Adherence problems have a great impact on auto-immune Rheumatic Diseases (AIRD). The COVID-19 pandemic may have worsened treatment adherence. The aims of this study were to measure treatment adherence to identify an earlier risk of poor adherence and measure families’ satisfaction with the health service during the pandemic.
MethodsProspective observational study with 50 parents/children and adolescents with recent AIRD diagnosis. Initially, they answered questions (demographic data, disease) and completed the Pediatric Rheumatology Adherence Questionnaire (PRAQ), after 6 months they completed the Morisky-Green Test (MGT), Brief Medication Questionnaire (BMQ), Compliance Questionnaire for Rheumatology (CQR-19) and Pediatric Quality of Life Inventory Questionnaire 3.0 (PedsQlTM-SSS). The patient's medical records from the previous 12 weeks were reviewed for global and medication adherence data.
ResultsThe mean global adherence score was 94.3 ± 10.0, for medication adherence 97.3 ± 9.3, and for PRAQ questionnaire 5.2 ± 1.5. The authors observed agreement between MGT, BMQ, CQR-19, PedsQLTM-SSS scores and medication adherence rate, but not with global adherence rate. There were no associations between demographic characteristics, disease diagnosis, and adherence. No associations between PRAQ scores and values and global/medication adherence rates were observed. No variables were shown to be predictors of good adherence. The mean PedsQLTM-SSS rate was 92.1 ± 6.8.
ConclusionThe high values of MGT, BMQ, CQR-19 questionnaire scores were in agreement with the medication adherence rate. Despite the pandemic, the global and medication adherence rates were good. It was not possible to demonstrate the PRAQ's predictive power. The authors weren't able to establish an association between families’ satisfaction and treatment adherence rates.
Although physiologically different, autoimmune rheumatic diseases (AIRD) have in common, long-term treatments with potential physical, social, and emotional impacts, for children/adolescents, and their caregivers. Children and adolescents with AIRD may not have immediate beneficial effects. The main objective of treatment is disease control, focusing on the maintenance of growth, physical, emotional, and social development. Thus, adherence to treatment is an issue that impacts its effectiveness, and favorable clinical outcomes.1,2
There are several medications indicated for AIRD. These drugs can be classified as conventional Disease-modifying Antirheumatic Drugs (DMARDs) such as methotrexate, leflunomide, and biologic DMARDs such as anti-tumor necrosis factor (anti-TNF), and anti-interleukin 6 (anti-IL6) agents. Other immunosuppressants such as cyclophosphamide, azathioprine, cyclosporin, are drugs that are also included in the treatment of these diseases.3
When talking about treatment adherence, there is no universal concept. According to the World Health Organization (WHO) and two other definitions by Haynes & Rand, adherence is defined as patient behavior that complies with recommendations and guidance and encompasses the intake of medications, diet follow-up, lifestyle changes, and orientation from health professionals.4-6
It is known that failures in adherence to treatment are the main cause of not obtaining all the benefits that medications could provide to patients.7 There is not a known method for assessing adherence to treatment, but there are tools for assessing adherence to drug treatment. As a direct tool of adherence evaluation, samples from the patient are required which are analyzed in laboratories to determine the presence of the drug.8 However, this method generates costs, presents difficulties in obtaining samples, requires the availability of dosing kits, and ends up not being the most used method.9
There are several indirect methods of assessing adherence to treatment. One of the most used methods to assess medication adherence is the Morisky-Green Test (MGT), which is validated in Brazil.10 The Brief Medication Questionnaire (BMQ), is another instrument validated in Portuguese, that assesses adherence, but in more than one aspect, through investigation of domains (posology, beliefs, medication reminder), identifying barriers from patients’ perspectives.11 The Compliance Questionnaire for Rheumatology (CQR-19) is an instrument that also assesses the adherence of patients monitored by a rheumatologist.12
The Pediatric Rheumatology Adherence Questionnaire (PRAQ) is a quantitative questionnaire developed in Brazil, which seeks to earlier detect the risk of adherence to treatment in children/adolescents with chronic rheumatic diseases.13
Low adherence, which includes missing appointments (medical or physical therapy), not taking medication, and not attending exams, occurs in approximately 50% of children and 65–90% of adolescents.14 With the advent of the COVID-19 pandemic, concerns about adherence reached greater proportions due to issues with social distancing, problems with transport to consultations and exams, and shortages of some medications.14
Therefore, the present study's objectives were: 1) To measure adherence to treatment of children and adolescents with AIRD throughout the pandemic, 2) To earlier identify the risk of poor adherence (using the PRAQ questionnaire) and adherence to treatment rates through data verification in the medical records of these patients (attendance to medical appointments, compliance with the physical therapy plan, attending requested exams and taking prescribed medications) and using specific questionnaires, 3) To study the correlation between adherence to treatment rates and the scores of the adherence questionnaires, and 4) To verify patients’ and caregivers’ satisfaction with the health service during the pandemic.
