To carry out a narrative review on the use of marketing strategies in child nutrition, as well as potential implications for health professionals and children.
Data sourceSearches were carried out on the PubMed, SciELO, and Google platforms, using the terms “child nutrition” or “industrialized baby food” or “infant formula” or “breast milk” or “breastfeeding” and “marketing”, with original articles, review articles, institutional reports, institutional position documents and websites considered relevant to the topic being analyzed.
Data synthesisChildren's food marketing started with the industrialization of food and the resulting actions aimed at increasing sales and meeting commercial interests. Since its inception to the present, infant formulas have been the most widely used products, which has impacted breastfeeding practices. International and national institutions, that care for children's health, are searching for strategies to limit the abusive marketing of industrialized children's foods. Marketing strategies interfere with medical knowledge and actions, potentially influencing the guidance provided by pediatricians to families, and finally, compromising healthy eating practices at a critical period in life, with possible long-term effects.
ConclusionsHealth professionals, especially pediatricians, must provide the best care for children and families, and need to maintain the search for quality scientific information, not influenced by conflicts of interest. Updated and critical knowledge on the part of healthcare professionals can curb marketing strategies that aim to influence their actions.
To establish the best feeding practices in the first years of life, national1 and international2 recommendations are that breastfeeding should be offered exclusively until the sixth month of life, and then fresh, locally available foods be introduced, while processed foods should not be offered.1,2 Evidence indicates that there is a change in traditional eating habits, through the total or partial replacement of fresh foods with processed foods, which are promoted as a practical option, making family life easier.2 There is evidence that the consumption of ultra-processed foods leads to the development of an unhealthy nutritional profile and is associated with the emergence of chronic non-communicable diseases.2,3 To advertise these and other food products, marketing techniques are used, aimed at family members and health professionals, informing about health benefits, which are sometimes inaccurate, such as, for instance, that industrialized baby food promotes better health or better intellectual performance of the child.2
In this narrative review, different aspects related to the marketing associated with children's nutrition will be addressed.
Child nutrition: marketing, conflicts of interest and impact on medical educationThe impact of marketing strategies on food consumption in childhood can be exemplified by the global trend of increasing consumption of industrialized baby formulas, to the detriment of adequate breastfeeding practices.4 The World Health Organization (WHO) expressed concern regarding the increase in the consumption of processed baby foods, pointing out the harmful potential of this consumption for the health of human beings and warning of the great influence of marketing on the occurrence of this phenomenon.4
The marketing of processed foods focuses on influencing two main groups: the group of consumers, family members or those responsible for feeding the children; and the group of health professionals, prescribers of baby food, and respected opinion leaders, whose endorsement is essential for the sale of products.
Medical marketing is associated with the so-called “conflicts of interest”, a historically effective way of getting doctors to endorse (consciously or unconsciously) industry products (pharmaceuticals or food). Conflicts of interest arise when an interest, recently acquired by receiving a benefit (material or not), has the potential to unduly influence the professional's judgment or action.5 Research confirms that receiving benefits, whether small or large, provided by medical marketing, generates conflicts of interest, and has the potential to compromise the best professional performance. A survey carried out by the WHO, published in 2022, with the title “How the marketing of baby formulas influence our decisions about infant feeding”, analyzed the marketing tactics used by the infant formula industries in eight countries. Among other findings, the research found evidence confirming the ability of marketing to influence pediatricians' knowledge and attitudes regarding the use of infant formulas.6
Many of the strategies used by the marketing of industrialized baby food, with the potential to influence medical knowledge and practice, are similar to the strategies used by the marketing of pharmaceutical industries and such strategies include providing promotional activities associated with continuing medical education events.7-9 Continuing medical education is essential for medical activity, and the marketing of industrialized baby foods quickly became aware of this fact, starting to make educational materials and events available, aimed at disseminating promotional information about their products. Although some doctors argue that participating in educational activities sponsored by industries does not interfere with their professional practice, good-quality scientific evidence refutes this possibility. A systematic review that evaluated the possible impact of the interaction of physicians with the activities offered by industries, found that the participation of professionals in sponsored lectures/symposiums influenced the participants’ behavior, leading them to prescribe more products from the sponsoring industries, even without there being sufficient evidence to support their superiority. The majority of participating physicians were unable to identify the presence of inaccurate information about products propagated by the industries.9
Regulation of the marketing of industrialized baby food in the world and in BrazilThe impact of industrialized baby food marketing has demonstrated its ability to modify natural eating practices since the beginning of its existence. One of the first infant foods to be produced industrially, infant formula, appeared in the 19th century, due to the need for a product that was safe to feed babies whose mothers were unable to breastfeed. After advances in industrial technologies that allowed cow's milk to evaporate and condense (making it safer for transportation and with longer shelf-life), the first milk formulas were developed. Soon, several companies launched products on the markets and started marketing campaigns. The campaigns promoted the products as safe substitutes for breastfeeding and highlighted the benefits for children and mothers.10 Under the influence of marketing messages, in the mid-20th century, the use of formula became the norm, and breastfeeding rates declined to previously unseen low rates. As a consequence of the interruption of breastfeeding and the inappropriate use of formulas, there was an alarming increase in infant mortality, especially in low-income countries.11
