To study the presenting clinical and demographic features, risk factors, and outcome of infants with late vitamin K deficiency bleeding.
MethodsOver a 5-year study period, the presenting clinical features and outcome of all 47 infants observed aged less than 6 months, who were diagnosed with late-onset primary and secondary VKDB by detailed history, physical examination, and laboratory findings were evaluated. Confirmed primary late VKDB was diagnosed when no cause other than breastfeeding could be found, while in the secondary subtype additional risk factors compromising the vitamin K effect were diagnosed.
ResultsSecondary late VKDB (83%, 39 patients) was more common than the primary subtype. The mean age of patients was 10.50 ± 5.75 and 9.74 ± 6.04 weeks in primary and secondary VKDB subtypes, respectively, and the age of infants did not have a significant difference (p > 0.05). The male to female ratio was 2.13:1. The residency, place and mode of delivery, gestational age, and types of feeding of patients did not have a significant difference between VKDB subtypes. The skin and gastrointestinal tract (GIT) (40.4%) followed by intracranial hemorrhage (ICH) (32%), were common sites of bleeding. Neurological complications were seen in 21% of patients; however, lethality was 23%, and the outcome of patients did not have a significant difference (p > 0.05) between VKDB subtypes.
ConclusionSecondary late VKDB is more common than the primary subtypes, and late VKDB is still a serious disease in developing countries, including Iraq, when vitamin K prophylaxis isn’t routinely used at birth.
The term vitamin K deficiency bleeding (VKDB) substituted for the wording of hemorrhagic disease of the newborn, as bleeding in the neonatal period is often not due to vitamin K deficiency and VKDB may occur in the postnatal period.1
There are various forms of VKDB and it can be classified by etiology and age of onset. The etiology may be considered either primary or secondary. In primary VKDB no cause other than breastfeeding can be found, while in the secondary type additional risk factors compromising the vitamin K effect are diagnosed, like malabsorption secondary to hepatobiliary and intestinal diseases, poor intake of vitamin K or antagonist of vitamin K by drugs. According to the age at onset, VKDB is usually categorized into three main types: early, classical, and late, with different pathogenic mechanisms and implying for VK prophylaxis. Early VKDB’s onset is at <24 h of age; classical VKDB begins in the first week excluding the first 24 h; and late VKDB occurs between the second week and the sixth month of life.1–3
The incidence of late VKDB in the absence of vitamin K prophylaxis ranges from 10.5 to 80 per 100,000 births.3,4 When intramuscular vitamin K prophylaxis is given at birth, the rate of late VKDB ranges from 0.24 to 3.2 cases per 100,000 live births.5,6
In late VKDB, the bleeding manifestations are severe and mainly involve the gastrointestinal tract and skin, but also the central nervous system.7,8 Intracranial hemorrhage (ICH) occurs frequently in cases of late VKDB and can lead to significant morbidity and mortality. In a pooled analysis, ICH was observed in 30–63% of late VKDB, with 14–20% mortality and 40% long-term neurological morbidity among survivors.9–11
Late VKDB is one of the important health problems in infant morbidity, mortality, and socio-economic problem of the country, which can be prevented by vitamin K prophylaxis.3,4 More attention should be paid by pediatricians and health authorities to prevent this serious disease. There are few case reports available on this aspect from the region.12 Herein, in this first study that is being conducted in Iraq, we present the initial clinical and demographic features, laboratory findings, and outcome with their significant difference among all 47 patients with primary and secondary late VKDB, who were observed over a 5-year study period at the Pediatric Ward of the Al-Sader Teaching Hospital in the Misan Province, south of Iraq.
Material and methodsThis prospective study was conducted from October 1st, 2014 to September 31st, 2019. All 47 infants with less than 6 months of age with late onset VKDB, who were observed and admitted to the pediatric ward in the Al-Sader Teaching Hospital in the Misan Province, Iraq during the study period were included. An especially designed questionnaire was used to collect initial sociodemographic, clinical, and laboratory data of the studied infants at diagnosis. The patients were diagnosed, treated, and followed up closely for at least 8 months, with a median duration of follow-up of 12 months (range 8–24 months). Informed consent was obtained from parents or guardians of patients, and this study protocol was approved by the Ethical Committee at the College of Medicine, University of Misan, Iraq.