MethodsStudy designObservational prospective study of children and adolescents with a recent diagnosis of AIRD.
Subject and data collectionThe data collection occurred between January 2020 and January 2021 with caregivers and their respective children or adolescents, with recent diagnoses (of up to four months, and a range of ± 2 months). Patients with the following diagnoses were included: Juvenile Idiopathic Arthritis (JIA), Juvenile Systemic Lupus Erythematosus (JSLE), Juvenile Dermatomyositis (JDM), Juvenile Systemic Sclerosis (JSSc), and Takayasu's Arteritis (TAK) according to the diagnostic or classification criteria applicable for each disease.15-19 Patients up to 18 years of age, who used at least one systemic medication, for longer than a month, followed up at a tertiary center for Pediatric Rheumatology, and agreed to participate in the research, through the signing of the Informed Consent Form, were included.
The project was divided into two steps. Initially, the subjects and their caregivers answered the PRAQ questionnaire7 and questions related to disease and demographic data (first evaluation).
Six months after the first evaluation, the instruments Morisky-Green test (MGT)10,11, Brief Medication Questionnaire (BMQ),11 Pediatric Quality of Life Inventory (PedsQLTM-SSS) version 3.0,20 and Compliance Questionnaire for Rheumatology (CQR-19)11 were applied to the same population (second evaluation).
Additionally, the adherence evaluation was measured through a detailed assessment of medical records in the 12 weeks prior to the second evaluation. This included attendance to medical appointments, compliance with physical therapy plans, where applicable, taking the prescribed medications, and performing the requested exams. The questionnaire administrator had no previous knowledge of the health status of the patients. This data set on treatment adherence was called Global Adherence and just the data related to taking the prescribed medications were named Medication Adherence.
Global Adherence, Medication Adherence data, and the questionnaire scores were compared.
The 4 question-MGT classifies the patient as adherent to the medication if they do not present any positive response, as moderate adherence if they present 1 to 2 positive answers, and low adherence if they present 3 to 4 positive answers; that is, the lower the score, the lower the degree of non-adherence.10,11
In the BMQ there are 3 domains (posology, beliefs, and medication reminder), and the patient is classified according to their answers, as adherent (no positive answer in the domains), as probable adherent (positive response in 1 domain), as probable low adherent (positive response in 2 domains), and as low adherent (positive response in 3 domains). For statistical purposes, the authors classified patients who scored up to 1 domain as ‘adherent’ and those who scored in 2 or 3 domains as ‘non-adherent’.11
The CQR-19 classifies the patient as adherent when they present ≤ 80% score, using a five-point Likert scale of agreement (0 = strongly disagree, 1 = do not agree, 2 = neutral, 3 = agree, and 4 = strongly agree, or not applicable – NA). The answered items are scored from 0–100, where 0, 1 = 25, 2 = 50, 3 = 75, 4 = 100, and the final score is obtained by adding up all the answers, divided by the total number of questions answered. If more than 50% of the questionnaire is answered as “NA”, it is disregarded.12
The PedsQLTM-SSS score is obtained using a five-point Likert scale, [1 = never satisfied, 2 = sometimes satisfied, 3 = often satisfied, 4 = almost always satisfied, and 5 = are always satisfied or “not applicable (NA)]”. The answered items are scored from 0 −100, where 0 = 0, 1 = 25, 2 = 50, 3 = 75, and 4 = 100, and the final score is obtained by adding up all the answers, divided by the total number of questions answered. The higher the score the greater the satisfaction with the service.20
The PRAQ has five blocks (socioeconomic factors, factors related to health team and system, factors related to health status, factors related to therapy, and factors related to patient/caregiver). For each question there are three answer options: “yes,” “no” and “not applicable”. For each “yes” answer obtained, a point is added and the higher the final score, the greater the chance of low adherence. The maximum number that can be obtained is 25 points, but it does not have a specific cut-off value.13
In this study, poor adherence was considered when the patient performed 80% or less of what was prescribed. This study was carried out throughout the pandemic, and it should be noted that some of the medical and rehabilitation consultations were carried out by telemedicine.
