Serum levels of creatinine in neonates are quite variable and suffer interference from the immature kidney and maternal creatinine concentration. The aim of this study was to measure novel biomarkers of glomerular and tubular function in healthy preterm neonates at 72h and 3 weeks of life.
MethodsUrine samples were collected in 40 preterm neonates with 28–34 incomplete weeks of gestational age. None of the participants had comorbidities, malformations and infections. The samples were collected at 72h of life and at 3 weeks after birth. Measurements of Calbindin, Collagen IV, FABP1, αGST, IP-10, KIM-1, Osteoactivin, Renin, TFF-3, TIMP-1, α-1-Microglobulin, Albumin, Clusterin, Cystatin C, EGF, Lipocalin-2/NGAL and Osteopontin were performed using panels 1 and 2 of multiplex kits of kidney injury. Data were analyzed using the software GraphPad Prism version 6.0.
ResultsThe preterm neonates included 55% of males with gestational age of 30±1 weeks. The most frequent maternal condition associated with preterm birth was preeclampsia (80%). Molecules related to glomerular function showed a significant increase in the concentrations obtained at 3 weeks of life compared to 72h of life. Markers related to tubular injury (KIM-1 and NGAL) also showed an increase. On the other hand, cystatin C did not change.
ConclusionThe elevation of molecules related to glomerular function indicates an increase of glomerular filtration rate from 72h up until 3 weeks of life, which was not clearly detected with the measurement of cystatin C.
Despite great advances in neonatal medicine over the past years, the accurate assessment of renal function in premature newborns remains challenging.1,2 Nephrogenesis is usually completed between the 34th and 36th week of gestation.3 In this way, infants born preterm before 36 weeks of gestation are in active nephrogenesis,4 which makes them susceptible to aggressions caused by the extra-uterine environment.5,6 This may result in a reduction of the total number of functioning nephrons throughout a lifetime,7 which can lead to heart and kidney diseases in adult life.8
The most used endogenous marker of renal function in clinical practice is serum creatinine concentration, especially to estimate glomerular filtration rate (GFR)9 However, creatinine is unreliable in preterm infants due to its dependence on muscle mass, renal immaturity and interference of maternal creatinine.3,10 After the first 72h of life, there is a reduction in circulating maternal creatinine. The rate of this decline depends on multiple variables, including weight and gestational age.11 Therefore, the measurement of serum creatinine levels is not considered accurate for the neonatal period in general, and even less for preterm neonates.12
In this context, it is extremely important to study other biomarkers of renal function in preterm infants, as a better understanding of their applications and limitations might help detect an acute kidney injury (AKI), allowing an early treatment and a betteroutcome.13–15 Preterm neonates are particularly vulnerable to having an AKI, as they are often in intensive care units being exposed to nephrotoxic drugs and having a higher risk of being infected.16
Therefore, the aim of this study was to measure and analyze markers of glomerular and tubular function in healthy preterm neonates at 72h and 3 weeks of life.
Patients and methodsStudy design and ethicsThis was a prospective observational study of preterm neonates (PTN) with gestational age between 28 and 34 incomplete weeks, who were born from June to December 2014 in a philanthropic hospital in Minas Gerais, Brazil. This hospital is one of the three largest marternity hospitals of Brazil, which is fully funded by the public national health service. The hospital assists a mean of 900 births per month, in which 70% of those are vaginal births and with about 200 newborns per month needing neonatal care. The hospital has a broad area of population coverage, most of them in the metropolitan area of Belo Horizonte, the capital of Minas Gerais. The infrastructure of the hospital consists of more than 85 obstetrics bed and about 100 beds for high-risk newborn progressive care. Infants who were admitted in the Neonatal Intensive Care Unit (NICU) and whose parents signed the free and informed consent were enrolled. Exclusion criteria were (i) 5-min Apgar score below 7 (ii) diagnosis of congenital malformations, syndromes and/or associated diseases; (iii) presence of an acute disorder, including sepsis or necrotizing enterocolitis at any of the time-points and (iv) death within the first three weeks of life.