In the clinical criteria for diagnosis of late-onset VKDB based on primary VKDB, no cause other than breastfeeding can be found, while in the secondary type additional factors compromising the vitamin K effect are diagnosed, like malabsorption secondary to hepatobiliary and intestinal diseases or preceding antibiotics use, and definite cases had to meet the laboratory criteria of a clearly prolonged prothrombin time (PT) or International Normalized Ratio (INR) >3.5, activated prolonged partial thromboplastin time (APTT) with normal fibrinogen level and platelet count with evidence of rapid normalization of these values (PT and APTT), and cessation of bleeding following VK administration.1 Laboratory investigations included the complete blood count (CBC), prothrombin time (PT)/INR, activated partial thromboplastin time (APTT), and fibrinogen level. Blood sample for CBC was collected in EDTA tubes and detected using ABX Micros ES 60 hematology analyzer (HORIBA Medical, France). For coagulation assays, venous blood samples were collected in tubes containing 0.109 M (3.2%) of trisodium citrate in a ratio of 4.5 mL of blood to 0.5 mL of sodium citrate and then centrifuged without delay at 1200 G for 15 min,13 and the assay was performed using Mindray C2000-2 Double Channel Coagulation Analyzer (China), with appropriate quality control materials and standard reagents (BIO-TP Prothrombin Time [PT] – BIOLABO, and BIO-CK APTT Kaolin – BIOLABO, France), within 3 h following the blood collection. The Clauss method was used for fibrinogen assay. Normal values were 10–12 s for PT, 26–40 s for APTT, and 1.8–3.6 g/L for fibrinogen.13 Cranial computed tomography (CT) scan was done for 18 infants with clinical manifestation of ICH. Regarding treatments, vitamin K (5 mg intravenously daily for 5 days) was given to all 47 observed infants; fresh frozen plasma (FFP) was