Statistical analysisIn order to verify the existence of associations between two categorical variables, Fisher's exact test was used. The agreement between the classification of adherence by criteria of TMG, BMQ and CQR-19 questionnaires, with global and medication adherence in the perception of the medical team, was evaluated using the Kappa coefficient. The linear association between adherence scores, and PRAQ and PedsQLTM-SSS, was assessed using Spearman's correlation. To assess the simultaneous effects of demographic and clinical characteristics (predictor variables) of patients on adherence (global and medication), univariate and multivariate logistic regressions were adjusted. Due to the sample size limitation, which caused the absence of events at the levels of some predictor variables, the Firth logistic regression model was adjusted.21
For all statistical tests, a significance level of 5% was used. Statistical analyses were performed using SPSS 20.0 and STATA 12 statistical software
EthicsThe study was approved by the Ethics Committee of UNIFESP (approval letter number 2,969,684) in 2018, under the name “Role of the doctor-patient relationship in adherence to the treatment of children and adolescents with AIRD”. Informed consent and assent forms were applied to all participants and caregivers.
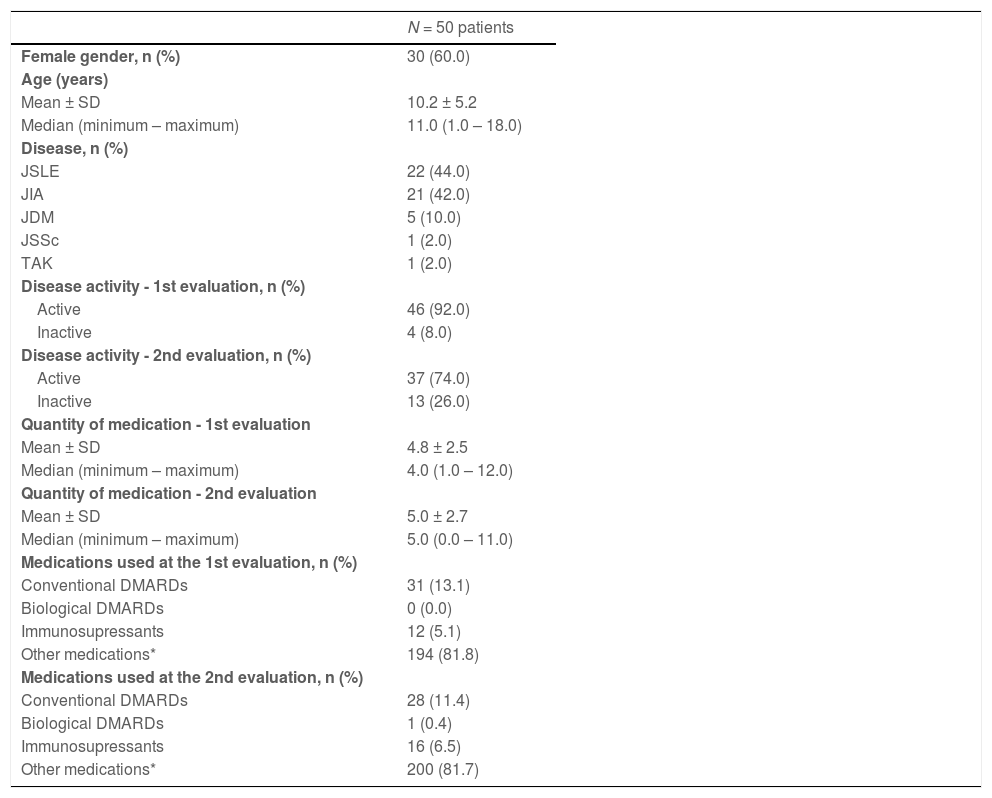
ResultsDemographics and main clinical aspectsFifty patients were included from a convenience sample of children [from 1 to 11 years old, n = 26] and adolescents [12 to 18 years old, n = 24], with a recent diagnosis of JIA, JSLE, JDM, JSSc or TAK. The sample was composed of 30 (60%) females, with a mean age at disease onset of 9.6 ± 5.1 years, a mean number of medications used of 4.8 ± 2.5 at the first evaluation, 5.0 ± 2.7 at the second evaluation, and a mean time of disease follow up of 5 months. Conventional DMARDs were used in 13,1% of patients at the first evaluation and in 11,4% of patients at the second evaluation. Only one patient was using biological