To evaluate cardiac function and structural changes in children of diabetic mothers in the fetal and neonatal period using Doppler-echocardiographic data.
MethodA prospective, descriptive observational study conducted in a private and tertiary care service for high-risk pregnant women. It included 48 children of mothers with gestational diabetes mellitus (GDM) considered clinically compensated during pregnancy, with a single fetus and absence of malformations. Myocardial thickness, shortening fraction, left ventricular (LVMPI) and right ventricular (RVMPI) myocardial performance index, and mitral and tricuspid valve E/A ratio were evaluated in 96 echocardiographic exams with Doppler.
ResultsThe hypertrophic cardiomyopathy was 29% vs 6% p = 0.006 in the prenatal and postnatal periods respectively. The shortening fraction was 0% vs 6% p = 0.242 in the fetuses and newborns respectively. The myocardial performance index of the right ventricle was 12% vs 54% p ≤ 0.001, and on the left ventricle 27% vs 60% p = 0.001 in the prenatal and postnatal periods respectively. The ratio of mitral valve E/A waves was 6% vs 50% p ≤ 0.001 and the ratio of tricuspid valve E/A waves was 0% vs 27% p ≤ 0.001 in the fetuses and newborns respectively.
ConclusionA decrease in the rate of myocardial hypertrophy and changes in cardiac function parameters were observed in the fetal and neonatal periods.
Gestational diabetes mellitus (GDM) is defined as glucose intolerance, which appears or is discovered during the first trimester of pregnancy.1 It is a chronic and evolutionary disease characterized by changes in the metabolism of carbohydrates, proteins and lipids. The diagnosis of hyperglycemia is established through laboratory tests that include fasting blood glucose or oral glucose tolerance test.2
GDM affects the fetal heart throughout the gestational period. In the initial period, it hinders the proper expression of genes for the correct development of the heart during embryogenesis, which brings structural problems as a consequence. When at more advanced gestational ages, fetal hyperinsulinemia resulting from inadequate maternal glycemic control increases the expression of insulin receptors in cardiac cells. Insulin, an anabolic hormone, causes hyperplasia and hypertrophy of myocardial cells, resulting in myocardial hypertrophy.3,4
GDM is a public health problem, and even with the prenatal control of diabetic pregnant women changes in fetal growth and other abnormalities persist. Children of mothers with GDM using insulin during the third trimester of pregnancy are 20.6 times more likely to exhibit cardiovascular changes than newborns of non-diabetic mothers.2
Since it is able to provide data that can be used in the prevention and treatment of cardiac disorders, the fetal Doppler echocardiogram has been used as a non-invasive test in the diagnosis of morphological and functional changes in the fetal and pediatric heart.
Every newborn of a diabetic mother should, if possible, undergo an echocardiogram in the first 12−48 h of life to assess cardiac function and the presence of structural malformations. Frequent functional heart problems such as myocardial hypertrophy can lead to congestive heart failure, low output and cardiomegaly.2
The increase in perinatal morbidity and mortality can be attributed to the excessive transfer of maternal glucose to the fetus. The consequences on the newborn are wide-ranging, such as prematurity, asphyxia, neonatal hypoglycemia, respiratory distress syndrome, polycythemia, and hypertrophic cardiomyopathy.5 Therefore, glycemic control can be of special importance for the reduction of perinatal complications. The aim of this study is to evaluate, through Doppler-echocardiographic data, the cardiac function and structural changes of the children of diabetic mothers in the prenatal and postnatal periods.
MethodsThis retrospective cohort was carried out in a high-risk pregnant woman care service at a private hospital. The study population was determined by a convenience sample. The research included mothers with gestational diabetes mellitus (GDM) considered clinically compensated with insulin during pregnancy who were evaluated by echocardiography. The examinations were performed in the neonatal period, specifically between 22 and 37 weeks of gestational age, and in the postnatal period, which comprised the first two months of life.
The diagnostic criterion for GDM was established by the American Diabetes Association, with fasting plasma glucose levels ≥92 mg/dL, ≥180 mg/dL in one hour, and ≥153 mg/dL in two hours, and the exam was performed between the 24th and the 28th week of gestation.6 Pregnant women with fasting blood glucose equal to or less than 90 mg/dL and postprandial equal to or less than 120 mg/dL were considered compensated.6
Pregnant women with compensated gestational diabetes mellitus with insulin, with a single fetus, without malformation and with the absence of other diseases that interfered with the formation of the newborn were included.
Pregnant women whose fetuses or newborns came to present some malformation diagnosed after inclusion, history of cardiomyopathy or congenital heart disease were excluded.
The pregnant women signed the Free and Informed Consent Form.
The variables analyzed were maternal characteristics such as: age, number of previous pregnancies, body mass index, gestational age at diagnosis, weight gain during pregnancy, fasting blood glucose, glycosylated hemoglobin, and gestational age at fetal echocardiogram. The echocardiographic data collected were: myocardial thickness, shortening fraction, the left ventricular (LVMPI) and right ventricular (RVMPI) myocardial performance index and the mitral and tricuspid valve E/A ratio.