Gestational age and birth weight, gender, Apgar scores, infant’s diagnosis during their admission in the NICU, conditions associated with the premature birth and antenatal exposure to glucocorticoids were collected from hospital data.
The study was approved by the Ethics Committee of both the Federal University of Minas Gerais and the Sofia Feldman Hospital (protocol CAAE 30382114.9.0000.5149.), and did not interfere with medical recommendations or the proposed treatment of infants in the NICU.
SubjectsSample size calculations were based on a previous study investigating the association of inflammatory marks of brain injury, neurothrophic factors and motor development in PTN.17 Considering a sample error of 5% and 95% of reliability, we obtained a number of 40 PTN for this study.
Study protocolAll study participants had urine samples collected at two time-points: 72h of life (T1) and 3 weeks after birth (T2). These urine samples were obtained using a newborn urinary collector. Afterwards, these samples were transferred to 15mL plastic tubes and immediately centrifuged (3800rpm, 5min, room temperature). The supernatant was collected and transferred to 1.5mL microtubes. Then, they were stored at a −80°C freezer until analysis.
Quantification of kidney injury biomarkers by immunoassayThe measurement of urinary levels and of biomarkers of kidney injury were performed simultaneously using the Milliplex/Luminex xMAP platform, according to information from the manufacturer (Millipore Corporation, MA, USA). The Human Kidney Injury Magnetic Bead Panel 1 kits were used, which simultaneously quantifies the levels of calbindin, osteoactivin, α-glutathione S-transferase (αGST), tissue inhibitor of metalloproteinase 1 (TIMP-1), kidney injury molecule 1 (KIM-1), protein induced by interferon (IP-10/CXCL10), renin, fatty acid binding protein (FABP-1), collagen IV and trefoil factor 3 (TFF-3); the Human Kidney Injury Magnetic Bead Panel 2 simultaneously analyzes the levels of epidermal growth factor (EGF), lipocalin, albumin, clusterin, cystatin C, osteopontin (OPN) and α-microglobulin.
Briefly, it captures microspheres coated with specific monoclonal antibodies for each analyte are added to the wells, along with urine samples and standards. After incubation and washing, a mixture of secondary biotinylated antibodies is added. Then, streptavidin conjugated to the fluorescent protein is incubated for a brief period. After washing, the supernatant was discarded and the precipitate containing the microspheres was resuspended in a buffer solution. The standards and samples were acquired in the MAGPIX microsphere analyzer (Luminex Corporation, Texas, USA) and the results were analyzed using the Milliplex Analyst program (MilliporeSigma) and represented in pg/mL.
The kidney injury biomarkers assay was performed in single samples. These assays were performed simultaneously and with the same batch of reagents to avoid interassay variability.
Statistical analysisStatistical analysis was performed using Prism® version 6.0. Qualitative variables were expressed in absolute frequencies and percentages. Gaussian distribution of quantitative variables was verified using the Shapiro Wilk test. Non-parametric variables were shown as a median and interquartile range (1st quartile – percentile 25 and 3rd quartile – percentile 75). Continuous variables were described using measures of central tendency and dispersion, and qualitative variables were expressed as absolute frequencies and percentages. In the time-point analysis the Friedman test was chosen, and for variables with values p<0.05 the Wilcoxon test with Bonferroni correction was utilized. The Spearman correlation coefficient was used for correlation analysis.