DMARD at the second evaluation. Table 1 presents demographic and clinical characteristics and number of medications used by patients. Regarding disease activity, the authors observed that 92% of patients were active at the beginning of the study whereas 74% were still active in the evaluation after 6 months. There were no reports of COVID-19 infection in the patients included in the study. The complete list of medications at the first and second evaluations is shown in the Supplementary Table.
Demographic, clinical characteristics and number of medications used by patients.
n, number; JSLE, Juvenile Systemic Lupus Erythematosus; JIA, Juvenile Idiopathic Arthritis; JSSc, Juvenile Systemic Sclerosis; JDM, Juvenile Dermatomyositis; TAK, Takayasu's Arteritis; NSAIDs, nonsteroidal anti-inflammatory drugs; DMARDs, disease-modifying antirheumatic drugs.
Other drugs = antimalarials, diuretics, antihypertensives, gastric protector, folic acid, anti-emetics, antidepressants, ferrous sulfate, calcium, vitamin D.
The mean Global Adherence Score, according to medical records, was 94.3 ± 10.0 (ranging from 62.5 – 100.0) with 45 patients (90%) being considered adherent, and 5 (10%) being considered non-adherent to treatment. The mean score for Medication Adherence, according to medical records, was 97.3 ± 9.3 (ranging from 50.0 – 100.0) with 47 patients (94%) adequately taking all the prescribed medications.
Prediction of adherence according to demographics, disease type, number of prescribed medications, questionnairesThe authors used the Firth logistic regression model to assess the prediction of Global and Medication adherence, but none of the variables were able to predict either good or poor adherence to treatment.
The mean score of the PRAQ questionnaire in the first evaluation (score ranging from 0 to 25) was 5.2 ± 1.5 (Table 2). The authors didn't observe a correlation between the PRAQ score and the rates of Global and Medication Adherences to treatment (p = 0.111 and p = 0.932, respectively).