Denouncing this scenario, the book “The Baby Killer” was released in 1974, written by journalist Mike Muller, in partnership with the philanthropic entity War and Want.12 The book reports on abusive marketing practices used in low-income countries and their consequences. “The baby killer” is considered a historic milestone in the fight to protect natural nutrition (breastfeeding) against marketing interests that compromise children's health. In 1995, the first Brazilian edition of the book was published, translated by Professor Fernando Figueira, president of the Institute of Children's Medicine of Pernambuco (IMIP, Instituto de Medicina Infantil de Pernambuco-Brazil) entitled “O matador de bebês”, and in August 2023, the third edition was launched, demonstrating the importance of this problem that is still experienced today.12
Due to pressure from society and considering the evidence of the damage caused by the marketing of industrialized formulas, the World Health Organization (WHO) launched, in 1981, the International Code of Marketing for Breastmilk Substitutes, the first document to regulate the advertising and promotion of sales of food products for children.11 National legislation, based on the Code, was created in several countries worldwide. In Brazil, the Brazilian Code of Marketing of Infant and Toddlers Food and Childcare-related Products, the NBCAL, was created, sanctioned as a Law in 2006, and regulated by the executive government as a Decree in 2018. The NBCAL corresponds to a set of regulations on commercial promotion and labeling of foods and products intended for newborns and children up to three years of age.13 After the regulations, marketing practices that directly targeted mothers were stopped or reduced in many countries, and an improvement in breastfeeding rates was observed.11 Although scientific evidence points to the harm caused by consuming industrialized formulas and the lack of breastfeeding, more babies are fed with formulas today than at any other time.14
In February 2023, the prestigious scientific magazine ‘The Lancet’ launched “The 2023 Lancet Series on Breastfeeding”, a series of three documents on how the importance of breastfeeding is undervalued by governments and public health, and how the vulnerability of women and children is exploited by the marketing strategies employed by the milk formula industries.14 In one of the articles in the series, entitled, “Marketing of commercial milk formula: a system to capture parents, communities, science, and policy”, Prof. Nigel Rollins et al. highlight that a strategy used by formula marketing is to simplify the challenges experienced by parents, transforming them into a series of problems and needs that can be solved by purchasing specific products. It also highlights the existence of strategies aimed specifically at health professionals and scientific institutions, through actions such as financial support, development of scientific research linked to the industry and medicalization of feeding practices aimed at infants and young children.15 Regarding the series published in The Lancet magazine, members of the IMIP board published a Letter to Readers, recording that Professor Fernando Figueira was a pioneer, and they close by highlighting that “Figueira's effort to protect breastfeeding, revealing the harmful commercial practices of companies producing breast milk substitutes, must be remembered in the face of an unequal fight against the marketing of milk formula industries”.16
In Brazil, it is the responsibility of the National Health Surveillance Agency (ANVISA, Agência Nacional de Vigilância Sanitária) and state and municipal surveillance organs to monitor compliance with the NBCAL; however, for this surveillance to be carried out successfully, it is necessary that civil society and professionals directly involved with health nutrition and child health, act as partners. In view of the need for groups that could support compliance with international standards for the protection of breastfeeding against abusive marketing, in 1979, IBFAN (International Baby Food Action Network) started worldwide, and in 1983, IBFAN Brazil, founded by sanitary doctor Marina Rea.13 Among the activities developed by IBFAN Brazil, the most important is NBCAL Monitoring, carried out annually, to verify whether self-regulation is being complied with by industries, commerce and health professionals.17
What marketing does not tell you: potential negative impacts of using processed foods early in lifeVery different from the claims frequently used by marketing as a promotional strategy for products (Table 118-26), processed baby foods are usually not healthy food options.4,15 Taking into account the new classification developed by researcher Carlos Monteiro to categorize foods according to the type of processing involved in their production, many industrialized baby foods are considered ultra-processed27 and, therefore, associated with potential negative impacts on human health in the short and long term.3,28 The effects of ultra-processed food consumption can be observed in all age groups but tend to be more of a matter of concern when consumption occurs at more vulnerable stages, such as at the beginning of life.29 Industrialized baby foods are not subject to the same standards that regulate the commercial promotion of medicines; manufacturing companies are not required to declare the potential adverse effects attributed to the use of the products, and they do not provide adequate information to health professionals.17 The fact that there is no transparency and simplified dissemination of this information, lead doctors to have little knowledge about the potential risks involved in the consumption of these products, which can compromise the quality of the medical procedure and the safety of children.30 Promotional information, attributing greater or better functionality to the consumption of processed baby foods, is one of the strategies most frequently used by marketing (Table 1). A recent study that analyzed the reliability of health claims propagated by infant formula industries in 15 countries, proved the inconsistency of several claims, having found that the same beneficial effect was attributed to different types of ingredients, and conversely, different benefits were attributed to the same ingredient. Most claims were not supported by scientific references and when there was such evidence, it was not good quality scientific evidence.30
Health claims propagated by the marketing of infant formulas and contestations from studies and scientific institutions.