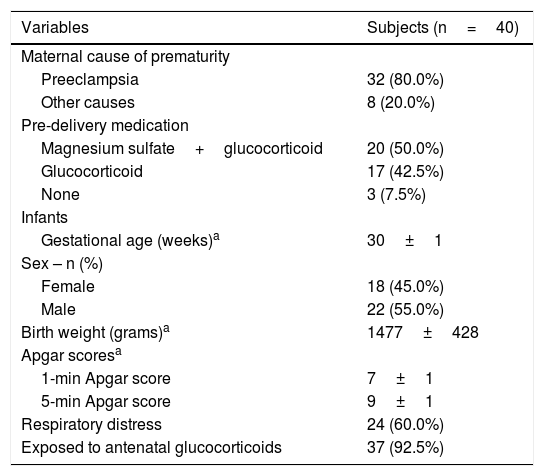
ResultsPopulationA total of 42 preterm neonates were initially selected, however, two families refused to participate in study protocol. Therefore, this study evaluated 40 preterm neonates, in which urine samples were properly collected and processed at 72h and 3 weeks of life. The study sample included 55% of males and 45% of females with gestational age of 30±2 weeks and birth weight of 1477±428g. All deliveries were by caesarean section, with 50% of mothers receiving magnesium sulfate and glucocorticoid as predelivery medication 24h before giving birth, and 42.5% were given only glucocorticoid, with a total of 92.5% of neonates exposed to antenatal glucocorticoids. Ten subjects had very low birth weight and 4 had extremely low birth weight. None of the PTN had any abnormalities detected before birth. Respiratory distress syndrome had an incidence of 60% of the PTN, requiring respiratory support as supplemental oxygen and/or continuous positive airway pressure (CPAP). Regarding maternal conditions, the most frequent one associated with premature birth was preeclampsia (80%), followed by other causes including premature placental abruption and rupture of the amniotic sac (20%).
Table 1 shows the general characteristics of the preterm neonates included in the study.
Demographic and clinical characteristics of the mothers and preterm neonates.
| Variables | Subjects (n=40) |
|---|---|
| Maternal cause of prematurity | |
| Preeclampsia | 32 (80.0%) |
| Other causes | 8 (20.0%) |
| Pre-delivery medication | |
| Magnesium sulfate+glucocorticoid | 20 (50.0%) |
| Glucocorticoid | 17 (42.5%) |
| None | 3 (7.5%) |
| Infants | |
| Gestational age (weeks)a | 30±1 |
| Sex – n (%) | |
| Female | 18 (45.0%) |
| Male | 22 (55.0%) |
| Birth weight (grams)a | 1477±428 |
| Apgar scoresa | |
| 1-min Apgar score | 7±1 |
| 5-min Apgar score | 9±1 |
| Respiratory distress | 24 (60.0%) |
| Exposed to antenatal glucocorticoids | 37 (92.5%) |
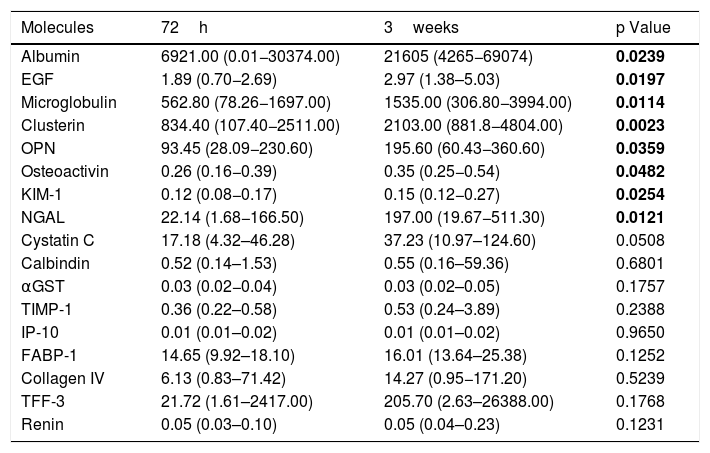
Measures of tested biomarkers are displayed on Table 2. Molecules related to glomerular function (Albumin, EGF, Clusterin, Microglobulin, OPN and Osteoactivin) showed a significant increase in the concentrations obtained at 3 weeks of life compared to 72h of life. Markers related to tubular injury (KIM-1 and NGAL) also showed an increase. Despite increased at 3 weeks of life if compared to 72h, Cystatin C levels did not reach statistical significant difference. In addition, Calbindin, GSTα, TIMP-1, IP-10, FABP-1, Collagen IV, TRF-3 and Renin did not vary in any of the urine samples collected at 72h and 3 weeks of life.