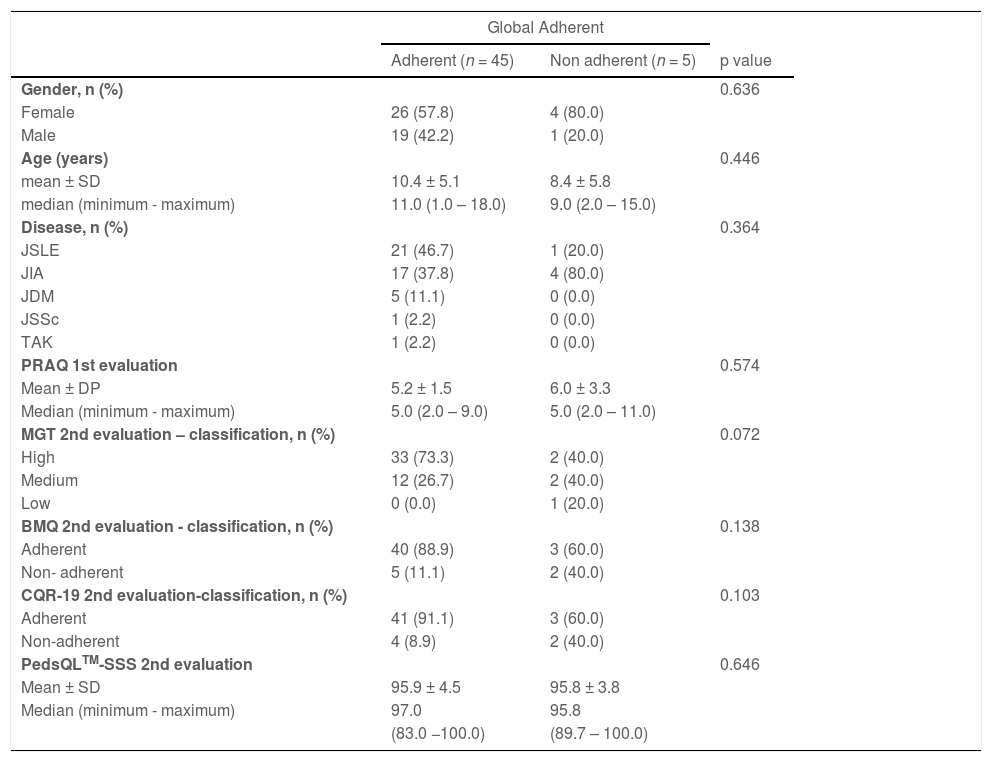
Associations between Global Adherence to treatment, and demographic data, disease type, PRAQ score, scores of TMG, BMQ, CQR-19 and PedsQLTM-SSS questionnaires.
p – descriptive level of Fisher's exact test or Mann-Whitney test. n total = 50.
JSLE, Juvenile Systemic Lupus Erythematosus; JIA, Juvenile Idiopathic Arthritis; JSSc, Juvenile Systemic Sclerosis; JDM, Juvenile Dermatomyositis; TAK, Takayasu's Arteritis; MGT, Morisky Green test; BMQ, Brief Medication Questionnaire; CQR-19, Compliance Questionnaire; PRAQ, Pediatric Rheumatology Adherence Questionnaire; PedsQLTM-SSS, Pediatric Quality of Life Inventory version 3.0.
Table 2 shows the associations between Global Adherence to treatment, and demographic data, disease type, PRAQ score, scores of TMG, BMQ, CQR-19, and PedsQLTM-SSS questionnaires. Although the authors observed high values in all questionnaires among the group of adherent patients (Global Adherence), there was no association between scores and rates of global adherence to treatment.