EFSA, European Food Safety Authority; DGKJ, German Society for Child and Adolescent Medicine; 2′-FL, 2′-fucosilactose; LNnT, lacto-N-neotetraose; HM, human milk.
In addition to unproven benefits, there are potential adverse effects associated with industrialized baby food. Although infant formula is the recommended option for feeding children whose mothers cannot or do not wish to breastfeed and is considered nutritionally adequate to guarantee the growth of babies, its industrial production requires that multiple components (nutritional and non-nutritional), coming from different production chains, be submitted to industrial processing (mechanical, chemical and physical). Some of the potential adverse effects attributed to the consumption of ultra-processed baby foods are related to the industrial processing involved in the manufacturing.31 The consequences for human health of the consumption of food products manufactured through multiple and intense industrial processes is a problem that has been better investigated and understood in recent years,28 therefore, it was not adequately addressed when the guidelines and standards for production, marketing and monitoring of baby foods were developed. Currently, all over the world, ways are still being studied to ensure greater safety in the production of industrialized baby food.
A few decades ago, it was understood that, for an industrialized food to be considered safe for children, it only needed to meet the child's nutritional needs. Thus, in the Codex Alimentarius published in 1981 and still in force today, the quantitative and qualitative parameters of nutritional and non-nutritional components were defined (a task that currently seems to be much more challenging than imagined in the past), for the composition of infant formulas (first products intended for artificial feeding in the beginning of life), adopting as a reference the hitherto known composition of human milk. The Codex also determined that for a new product to prove “nutritional safety and adequacy”, it would need to demonstrate, through scientific evidence, that it was capable of guaranteeing adequate “growth and development in childhood”.32 For that, the manufacturing company would need to prove, through the evaluation of some anthropometric parameters, that a group of children exclusively submitted to consumption of the formula, had an anthropometric evolution similar to that of a control group, over a short period of time (about 15 weeks), and that its consumption was not associated with a greater frequency of clinically identifiable adverse effects.32 The criteria defined for the safety assessment of new infant formulas were simple to achieve, which allowed several companies to launch dozens of infant formulas around the world. The safety criteria became more limited as knowledge advanced about the particularities of early life, and about the existence of specific components in human milk, which were not known in the past.
