fetal organism.1,2 Despite a large body of evidence that maternal nutrition and fetal nutrient supply (in excess or deprivation) are intimately correlated with offspring features,3 few studies have emphasized the influence of maternal immunity on fetal growth and development.4,5 Systemic inflammation is increased in normal pregnancy, an event that is related to the parturition process.6 However, deregulated maternal immunity can result in intrauterine growth restriction and preeclampsia.5,7
Considering that maternal metabolic inflammation may affect the intrauterine environment 8 and have an impact on newborn size,9 the authors sought to assess the relationship of cytokine concentrations in maternal and cord blood with newborn anthropometry and body composition.
Materials and methodsThis cross-sectional study is a part of a large prospective epidemiological study (“The Araraquara Cohort Study”) that selected pregnant women from Public Health Units and the Municipal Maternity Hospital in Araraquara city, São Paulo, Brazil. All pregnant women with a gestational age ≤15 weeks at the first interview, who attended all follow-up examinations and who had an expected delivery date between November 2017 and May 2018, were considered to be eligible. Pregnant women were excluded if they experienced a miscarriage or gave birth to a baby with a congenital disease that compromised postnatal feeding. After recruiting the study participants, their gestational ages were checked by transabdominal ultrasound.
The study was approved by the Ethics Committee of the University of São Paulo (protocol number 1.885.874) and was conducted according to the guidelines of the Declaration of Helsinki. Written informed consent was obtained before any data collection.
Mothers provided information about demographic and socioeconomic (age, ethnicity, marital status, educational level and per capita income), lifestyle (smoking and alcohol consumption), maternal morbidity, and nutritional (pre-gestational weight and body mass index- BMI) factors on enrollment in the study, using questionnaires administered by a team of trained researchers and checked in the medical records. In the third trimester of gestation, maternal weight and body composition were determined by bioelectrical impedance analysis (BIA). Height was measured using a Seca 206 stadiometer (Seca®, Hamburg, Germany).
Newborn anthropometric measurements were recorded at birth. Newborn length was measured using a Seca® 416 infantometer (Seca®, Hamburg, Germany), and head circumference was measured using a Seca® 2 201 flexible tape (Seca®, Hamburg, Germany). Newborn ponderal index was calculated by weight (g) × 100/length (cm3). A trained research team performed all measurements to ensure accuracy and reproducibility of the data. Body composition was evaluated within 12–72 hours after delivery by air displacement plethysmography (Pea Pod®, Cosmed, San Francisco, CA, USA).
Maternal blood samples were collected from pregnant women between 30 and 36 weeks (third trimester), and umbilical cord blood samples were collected after placenta discharge. The concentration of cytokines was determined in plasma samples by ELISA following manufacturer instructions. The detection limits were 156.3 pg/mL for TGF-β1, 2 pg/mL for IL-6, 4 pg/mL for TNF-α, and 7 pg/mL for CCL2 (eBioscience, San Diego, CA, USA).
Multiple linear regression models were used to assess associations between maternal and cord blood cytokine concentrations and newborn anthropometry and body composition measurements. The outcome measures were newborn weight, length, head circumference, BMI, fat mass, and fat-free mass. Confounding variables included pre-gestational BMI, gestational weight gain, gestational age, newborn sex, hours after delivery, smoking and alcohol consumption. Statistical significance was set at p < 0.05. All analyses were performed using the SPSS 18.0 software (SPSS, Chicago, IL, USA).