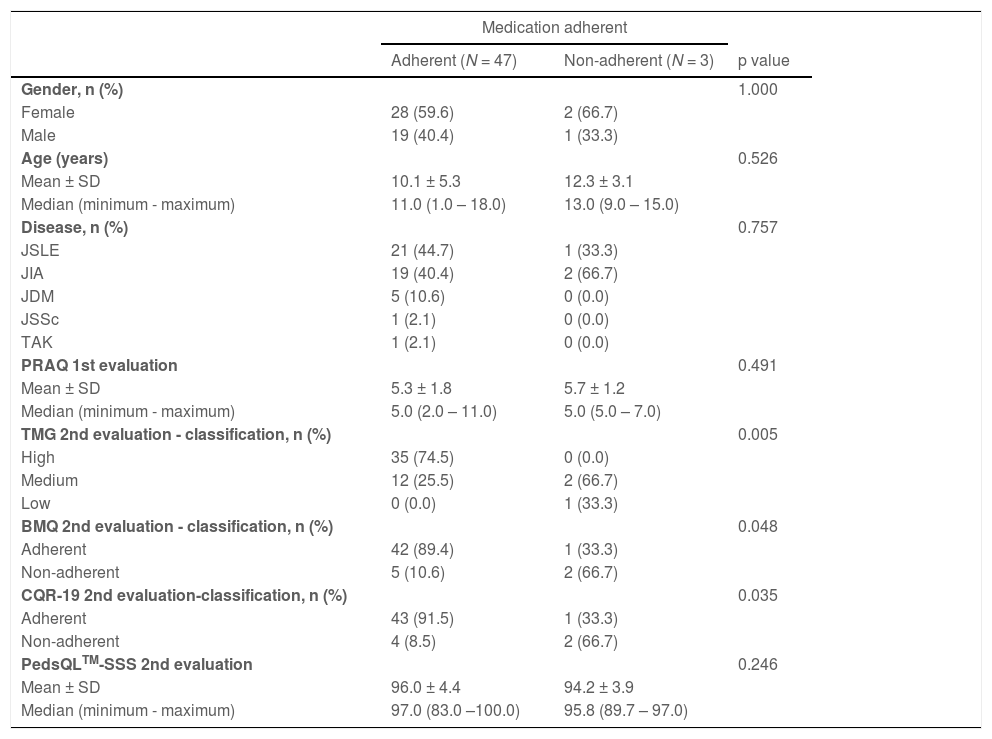
Table 3 shows the relationship between Medication Adherence, and demographic data, disease type, PRAQ score and the scores of TMG, BMQ, CQR-19 and PedsQLTM-SSS questionnaires. An association between the scores of the TMG, BMQ and CQR-19 questionnaires and Medication Adherence (p = 0.005, p = 0.048 and 0.035, respectively) was identified.
Associations between Medication Adherence, and demographic data, disease type, PRAQ score, scores of TMG, BMQ, CQR-19 and PedsQLTM-SSS questionnaires.
p – descriptive level of Fisher's exact test or Mann-Whitney test. n total = 50.
JSLE, Juvenile Systemic Lupus Erythematosus; JIA, Juvenile Idiopathic Arthritis; JDM, Juvenile Dermatomyositis; JSSc, Juvenile Systemic Sclerosis; TAK, Takayasu's Arteritis; MGT, Morisky Green test; BMQ, Brief Medication Questionnaire; CQR-19, Compliance Questionnaire; PRAQ, Pediatric Rheumatology Adherence Questionnaire; PedsQL-SSS, Pediatric Quality of Life Inventory version 3.0.
Adherence to treatment in children and adolescents is less studied than in the adult population.22 In general, the measurement of adherence rates is complex and there is no gold standard to directly measure medication adherence. Indirect methods, with emphasis on specific questionnaires, developed for adults, are inexpensive.9 Among them, MGT, BMQ, and CQR-19 questionnaires stand out. In the present study, which was the first to use such tools to assess pediatric adherence during the pandemic period, the authors observed an association between the Medication Adherence rate and these questionnaire scores at the second evaluation.
Patients with AIRD have been using several medications, the conventional DMARDs being the common ones. These drugs (as well as biological DMARDs and immunosuppressants) are distributed by the public health system, which facilitates access to treatment and consequently improves adherence.
Even during the COVID-19 pandemic, the authors observed high rates of treatment adherence in this sample.