Comparison between biomarkers of kidney injury collected at 72h of life and at 3weeks of life in healthy preterm neonates.
| Molecules | 72h | 3weeks | p Value |
|---|---|---|---|
| Albumin | 6921.00 (0.01−30374.00) | 21605 (4265−69074) | 0.0239 |
| EGF | 1.89 (0.70−2.69) | 2.97 (1.38–5.03) | 0.0197 |
| Microglobulin | 562.80 (78.26−1697.00) | 1535.00 (306.80−3994.00) | 0.0114 |
| Clusterin | 834.40 (107.40−2511.00) | 2103.00 (881.8−4804.00) | 0.0023 |
| OPN | 93.45 (28.09−230.60) | 195.60 (60.43−360.60) | 0.0359 |
| Osteoactivin | 0.26 (0.16−0.39) | 0.35 (0.25−0.54) | 0.0482 |
| KIM-1 | 0.12 (0.08−0.17) | 0.15 (0.12−0.27) | 0.0254 |
| NGAL | 22.14 (1.68−166.50) | 197.00 (19.67−511.30) | 0.0121 |
| Cystatin C | 17.18 (4.32–46.28) | 37.23 (10.97–124.60) | 0.0508 |
| Calbindin | 0.52 (0.14–1.53) | 0.55 (0.16–59.36) | 0.6801 |
| αGST | 0.03 (0.02−0.04) | 0.03 (0.02–0.05) | 0.1757 |
| TIMP-1 | 0.36 (0.22–0.58) | 0.53 (0.24–3.89) | 0.2388 |
| IP-10 | 0.01 (0.01–0.02) | 0.01 (0.01–0.02) | 0.9650 |
| FABP-1 | 14.65 (9.92–18.10) | 16.01 (13.64–25.38) | 0.1252 |
| Collagen IV | 6.13 (0.83–71.42) | 14.27 (0.95−171.20) | 0.5239 |
| TFF-3 | 21.72 (1.61–2417.00) | 205.70 (2.63–26388.00) | 0.1768 |
| Renin | 0.05 (0.03–0.10) | 0.05 (0.04–0.23) | 0.1231 |
EGF, endothelial growth factor; OPN, osteoprotegenin; KIM-1, kidney injury molecule 1; NGAL, Neutrophil gelatinase-associated lipocalin; αGST, alpha-Glutathione S-transferase; TIMP-1, Tissue Inhibitor of Metalloproteinases 1; IP-10, Interferon gamma-induced protein 10; FABP-1, Fatty Acid- Binding Protein 1; TFF-3, Trefoil factor 3. The values were quantified in pg/mL and expressed as medians and (Percentile 25 – Percentile 75). P values were obtained by Wilcoxon test with Bonferroni correction. Bold values are for p<0.05.
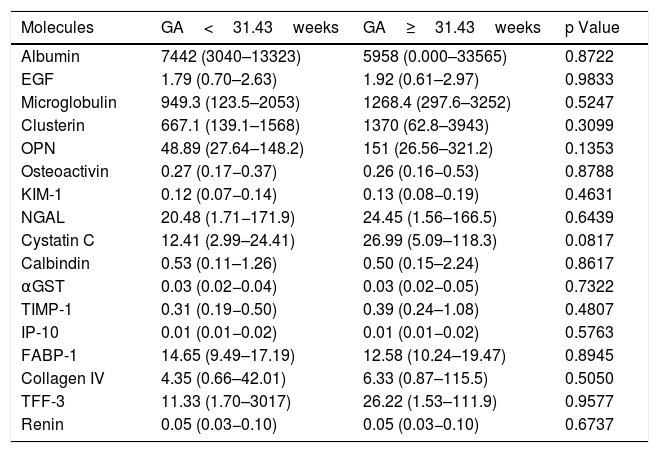
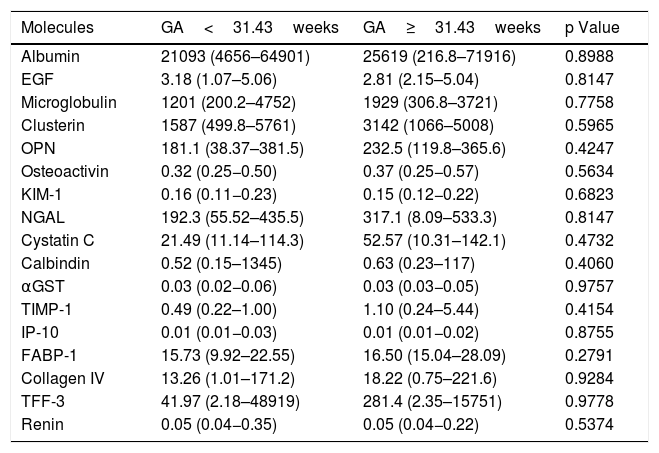
To evaluate if gestational age affected the urinary concentrations of the biomarkers, we compared levels in urine each molecule in two subgroups of preterm neonates according to the median of gestational age (GA): GA<31.43 weeks (n=21) versus GA≥31.43 weeks (n=19). These comparisons were made in both time-points: at 72h of life (Table 3) and at 3 weeks of life (Table 4). No differences were obtained for all comparisons at both time-points.