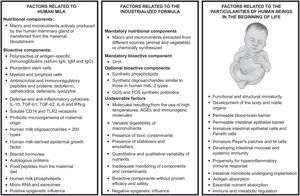
To understand the risk associated with a suboptimal diet at the beginning of life, it is necessary to consider the specific demands of the human body during this period (Figure 1). In the first months of life, the human infant (more fragile and immature than other mammals) will depend on the food offered to them, which must be capable of providing all the components (nutritional and functional) essential for the occurrence of the various transformations that will lead them to reach the capabilities of a healthy adult organism.33 As organs and systems are undergoing accelerated development, and as the need for food per kilogram of body weight is greater than at any other time, this is the period during which humans are most vulnerable to problems associated with nutrition. It is noteworthy that during this period, food must support better development of noble structures and functions, such as brain development, adequate growth of body structures and compartments, the development and regulation of the immune system, and the healthy development of the endocrinological and metabolic systems.29
Considering the gastrointestinal tract at the beginning of life, the environment where food will be introduced, it is necessary to consider that several structures and functions will also be undergoing a state of postnatal transformation, when nutrition ceases to be passive and safe (as occurred in the intrauterine environment), becoming active and challenging. Food particles (and non-food particles) can enter the human body through the intestinal mucosa and have a systemic effect. At birth, the intestinal mucosa will be formed by an incipient microbiota (where newly arrived microorganisms can establish themselves, creating communities that proliferate, or be excluded by competition), a thin (or non-existent) layer of mucus, and a layer of epithelial cells, with greater permeability than in adult life.34 Below the epithelial layer, in the lamina propria, a large number of immune cells will be in the development and differentiation phase.34 Evidence suggests that the immature mucosa at the beginning of life is appropriate for the necessary stimulation of the immune system that adapts to the external environment, allowing greater transfer of environmental antigens, which whether coupled (in the case of human milk) or not (in the absence of human milk) to maternal antibodies, will influence the activation and maturation of the lamina propria immune system.29
Analyzing human milk, a natural food perfected throughout human evolution, increasing scientific evidence reveals its complexity and the specificity of the components intended to help the immature human organism, increasing knowledge of the gap between it and industrially manufactured foods.26,33 In relation to nutritional components, synthesis by the human mammary gland cells of nutrients that are specific to humans, with structural and functional characteristics that cannot be found in milk from other mammalian species, stands out.26 In relation to functional components (Figure 1), discoveries are growing regarding the components directly linked to the evolutionary superiority of human beings, capable of modulating the expression of genes, and the development of noble structures and functions such as the central nervous system, immunity and microbiota.26,33
Aiming to get closer to human milk functions, in recent years, infant formula industries have started to add so-called bioactive compounds.35 In human milk, the compounds are produced by the mammary gland itself, having been shaped throughout human evolution; for industrial use, a few similar compounds are extracted from animal and/or vegetable ingredients, or synthesized by chemical processes. Although some compounds (such as prebiotics and lipids) have been approved for optional inclusion in infant formulas and are generally considered safe, they are not mandatory components, as there is yet no scientific proof that they result in health benefits. Despite the lack of scientific evidence, the marketing of infant formulas often uses these optional components to make claims of different benefits, clearly violating the Code, making comparisons between the synthetic components in formulas and the benefits of natural components in human milk.30
The incorporation of components aimed at manipulating the intestinal microbiota at the beginning of life (for instance, through the use of synthetic prebiotics) has been one of the strategies most often used by companies to propagate advances in the composition of formulas, using the discourse that this addition would make the products more similar to breast milk.30,36 The contrast between the limited potentially microbiota-modulating components used by industries, and the diversity of those found in breast milk, is great (Figure 1). Maternal breastfeeding transfers several live microorganisms (many unknown) and hundreds of prebiotics (more than 200 types of oligosaccharides), which reflect the interference of evolutionary forces and the exposures experienced by the maternal organism throughout her life.37 This wealth of factors, to date, cannot be reproduced artificially.38,35
Analyzing the potential efficacy and safety of synthetic prebiotics used in infant formulas, a systematic review published in 2018 concluded that, although the research did not raise concerns about safety, there was yet no robust evidence to recommend the preferential use of formulas supplemented with prebiotics.38 Regarding the specific use of synthetic oligosaccharides similar to those in human milk, experts have expressed some concern about how the baby's microbial community will be affected by the consumption of just a few oligosaccharides, rather than the complex mixture found in breast milk.33 In a statement, the Nutrition Committee of the German Society for Child and Adolescent Medicine highlights that existing data on the supplementation of infant formulas with synthetic oligosaccharides are too limited to make recommendations for their use.37 The Committee concludes that the use of terms such as “human milk oligosaccharides” and abbreviations such as “HMO” in the promotion of infant and transition formulas is unacceptable, as they do not represent what is present in the product, suggesting a non-existent similarity with human milk, which could weaken confidence in the superiority of breastfeeding, and calls on infant formula manufacturers to end this practice.37 It calls on regulatory agencies to prevent potential violations of existing legal restrictions on the marketing of infant formula, and on pediatricians to inform families that the synthetic oligosaccharides contained in the products are not comparable to those found in human milk.37