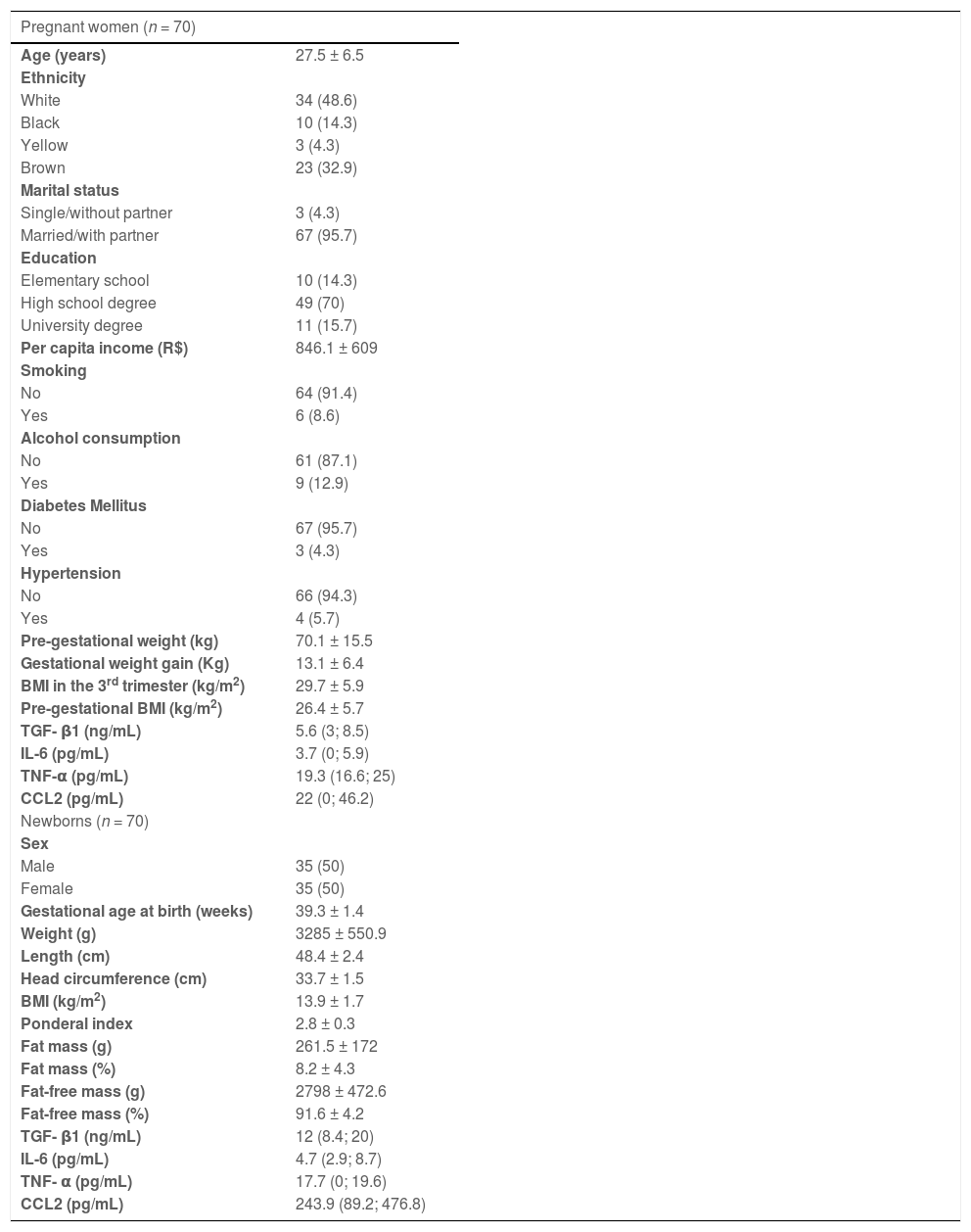
ResultsThe authors randomly selected 70 cases with sufficient plasma samples for cytokine measurements from the original study population. Table 1 shows the characteristics of pregnant women and their newborns, as well as the cytokine concentrations in maternal and cord blood. Most of the pregnant women were white, married or with a partner and had a complete high school degree. The women had mean pre-gestational BMI of 26.4 ± 5.7 kg/m2, weight gain of 13.1 ± 6.4 kg, and BMI in the third trimester of pregnancy of 29.7 ± 5.9 kg/m2. Overall, 60% (n = 54) of the women were overweight or obese (data not shown). None of the women had an infectious disease or had used illicit drugs during pregnancy. Among neonates, the mean BMI was 13.9 ± 1.7 kg/m2, and 71.4% showed adequate weight for gestational age (data not shown).