Comparison between biomarkers of kidney injury collected in healthy preterm neonates with gestational age (GA) lower than 31.43weeks and higher or equal to 31.43weeks at 72h of life.
| Molecules | GA<31.43weeks | GA≥31.43weeks | p Value |
|---|---|---|---|
| Albumin | 7442 (3040–13323) | 5958 (0.000–33565) | 0.8722 |
| EGF | 1.79 (0.70–2.63) | 1.92 (0.61–2.97) | 0.9833 |
| Microglobulin | 949.3 (123.5–2053) | 1268.4 (297.6–3252) | 0.5247 |
| Clusterin | 667.1 (139.1–1568) | 1370 (62.8–3943) | 0.3099 |
| OPN | 48.89 (27.64–148.2) | 151 (26.56–321.2) | 0.1353 |
| Osteoactivin | 0.27 (0.17−0.37) | 0.26 (0.16−0.53) | 0.8788 |
| KIM-1 | 0.12 (0.07−0.14) | 0.13 (0.08−0.19) | 0.4631 |
| NGAL | 20.48 (1.71−171.9) | 24.45 (1.56–166.5) | 0.6439 |
| Cystatin C | 12.41 (2.99–24.41) | 26.99 (5.09–118.3) | 0.0817 |
| Calbindin | 0.53 (0.11–1.26) | 0.50 (0.15–2.24) | 0.8617 |
| αGST | 0.03 (0.02−0.04) | 0.03 (0.02−0.05) | 0.7322 |
| TIMP-1 | 0.31 (0.19−0.50) | 0.39 (0.24–1.08) | 0.4807 |
| IP-10 | 0.01 (0.01−0.02) | 0.01 (0.01−0.02) | 0.5763 |
| FABP-1 | 14.65 (9.49–17.19) | 12.58 (10.24–19.47) | 0.8945 |
| Collagen IV | 4.35 (0.66–42.01) | 6.33 (0.87–115.5) | 0.5050 |
| TFF-3 | 11.33 (1.70–3017) | 26.22 (1.53–111.9) | 0.9577 |
| Renin | 0.05 (0.03−0.10) | 0.05 (0.03−0.10) | 0.6737 |
EGF, endothelial growth factor; OPN, osteoprotegenin; KIM-1, kidney injury molecule 1; NGAL, Neutrophil gelatinase-associated lipocalin; αGST, alpha-Glutathione S-transferase; TIMP-1, Tissue Inhibitor of Metalloproteinases 1; IP-10, Interferon gamma-induced protein 10; FABP-1, Fatty Acid-Binding Protein 1; TFF-3, Trefoil factor 3. The values were quantified in pg/mL and (Percentile 25 – Percentile 75). P values were obtained by Mann Whitney test.