The Philips EnVisor C echocardiograph was used with an S4 sectoral transducer (2–4.2 MHz). The examination was performed by the same trained observer, with experience in pediatric and fetal echocardiography and without knowledge of clinical and laboratory data. Three sequential measurements were performed, and the mean was used for analysis.
The septum and posterior LV wall measurements were obtained in a short axis view of the left ventricle using the M mode. The shortening fraction was obtained by the LV measurements in systole and diastole using the M mode. The Doppler of the LV entry flow and RV was obtained at the point of coaptation of the mitral and tricuspid valves, respectively, in the position of four apical chambers. There were measures taken:7
- •
E wave - interval from the baseline to the peak of the E wave, expressed in meters per second;
- •
A Wave - interval from the baseline to the peak of wave A, expressed in meters per second;
- •
E/A ratio - dividing the speed of the E wave by the speed of the A wave.8
The myocardial performance index was obtained using the formula: MPI = (a−b)/b. Variable a corresponds to the interval, in seconds, from the end of A wave of the mitral or tricuspid valve flow to the beginning of the next E wave, and corresponds to the sum of the isovolumetric contraction time, the isovolumetric relaxation time, and the ejection expressed in seconds. Variable b corresponds to the ejection time through the aortic valve or the pulmonary valve, obtained by flow Doppler in the left or right ventricular outflow tract expressed in seconds.9
Values were considered normal when they followed the standards provided in the literature.7–9
The study was conducted in accordance with local regulations for good clinical practice, the National Resolution of the National Health Council (CNS, 466/12) specifically. The research was carried out after authorization by the Ethics and Research Committee of the Hans Dieter Schmidt Regional Hospital by the number 1.572.265 CAAE: 55715216.7.0000.5363.
We declare that none of the researchers has any direct or indirect relationship with the pharmaceutical or equipment industry used in this study.
Quantitative variables were processed by calculating means and standard deviations. For qualitative variables were calculated absolute and relative frequencies. To test the homogeneity of the groups in relation to the proportions, the Chi–squared test or Fisher’s exact test was used for frequencies less than 5. Values were considered significance when p < 0.05.
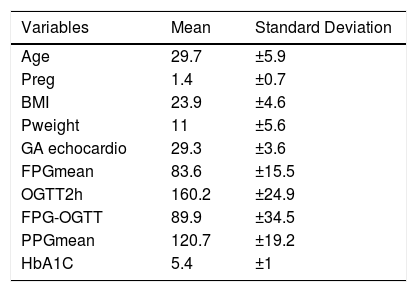
ResultsThe research carried out in a database in 2018 included 96 exams of 48 children of mothers with gestational diabetes. The maternal characteristics showed a population with an average age of 30 years, multiparous, with a BMI suitable for gestational age (24–28 weeks), with an acceptable weight gain of 11 kg (Table 1).
Maternal characteristics.
| Variables | Mean | Standard Deviation |
|---|---|---|
| Age | 29.7 | ±5.9 |
| Preg | 1.4 | ±0.7 |
| BMI | 23.9 | ±4.6 |
| Pweight | 11 | ±5.6 |
| GA echocardio | 29.3 | ±3.6 |
| FPGmean | 83.6 | ±15.5 |
| OGTT2h | 160.2 | ±24.9 |
| FPG-OGTT | 89.9 | ±34.5 |
| PPGmean | 120.7 | ±19.2 |
| HbA1C | 5.4 | ±1 |
Preg, number of previous pregnancies; BMI, body mass index; Pweight, weight gain during pregnancy; GA echocardio, gestational age at which fetal echocardiography was performed; FPGmean, mean of fasting blood glucose at home; OGTT2h, mean of glucose after 2 h since the oral glucose tolerance test; FPG-OGTT, mean fasting blood glucose after oral glucose tolerance test; PPGmean, mean of postprandial glucose; HbA1C, glycosylated hemoglobin.
The results of laboratory tests confirm the presence of gestational diabetes with an average OGTT2h value of 160 mg/dL, while the FPGmean, PPGmean, and HbA1C parameters, with values of 83 mg/dL, 120 mg/dL, and 5.9%, respectively, demonstrated good clinical control of pregnant women with insulin-dependent GDM (Table 1).
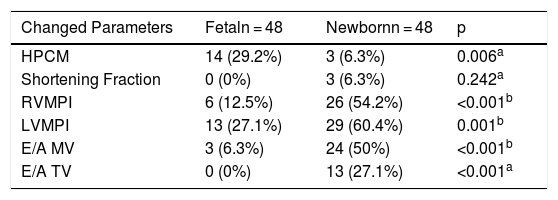
Among the echocardiographic variables, in the neonatal period the increase in the thickness of the interventricular septum occurred in 14 cases, corresponding to 29% of fetuses. In the evaluation carried out in the postnatal period, only 3 cases presented hypertrophic cardiomyopathy, showing an incidence of 6% (p = 0.006) (Table 2).