given in a dose of 15 mL/kg of body weight in life-threatening bleeding situations, and whole blood transfusion (20 mL/kg) was given in cases of severe anemia. Statistical analyses were reported as mean estimation ± standard deviation using the Statistical Package for the Social Sciences (SPSS) version 23 for Windows. Comparison of categorical data was carried by t-test and Fisher's test, and a p value of <0.05 was considered statistically significant.
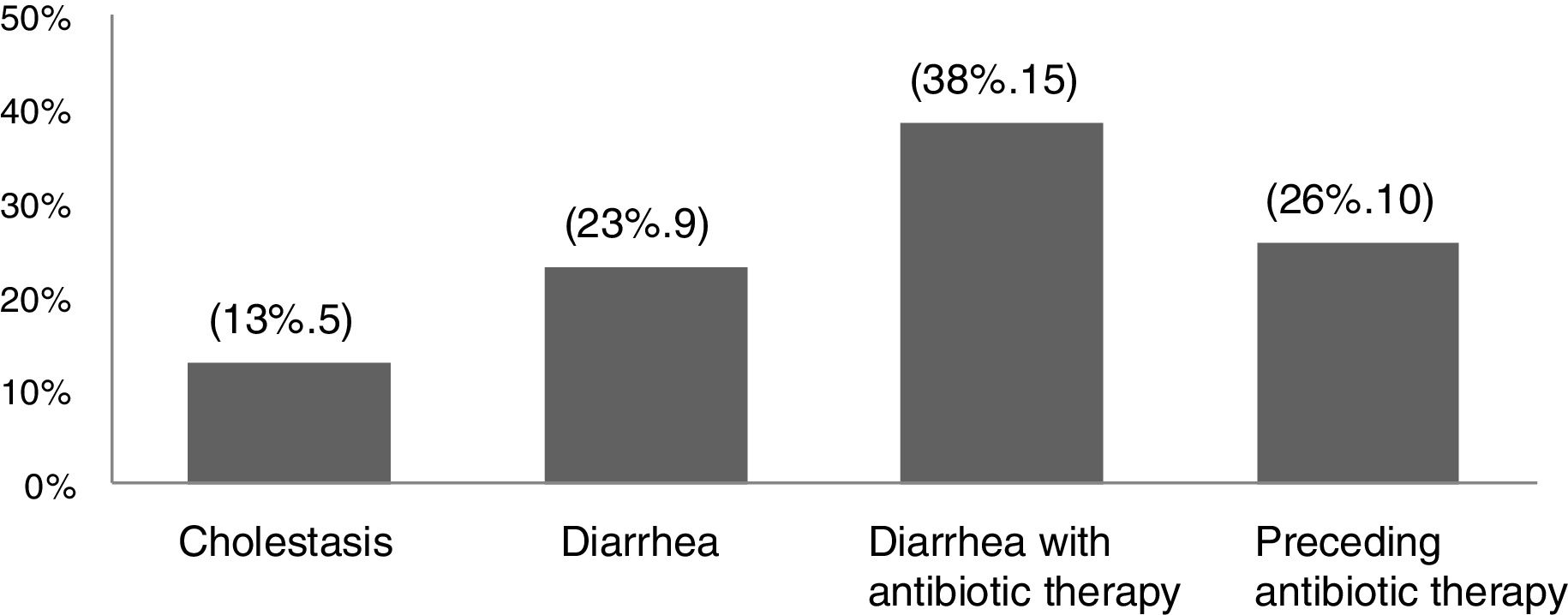
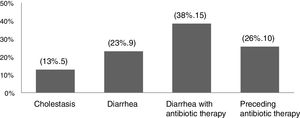
ResultsThere were 47 infants who met the criteria of late VKDB observed during the five-year study period. Secondary late VKDB was more commonly observed (83%, 39 patients) than the primary subtype (17%, 8 patients). The underlying causes and risk factors other than breastfeeding reported among secondary VKDB patients were cholestasis (13%, 5 patients), diarrhea (23%, 9 patients), diarrhea with antibiotic therapy (38%, 15 patients), or preceding antibiotic therapy (26%, 10 patients) (Fig. 1).
The age (mean ± SD) of patients at onset of symptoms was 10.50 ± 5.75 weeks (range, 2–21 weeks) in primary VKDB, while in the secondary subtype it was 9.74 ± 6.04 weeks (range, 4–20), and the age at onset of presentation did not have a significant difference between the subtypes of VKDB (p value 0.7).
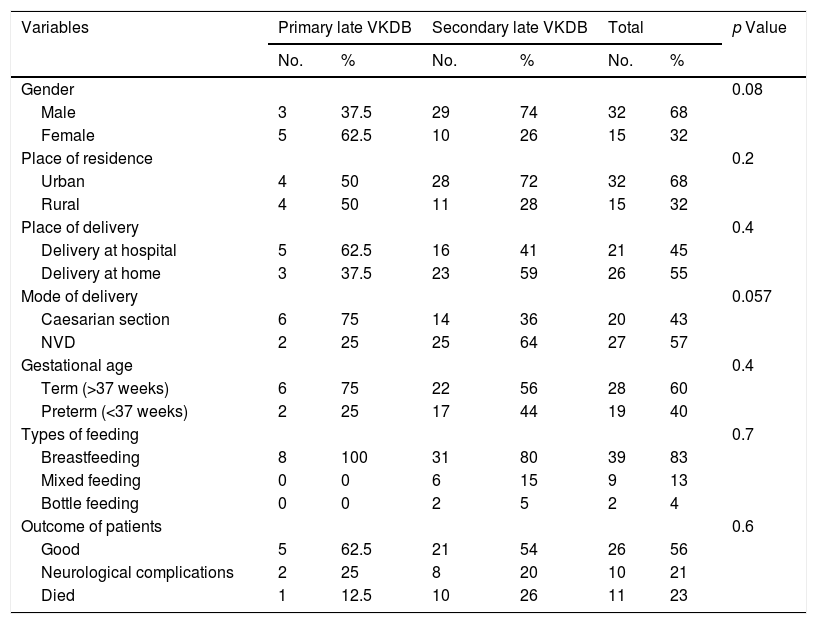
In the current study, late VKDB was more common among males (68%, 32 patients), with a male to female ratio of 2.13:1, and the gender of patients did not have a significant difference between the types of VKDB (p value = 0.08). As for the place of residence of the studied infants, more than two-thirds of the studied patients (68%, 32 patients) lived in urban areas, and the place of residence of patients did not significantly differ between the types of late VKDB (p value = 0.2) (Table 1).