In the present study, the authors evaluated adherence only in pediatric patients with a recent diagnosis and at the beginning of their treatment, in a single public service, which is a reference in Pediatric Rheumatology in the analyzed country, as opposed to what is found in the literature. Maybe this good adherence is due to the fact that patients were at the beginning of their treatment and therefore more concerned about the newly diagnosed disease. Some factors, such as the use of telemedicine for both medical and physical therapy consultations, certainly contributed to the higher rates of adherence. A study evaluated the satisfaction with telemedicine among parents or guardians of children or adolescents from the pediatric population, and demonstrated high satisfaction among them, throughout the pandemic. Telemedicine proved not to be a barrier to good contact between health professionals and the patients.23
A recently published study carried out in India, on treatment adherence among children up to 16 years of age, with rheumatic diseases, during the COVID-19 pandemic, indicated that a third of patients had stopped taking or had interrupted their treatment. Part of this result may be associated with the fear of taking prescribed immunosuppressive drugs and consequently acquiring the severe form of SARS-CoV-2.24 Fernández-Avila et al., through a questionnaire directed to 1097 adult rheumatologists in Latin America, observed a reduction in adherence to drug treatment in the reports of 50% of these specialists.25 Rebić et al. performed a systematic review that included 31 articles related to the adherence of adult patients with rheumatic diseases and observed a non-adherence to drug treatment of 14.8%.26 This finding was comparable to ours, where the authors observed 10% of non-adherence. However, all the articles included in this systematic review only included adults, and not at the beginning of treatment.
Contrary to the findings of the present study, Naddei et al. found that there was a worsening in the relapse rate in Italian children with JIA during the lockdown due to COVID-19, when comparing this population to a cohort one year prior to the lockdown, demonstrating the need for changes to home and health management during isolation.27 This finding may be related to the fact that medical follow-up was stricter during the lockdown in Italy. When compared to the analyzed country, despite the lockdown, neither the follow-up of the Brazilian patients nor the supply of medications in the outpatient clinic and high-cost medication dispensing centers, were interrupted during the pandemic.
Silva et al. developed the PRAQ questionnaire to assess adherence risk in children and adolescents beginning treatment for chronic rheumatic diseases.12 In a study carried out in this same service, it was observed that factors such as patient age, clinical status, psychological factors, sociocultural factors, economic factors, and family factors were associated with poor adherence.28 Since poor adherence to treatment is one of the major causes of unfavorable prognosis,1,24,29,30 the detection of families with problems in adherence to treatment should be considered a priority in care models. In the present series, the PRAQ questionnaire was not able to predict poor adherence to treatment, probably due to the small sample.
Another observation that deserves attention is the high levels of satisfaction reported by the parents of patients under medical care, measured by the PedsQL-SSS questionnaire, a tool translated and validated for this environment by the team.20 Although it was not possible to establish an association between high levels of satisfaction with the health service and adherence to treatment, the authors believe that this is a factor that impacts treatment adherence and that the high degree of commitment of the medical and multi-professional teams resulted in higher rates of adherence to global and medication treatment (90% and 94%, respectively). These results surprised us because data published before the pandemic had already shown that about 50% of children and between 65–90% of adolescents were not adhering to treatment.9 All evaluated patients were newly diagnosed, and concerns about COVID-19 infection in immunosuppressed patients were present, leading to better adherence.
These positive results in this service may be associated with certain factors, for example, having the same medical professional always attending to the same patient, and forming a bond with patients and family members. In addition, the rheumatology service seeks to offer comprehensive care to patients with physical therapy, nutrition, social assistance, psychological, and dental services, with the support of a non-governmental organization, which provides prescribed medications that are not provided by the government.
There were some limitations to this study, that should be highlighted. The first was the sample size, as the authors selected the first 50 patients scheduled in the outpatient clinic, all of them with a recent diagnosis and beginning treatment at the time of inclusion. A larger sample with a more diverse population and for a longer period of follow-up in the future would be of great value, to verify if the patients might maintain the high rates of treatment adherence found at the beginning of treatment and to demonstrate the predictive power of PRAQ. A second limitation was the use of instruments (MGT, BMQ and CQR-19) not validated for the pediatric age group. Additionally, the timing of the present study during the pandemic, and other studies carried out at different points in time, may result in other findings.
In conclusion, the high values of MGT, BMQ, and CQR-19 questionnaire scores were in agreement with medication adherence rates. Despite the pandemic, the global and medication adherences were good. It was not possible to demonstrate the PRAQ's predictive power. The authors could not establish an association between families’ satisfaction and treatment adherence rates.
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Institution or service with which the work is associated for indexing in Index Medicus/MEDLINE: Universidade Federal de São Paulo (UNIFESP).