Characteristics of pregnant women and newborns.
Mean ± SD, median ± interquartile range, or number of individuals (percentage). 1US$ = approximately R$3.7.
BMI, body mass index.
Maternal plasma TGF-β1 concentration was inversely associated with newborn weight (β = -43.0; p = 0.012), length (β = -0.16, p = 0.028), head circumference (β = -0.13, p = 0.004) and ponderal index (β = -0.32, p = 0.011). However, the association persisted just for head circumference (β = -0.26, p = 0.030) and ponderal index (β = -0.28, p = 0.028) after adjusting for pre-gestational BMI, gestational weight gain, gestational age, newborn sex, smoking, and alcohol consumption (Table 2).
Linear regression model showing associations of maternal plasma TGF-β1 concentration with newborn head circumference and ponderal index.
BMI, body mass index; CI, confidence interval.
There were no associations between cord blood cytokines and newborn size (data not shown).
DiscussionThis study showed that higher TGF-β1 concentrations were associated with lower head circumference, independent of pre-gestational BMI, weight gain during pregnancy, gestational age and sex. This finding indicates that maternal metabolic inflammation may affect the intrauterine environment and have an impact on newborn size, particularly on head circumference.
Nazzari et al. (2019) reported an inverse association between maternal IL-6 concentrations in the third trimester and head circumference of the newborns.10 Our results showed no association between maternal IL-6 and head circumference. These contrasting findings might be explained by the fact that in our study the mean concentration of IL-6 was 2.5 times higher than in the Nazzari study and also by the use of a different ELISA kit.10
Ragsdale et al. (2019) observed that maternal TNF-α, IL-10 and IL-6 (n = 407) were not associated with newborn anthropometry, while the IL-6/IL-10 ratio had an inverse association with weight and length at birth.11 Indeed, Yeates et al. (2020) did not find associations of maternal Th1 and Th2 cytokines, including MCP- 1 (CCL2), TNF-α, and IL-6, with newborn weight, length, and head circumference.12
Although an inverse correlation of umbilical cord blood TGF-β1 (n = 98) with weight at birth was previously demonstrated,13 in our study, the quantification of TGF-β1, IL-6, TNF- α, and CCL2 in umbilical cord blood showed no association with newborn anthropometry and body composition.
Growth factors such as TGF-β1 are known to be involved in tissue growth and differentiation,14 especially during pregnancy, playing an important role at the maternal-fetal interface.15 Elevated levels of TGF-β1 have been demonstrated in intrauterine growth restriction16 and in preeclamptic women,17 but the role of this cytokine in these conditions is uncertain.18 Some authors postulate that TGF-β1 may control fetal growth via its influence 1) on the proliferation of fetal hepatocytes, because fetal liver growth in the third trimester is one of the main determinants of size at birth or 2) on placental bed blood vessels.19
Therefore, maternal plasma TGF-β1 concentration may be involved in the regulation of newborn size, particularly head circumference and ponderal index. However, a limitation of this study is the fact that the mothers and respective newborns were selected at random from the samples that had sufficient plasma volume for cytokine quantification.
Further cohort studies are necessary to investigate the role of TGF-β1 and other cytokines in the three trimesters of normal and complicated pregnancies and their effect during the early stages of fetal development.
FundingThis study was supported by the São Paulo Research Foundation (FAPESP) (grant numbers 2015/03333-6; 2017/07143-2; 2018/17824-0 and 2018/19638-9), CNPq (grant number 145350/2018-5), and “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brasil” – FinanceCode 001.
The authors would like to thank the São Paulo Research Foundation (FAPESP) (grant numbers 2015/03333-6; 2017/07143-2; 2018/17824-0 and 2018/19638-9), CNPq (grant number 145350/2018-5), and “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brasil” – Finance Code 001.