Demographic and clinical features and outcome of patients with late VKDB.
| Variables | Primary late VKDB | Secondary late VKDB | Total | p Value | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Gender | 0.08 | ||||||
| Male | 3 | 37.5 | 29 | 74 | 32 | 68 | |
| Female | 5 | 62.5 | 10 | 26 | 15 | 32 | |
| Place of residence | 0.2 | ||||||
| Urban | 4 | 50 | 28 | 72 | 32 | 68 | |
| Rural | 4 | 50 | 11 | 28 | 15 | 32 | |
| Place of delivery | 0.4 | ||||||
| Delivery at hospital | 5 | 62.5 | 16 | 41 | 21 | 45 | |
| Delivery at home | 3 | 37.5 | 23 | 59 | 26 | 55 | |
| Mode of delivery | 0.057 | ||||||
| Caesarian section | 6 | 75 | 14 | 36 | 20 | 43 | |
| NVD | 2 | 25 | 25 | 64 | 27 | 57 | |
| Gestational age | 0.4 | ||||||
| Term (>37 weeks) | 6 | 75 | 22 | 56 | 28 | 60 | |
| Preterm (<37 weeks) | 2 | 25 | 17 | 44 | 19 | 40 | |
| Types of feeding | 0.7 | ||||||
| Breastfeeding | 8 | 100 | 31 | 80 | 39 | 83 | |
| Mixed feeding | 0 | 0 | 6 | 15 | 9 | 13 | |
| Bottle feeding | 0 | 0 | 2 | 5 | 2 | 4 | |
| Outcome of patients | 0.6 | ||||||
| Good | 5 | 62.5 | 21 | 54 | 26 | 56 | |
| Neurological complications | 2 | 25 | 8 | 20 | 10 | 21 | |
| Died | 1 | 12.5 | 10 | 26 | 11 | 23 | |
VKDB, vitamin K deficiency bleeding; NVD, normal vaginal delivery.
Regarding delivery histories, more than half of the infants (55%, 26 patients) were delivered at home; 27 infants (57%) were delivered by normal vaginal delivery, and 28 infants (60%) were delivered at term; place and mode of delivery and maturity of infants did not have a significant difference between the types of VKDB (p value >0.05). More than 3/4 of patients (83%, 39 patients) with late VKDB were exclusively breastfed, including all infants with the primary subtype, and 4% (2 patients) were bottle-fed, and there was no significant difference between VKDB subtypes regarding types of feeding (p value = 0.7) (Table 1). All infants with late VKDB were not administered vitamin K at birth.
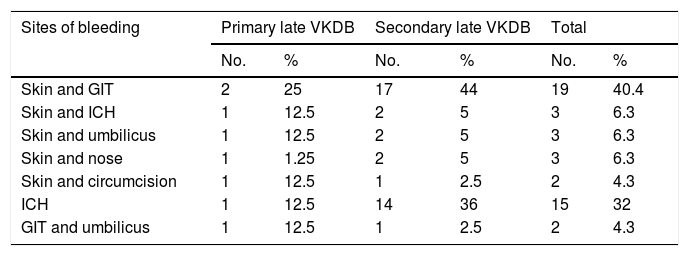
The most common sites of bleeding at presentation were the skin and the gastrointestinal tract (GIT) (40%, 19 patients), followed by ICH (32%, 15 patients) (Table 2).
Sites of bleeding in infants with late VKDB.
| Sites of bleeding | Primary late VKDB | Secondary late VKDB | Total | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Skin and GIT | 2 | 25 | 17 | 44 | 19 | 40.4 |
| Skin and ICH | 1 | 12.5 | 2 | 5 | 3 | 6.3 |
| Skin and umbilicus | 1 | 12.5 | 2 | 5 | 3 | 6.3 |
| Skin and nose | 1 | 1.25 | 2 | 5 | 3 | 6.3 |
| Skin and circumcision | 1 | 12.5 | 1 | 2.5 | 2 | 4.3 |
| ICH | 1 | 12.5 | 14 | 36 | 15 | 32 |
| GIT and umbilicus | 1 | 12.5 | 1 | 2.5 | 2 | 4.3 |
VKDB, vitamin K deficiency bleeding; GIT, gastrointestinal tract; ICH, intracranial hemorrhage.
There were 18/47 (38%) patients with ICH; among them, in 3 patients (16.7%) it was associated with skin bleeding (petechiae and bruising), and 15 patients (83.3%) had isolated ICH. A head CT scan was performed for all cases (18 infants) with ICH. The brain CT scan showed intraparenchymal hemorrhage in 7 cases (38.9%), and intraparenchymal with intraventricular hemorrhages in 6 cases (33.3%); 4 patients (22.2%) had subarachnoid with intraventricular hemorrhages, and one patient (5.6%) had parenchymal with subarachnoid hemorrhage.