Comparison between biomarkers of kidney injury collected in healthy preterm neonates with gestational age (GA) lower than 31.43weeks and higher or equal to 31.43weeks at 3weeks of life.
| Molecules | GA<31.43weeks | GA≥31.43weeks | p Value |
|---|---|---|---|
| Albumin | 21093 (4656–64901) | 25619 (216.8–71916) | 0.8988 |
| EGF | 3.18 (1.07–5.06) | 2.81 (2.15–5.04) | 0.8147 |
| Microglobulin | 1201 (200.2–4752) | 1929 (306.8–3721) | 0.7758 |
| Clusterin | 1587 (499.8–5761) | 3142 (1066–5008) | 0.5965 |
| OPN | 181.1 (38.37–381.5) | 232.5 (119.8–365.6) | 0.4247 |
| Osteoactivin | 0.32 (0.25−0.50) | 0.37 (0.25−0.57) | 0.5634 |
| KIM-1 | 0.16 (0.11−0.23) | 0.15 (0.12−0.22) | 0.6823 |
| NGAL | 192.3 (55.52–435.5) | 317.1 (8.09–533.3) | 0.8147 |
| Cystatin C | 21.49 (11.14–114.3) | 52.57 (10.31–142.1) | 0.4732 |
| Calbindin | 0.52 (0.15–1345) | 0.63 (0.23–117) | 0.4060 |
| αGST | 0.03 (0.02−0.06) | 0.03 (0.03−0.05) | 0.9757 |
| TIMP-1 | 0.49 (0.22–1.00) | 1.10 (0.24–5.44) | 0.4154 |
| IP-10 | 0.01 (0.01−0.03) | 0.01 (0.01−0.02) | 0.8755 |
| FABP-1 | 15.73 (9.92–22.55) | 16.50 (15.04–28.09) | 0.2791 |
| Collagen IV | 13.26 (1.01–171.2) | 18.22 (0.75–221.6) | 0.9284 |
| TFF-3 | 41.97 (2.18–48919) | 281.4 (2.35–15751) | 0.9778 |
| Renin | 0.05 (0.04−0.35) | 0.05 (0.04−0.22) | 0.5374 |
EGF, endothelial growth factor; OPN, osteoprotegenin; KIM-1, kidney injury molecule 1; NGAL, Neutrophil gelatinase-associated lipocalin; αGST, alpha-Glutathione S-transferase; TIMP-1, Tissue Inhibitor of Metalloproteinases 1; IP-10, Interferon gamma-induced protein 10; FABP-1, Fatty Acid- Binding Protein 1; TFF-3, Trefoil factor 3. The values were quantified in pg/mL and (Percentile 25 – Percentile 75). P values were obtained by Mann Whitney test.
The general idea of the present study was to investigate dynamic changes on molecules related to glomerular and tubular dysfunction in healthy preterm neonates. To date, this is the first study in Brazil to analyze this group of biomarkers in this specific population of preterm neonates. We found that almost half of the molecules tested showed significant differences between the very beginning of the neonates' life (at 72h) and at their three weeks of life. Among them all, molecules related to glomerular function were the ones that stood out. In this regard, Cystatin C concentrations did not significantly change at both evaluated time-points. Studies have indicated that Cystatin C may cross the placenta at less intensity than creatinine and, because of that, this molecule might be a better marker of renal function for the initial postnatal period.12,18 However, despite the increase in urinary concentrations of Cystatin C at 3 weeks of life, the difference with values at 72h of life did not reach statistical significance. There are some possibilities to explain the absence of difference. First, the relatively limited sample size and secondly, the use of urinary measurement of Cystatin C rather than serum levels of this molecule. Urinary levels might reflect some tubular dysfunction due to immaturity present in this age group, considering that Cystatin C is almost freely filtered by the glomerulus and almost completely reabsorbed and catabolized by the proximal tubule. Therefore, further evaluation and comparison with serum levels of Cystatin C would be complementary to investigate the role of this molecule as a biomarker of glomerular function in preterm neonates.