Demonstrating how much the existing knowledge about the use of bioactive compounds in industrialized baby foods is still incipient, in April 2023, the American agencies NIH (National Institutes of Health) and FDA (Food and Drug Administration) published a summary of the workshop where a panel of experts carried out an initial analysis of the safety of using bioactive components in infant formulas.35 Bioactive ingredients were defined as “ingredients of non-human origin that can imitate components typically present in human milk, not traditionally considered essential nutrients, but which are believed to have physiological activity, associated with clinical relevance”. The document highlights the need to develop research with appropriate methodology, which can provide reliable results on efficacy and safety, short and long-term effects, and the occurrence of serious or irreversible adverse effects.35 The panel recommends that, when selecting variables to measure outcomes, such as neurodevelopment, bioindicators should be evaluated, which detect the response of the specific component in the biological system, such as the brain. To improve the accuracy of information, such as the time of use to obtain the effects, it recommended that the results should be repeatedly evaluated, throughout and at the end of the intervention in childhood, as well as after childhood.35
It is known that one of the problems (not resolved with currently existing technological resources) regarding the quality of nutritional components is the fact that they are heterologous to humans, being obtained from different sources (animals, vegetables, chemical processes), unlike what occurs in breast milk, which has species-specific components, actively produced by the human mammary gland cells. Regarding quantities, the Codex establishes minimum and maximum limits for macro and micronutrients, and it is the responsibility of the manufacturing companies to ensure that products are maintained within these parameters during all stages of product manufacturing and distribution (from the finished product in the industry, through shelf life, until the product is reconstituted for consumption). Although the quality and quantity of nutritional components in products are often claimed (which are usually just complying with existing recommendations), studies indicate the existence of discrepancies between the values reported by the manufacturer and those found in the performed analysis.39
Another problem, which is very little reported, is the existence of potentially harmful substances (in smaller or larger quantities), such as contaminants and chemical residues, introduced (intentionally or unintentionally) throughout the production chain.40 Even in relation to the infant formula production process, which has regulations to minimize the risk of contamination, studies indicate that there are flaws so potentially harmful levels have been identified in studies carried out in different countries.41,42 A fact that can generate undesirable results in the composition of infant formulas is the need to undergo several stages of industrial processing, which aim to guarantee aspects related to the safe consumption of the product over time (such as reducing microbiological and chemical contamination), but also related to commercial interests (improving appearance, facilitating transportation and increasing shelf life).43 It is known that such processing, especially the use of high temperatures, can modify the structure of nutrients (interfering with their bioavailability) and result in the formation (through the Maillard reaction) of potentially toxic contaminants, such as advanced glycation end products (AGEs), associated with long-term consequences, such as diabetes, kidney failure, neurodegenerative and eye diseases.31 Contrary to expectations, AGEs are present in infant formulas at even higher levels than in other dairy products.41
International agencies that regulate food production are aware of the need to restrict and monitor the levels of chemical contaminants in foods, especially those consumed at the beginning of life, recognizing that there are still flaws in this surveillance and that the capacity to monitor the safety of foods produced in each country may vary depending on the available resources.40 In 2021, due to alarming news published in the media about the presence of toxic levels of heavy metals in many baby foods, the American Congress launched “The Baby Food Safety Act”, proposing to turn into a Law that control the presence of these metals in food for babies and young children. In 2022, the FDA launched the “Closer to Zero” program aiming, through research, regulation, and consultancy, to reduce the presence of contaminants to close to zero, especially in baby foods; however, to date, little or no involvement has been recorded on the part of the producing industries.44
Final considerationsThe human body in the maturation and development phase needs to receive adequate care, so that the growth and development process can occur satisfactorily, with nutrition playing a fundamental role in this process. To achieve this, an entire support structure needs to be present and offer the child optimal conditions to become a healthy adult. The family and the health sector (the pediatrician in particular) play an important role in this initial support network.
Pediatricians need to have good humanistic and scientific training so that they can make the best choices in relation to the children (and families) for whom they care. The initial training is important, but the speed at which new information appears in the field of medicine requires constant updating. Therefore, one needs to pay attention to the sources where they seek information and have a critical spirit to incorporate quality information as knowledge.
Medical marketing needs to be ethical, but it is often distorted and becomes advertising. In relation to baby food, it has been observed in recent years that the marketing of baby food industries has started to occupy a space similar to that already occupied by pharmaceutical industries, including the use of similar tactics. Sometimes a relationship is established between industry representatives and the physician that discloses a conflict of interest. To prevent this from happening, limits must be observed. How to recognize and incorporate these limits? The way forward is to have good medical and scientific training and the ability to criticize.
Exclusive breastfeeding in the first six months of life, and continued until two years or more is one of the strongest consensuses in pediatrics. A healthy complementary diet, with fresh foods, is the way to continue nurturing the good transformations that started with breastfeeding. There are exceptional situations, where an industrialized food (such as infant formula) may be necessary. In these situations, the pediatrician must be prepared to provide the best guidance, based on the best possible sources of information. The pediatrician is the subject of the action. If this rule is followed, the impact of marketing on infant nutrition will be minimized.