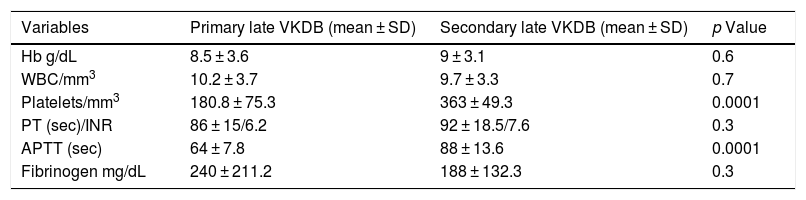
In the current study, the distribution of variables was normal, and we used mean ± standard deviation (SD) to define normality of the distribution. The mean Hb level, WBC count, PT, and fibrinogen level did not have a significant difference (p value >0.05), but platelet count and APTT had a significant difference (p value <0.05) between VKDB subclasses (Table 3).
Laboratory data of infants with late VKDB.
| Variables | Primary late VKDB (mean ± SD) | Secondary late VKDB (mean ± SD) | p Value |
|---|---|---|---|
| Hb g/dL | 8.5 ± 3.6 | 9 ± 3.1 | 0.6 |
| WBC/mm3 | 10.2 ± 3.7 | 9.7 ± 3.3 | 0.7 |
| Platelets/mm3 | 180.8 ± 75.3 | 363 ± 49.3 | 0.0001 |
| PT (sec)/INR | 86 ± 15/6.2 | 92 ± 18.5/7.6 | 0.3 |
| APTT (sec) | 64 ± 7.8 | 88 ± 13.6 | 0.0001 |
| Fibrinogen mg/dL | 240 ± 211.2 | 188 ± 132.3 | 0.3 |
VKDB, vitamin K deficiency bleeding; SD, standard deviation; Hb, hemoglobin; WBC, white blood cell; PT, prothrombin time; INR, international normalized ratio; APTT, activated partial thromboplastin time.
All infants in this study were treated at the hospital with vitamin K (5 mg) intravenously daily for 5 days; 22 patients (47%) who presented with life-threatening bleeding situations also received 15 mL/kg of fresh frozen plasma (FFP), and a whole blood transfusion of 20 mL/kg was given to 29 patients (61.7%) who had anemia.
Regarding the outcome of patients with VKDB, our study has shown that 26 patients (56%) had a good outcome; 11 patients (23%) died; and 10 infants (21%) had neurological complications in the form of cerebral palsy (50%, 5 patients), microcephaly with seizure disorders (30%, 3 patients), and hydrocephaly with sensory deafness (20%, 2 patients) throughout the follow-up period, that extended over 6 months. There were no significant differences (p value = 0.7) regarding the outcome of patients between VKDB subtypes (Table 1).
DiscussionBleeding tendency in infants, which is now classified as late VKDB, was first described by Bhanchet et al. in 1977, when they summarized in their study of 93 affected Thai infants.14 We have presented 47 infants with confirmed late VKDB with no history of vitamin K administration at birth. This small sample of patients was observed over a 5-year study period because of the rarity of late VKDB.3 It is important to take into consideration that VKDB occurs more commonly in the Asian population compared to the European population. This may be explained by the 6-fold higher incidence of biliary atresia in Asia compared to Western Europe.15
In our study, predisposing diseases for late VKDB were common before bleeding begins, which is similar to what Ijland et al.5 observed when they reported that infants with late VKDB (88.3%) often have signs and symptoms of predisposing disease; however, in the second and third nation-wide surveys in Japan of vitamin K deficiency in infancy, it was found that 10.5% and 16% respectively had bleeding episodes due to VKDB associated with a clear pathological cause,16,17 and Chuansumrit et al.18 reported that 3.7% of infants with bleeding were diagnosed with secondary VKDB. Liver diseases are known to be a major cause of late VKDB, because they cause maldigestion of fat; the decrease in fat absorption leads to a deficiency of fat soluble vitamins.19,20 Busfield et al.6 and Sutor et al.11 reported that 27% and 37%, respectively, of infants with late VKDB had liver disease, which is higher than our findings.