Considering all the limitations in using serum creatinine concentration to evaluate renal function, which are even more relevant in preterm neonates,1–3,10–12 the search for novel biomarkers of renal function is of utmost importance. Furthermore, biomarkers that may allow early detection of changes in renal function, with accuracy and in a non-invasive way are the ideal goal of recent studies in this field.11–15
The incidence of acute kidney injury (AKI) in very preterm neonates is estimate at 20–40%, with the vast majority of cases of pre-renal etiology associated with hypovolemia or reduction of renal perfusion, whether or not they are related to the presence of an infection.19 This incidence can reach 56% in asphyxiated newborns. Prematurity, extreme low birth weight, congenital abnormalities of kidney and urinary tract, congenital heart diseases, sepsis and use of nephrotoxic drugs are factors that can lead to AKI.19,20
In neonatal intensive care unities (NICU), where most of the very preterm newborns are found, renal impairment can be an important trigger of clinical decompensation, and it is associated with significant morbidity and mortality of these patients. Even with appropriated treatment, AKI is associated with 25–50% of mortality in neonates and is an independent risk factor for this outcome.19,20 In addition, AKI can also cause long-term impairment of kidney function, leading to increased risk of chronic kidney disease in adulthood.19,20 Considering the great impact of AKI in preterm newborns, it assumes utmost importance to find assertive, less invasive and early biomarkers of kidney dysfunction in this age range.11–15
Regarding AKI, Askenazi et al. found significant changes of NGAL, OPN, clusterin, Cystatin C and alpha-GST in a cohort of 113 neonates with birth weight less than 1200g.21 These molecules had 1.7–3.7 bigger values in neonates with AKI in contrast with those without this complication. Similar results were also found in other studies.19,20,22–24 There was also an inverse relation with EGF found, with the levels of this molecule lower in preterm neonates with AKI.21,22 In our study, all of these molecules, except Cystatin C and α-GST, exhibited significant differences according to the time of urine collection. The α-GST is a proximal tubular cell molecule that is released in urine when there is cell damage. This molecule may increase in situations of tubular injury.25,26 In this case, as we did not identify any significant difference, and we were not able to hypothesize the role of this biomarker for this population. Glomerular markers as OPN, clusterin and EGF and the tubular marker NGAL significantly increased from 72h to 3 weeks of life. Regarding microglobulin, albumin and osteoactivin, another recognized markers of glomerular function, we also found significant increase between the two evaluated time-points, but their role during injury has not yet been established.21,22,24 Dynamic changes of these molecules may not only reflect kidney injury, but also the physiological maturation of renal function in healthy preterm neonates.
As occurred with NGAL, a marker of tubular function, our results show that KIM-1 also had a significant increase from 72h to 3 weeks of life and might reflect the increase in tubular function during the first month after birth. Although when tested as a marker of kidney injury, studies so far did not find sufficient evidence for the predictive role of these molecules in evaluating renal function physiological maturation.19–21,24
In contrast with other studies,18–20,22,23 this study had two samples of the same patient, a strict selection criterion to find health preterm infants and used panels with multiple biomarkers. As also observed in other studies, the relatively limited number of patients was a limitation of our study, suggesting that the need for further research in this area. The simultaneous analysis of serum creatinine and serum Cystatin C with these urinary biomarkers could also bring some light to better understand kidney function in preterm neonates, as well the analysis of these biomarkers in neonates with acute kidney injury. Another limitation was the fact that we did not measure urinary creatinine in order to express the biomarkers in relation to creatinine. Unfortunately, the volume of collected urine was not sufficient to measure creatinine in all samples and at both time-points for the 40 preterm neonates. On the other hand, it should be mentioned that urinary creatinine concentrations are quite variable in preterm neonates secondary to tubular secretion.3,10,12 Therefore, the relative quantification of each biomarker in relation to urinary creatinine might have also some limitations.
The evaluation of molecules related to glomerular function indicates an intense increase of glomerular filtration rate from 72h until 3 weeks of life, which was not possible to detect with the measurement of Cystatin C. On the other hand, molecules related to glomerular and tubular function significantly increased according to age, suggesting a possible physiological maturation of the kidneys of the healthy preterm neonates. These results corroborate the potential of urinary biomarkers of renal function, and bring the possibility that they might be used in the future as markers of kidney injury as well.
FundingThis work was partially supported by Brazilian National Council of Research Development (CNPq - Grant # 302153/2019-5), Coordination of High Education Level Personnel (CAPES) and Foundation of Research of Minas Gerais (FAPEMIG).
Conflicts of interestThe authors declare no conflicts of interest.