Late VKDB is characterized by bleeding in infants between the 8th day and 6 months of life, and it has a peak incidence between the 3rd and 8th weeks of life.1,3 Corresponding to our results, Schulte et al.21 found that the mean age of infants with late VKDB was 10.3 weeks (range, 7–20 weeks). However, Ijland et al.5 reported that the mean age was 3–7 weeks, and Sutor et al.11 reported that the peak age was 4 weeks, and that the majority (79%) of the infants were between 3 and 7 weeks old.
At variance with our finding, Shearer3 in their study mentioned that late VKDB was higher in rural areas, while most of our patients came from urban areas, but the place of residence did not significantly differ between VKDB subtypes. Similar to other studies,11,18 male infants have accounted for the majority of our patients, but the gender of patients did not have a significant difference between VKDB subclasses. Although this striking gender discrepancy is not yet clarified, results from a previously reported study suggested that male infants may require more dietary phytomenadione than females with the same body weight.22 Regarding mode of delivery, our results are unlike the findings of Schulte et al.21 and Zurynski et al.,23 who reported that 28.6% and 22% of infants respectively were delivered at home.
Although prothrombin levels are lower than in term babies at birth.20 Recent studies have certainly not provided any support to the belief that vitamin deficiency bleeding is more common in preterm babies.24 In agreement with our results, Bör et al.25 reported that all infants with late VKDB were born at term, as well as Zurynski et al.,23 who found that 93% of patients were full-term babies.
Exclusive breastfeeding has emerged as a matter of concern in developing countries, where exclusive breastfeeding is vigorously advocated to promote optimal health in the infant. But vitamin K is poorly transmitted across the placental barrier, and its stores are low at birth, with levels often below the detection limit of 0.02 ng/mL; therefore, breastfed infants are at risk because of low concentrations in human milk,1,3 while bottle-fed babies are at almost no risk because almost all infant milk is artificially fortified.19 Our results regarding types of feeding are in agreement with other studies.11,18,23,24,26
Similar to our findings, Zurynski et al.23 observed in their study that skin and GIT, followed by ICH, were the most common sites of bleeding. However, Mihatsch et al.20 mentioned that ICH, skin and GIT respectively were common sites in late VKDB. A major feature of late VKDB is a much higher incidence of ICH (30–88%) in patients, with serious ICH leading to high morbidity and subsequent mortality.3,11,12,18,23,25,27–29 Our results are similar to those reported in other studies that observed that the most common site was intracerebral, followed by multiple ICH.3,27,28 However, Ozdemir et al.12 demonstrated that subdural hemorrhage was the most common type of ICH reported, followed by intracerebral and subarachnoid hemorrhages, while Martín-López et al.30 mentioned that the majority of the patients (75%) showed ICH at more than one site. Late VKDB subtypes were associated with severe life-threatening bleeding, mainly ICH, which resulted in low hemoglobin level and anemia among our studied infants, which is similar to the findings reported by others.18,21,25,26
Throughout the follow-up period, we observed that VKDB subtypes were associated with morbidity and lethality among studied patients, and the outcome of patients did not have a statistically significant difference between the subtypes of VKDB. In their study, Sutor et al.11 found that mortality was 19% and neurological sequelae 21% in survivor infants with VKDB. Out of a total of 691 cases of late idiopathic VKDB reported in Thailand, mortality rate was 24%, and permanent neurological deficits were found in 142/257 (55%); they were: seizure disorders (64%); muscle weakness (21%); mental retardation (15%); hemiparesis (13%); hydrocephalus (7%); and microcephaly 5%; however, neurological sequelae in secondary late VKDB were found in 7/25 (28%) survivors, and the mortality rate was 26%,18 while in Aydinli et al.26 seizure disorders (73%), severe psychomotor retardation (46%), cerebral palsy (46%), microcephaly (46%), and hydrocephalus (27%) were observed after a follow-up period ranging from 6 to 48 months of infants with late VKDB, but no fatality was mentioned.
In conclusion, secondary late VKDB is more common than the primary subtype, and late VKDB is still an important cause of morbidity and mortality in developing countries including Iraq, where vitamin K prophylaxis is not routinely practiced. The present study highlights the importance of prompt diagnosis of this clinical condition by proper history taking, clinical examination, and relevant investigations. Vitamin K prophylaxis should be offered to all newborns who are exclusively breastfed.
Conflicts of interestThe author declares no conflicts of interest.
Much obliged to Dr. Hmood MH, for his assistance in performing the statistical analysis.