Cardiac function data assessed by echocardiogram.
| Changed Parameters | Fetaln = 48 | Newbornn = 48 | p |
|---|---|---|---|
| HPCM | 14 (29.2%) | 3 (6.3%) | 0.006a |
| Shortening Fraction | 0 (0%) | 3 (6.3%) | 0.242a |
| RVMPI | 6 (12.5%) | 26 (54.2%) | <0.001b |
| LVMPI | 13 (27.1%) | 29 (60.4%) | 0.001b |
| E/A MV | 3 (6.3%) | 24 (50%) | <0.001b |
| E/A TV | 0 (0%) | 13 (27.1%) | <0.001a |
HPCM, hypertrophic cardiomyopathy; RVMPI, right ventricular myocardial performance index; LVMPI, left ventricular myocardial performance index; E/A MV, ratio between E and A waves of the mitral valve; E/A TV, ratio between E and A waves of the tricuspid valve.
It is noteworthy that of the 14 fetuses with HPCM, only one evolved with the change in the postnatal period, while the rest improved. In echocardiograms performed in the postnatal period, it was found that two newborns evolved with HPCM only after birth.
The shortening fraction was normal in 100% of the fetuses’ cases, and in the pediatric analysis 3 cases showed changes, making up 6% of the echocardiograms in the postnatal period (p = 0.242) (Table 2).
The myocardial performance index of the right ventricle was altered in 6 fetuses and in 26 newborns, equivalent to 12% of fetal echocardiograms and 54% of pediatric echocardiograms with these changes (p < 0.001). The myocardial performance index of the left ventricle was altered in 27% of cases in the prenatal period and in 60% in the postnatal period, corresponding to 13 fetuses and 29 newborns (p = 0.001) (Table 2).
The ratio of mitral valve E/A waves was modified in 6% of the fetal echocardiograms, corresponding to 3 fetuses with this cardiac alteration, and in the pediatric echocardiograms observed there were changes in 50%, corresponding to 24 newborns with this cardiac alteration (p ≤ 0.001). The ratio of tricuspid valve E/A waves was normal in 100% of fetal cases, and it was altered in 27% of the pediatric cases, corresponding to 13 newborns (p < 0.001) (Table 2).
DiscussionAlthough the pregnant women were in good clinical, laboratory, and ultrasound control, an increase in fetal myocardial thickness was found in the prenatal period in this research, with the parameters of cardiac function (myocardial performance index, shortening fraction and E/A ratio) altered. However, in the pediatric evaluation an inversion of the changes was observed, since a low rate of hypertrophic cardiomyopathy was found in neonates as well as altered cardiac function. However, the newborns did not present a clinical manifestation of cardiac congestive insufficiency, presumably due to the fact that these changes are discrete and insufficient.
Hypertrophic cardiomyopathy (HPCM) as a cardiac disorder in fetuses of pregnant women with GDM is already a diagnosis of comprehensive knowledge in the world literature.10–12 This change involves the right ventricle and the left ventricular wall, but septal hypertrophy is more evident due to the large amount of insulin receptors in this cardiac area.13,14
Hyperglycemia, in temporary peaks, occurring in the third trimester of pregnancy is essential for the development of myocardial hypertrophy and diastolic abnormalities. These findings occur both in the fetuses of mothers with pre-gestational diabetes and in those with gestational diabetes.15 In a study carried out on fetuses of mothers with gestational diabetes before treatment, the high prevalence and early occurrence of HPCM in this population was shown. These data indicate that myocardial hypertrophy is one of the first effects of maternal diabetes on fetuses.13
Other authors have shown that the thickness of the interventricular septum above two standard deviations is predominant in all gestational ages, and cardiac function was altered between 24–27 weeks when the cardiac output of fetuses with HPCM was evaluated.13 On the other hand, HPCM can present mild intensity and appear only in the last trimester of pregnancy, without necessarily altering myocardial function.16 According to Gardiner, in 2005, hypertrophic cardiomyopathy in the fetus of a diabetic mother can be considered a functional adaptive process and not a primary cardiac dysfunction.17 Research has shown that myocardial hypertrophy is of a transitory nature and may disappear in about six months to two years after birth.18
In gestational diabetes, the main changes resulting from HPCM are transient subaortic stenosis and congestive heart failure.15 In this research, possibly, the treatment of gestational diabetes mellitus reduced the number of HPCM in the postpartum period.
We also observed diastolic dysfunction of the right ventricle even in newborns without HPCM, indicating that although few fetuses have altered cardiac function, it is already present in the intrauterine. Research has shown that the inversion of the E/A ratio in the children of diabetic mothers occurs later in comparison to the neonates of normal pregnant women. It also observed that neonates of pregnant women with gestational diabetes with controlled glycemia have prolonged deceleration time, which suggests a slight chance of ventricular relaxation in these patients.15
At birth, in both ventricles, the myocardial performance index increases temporarily, then decreases, and stabilizes after 24 h of life. This index in fetuses of diabetic pregnant women between 27 and 40 weeks of gestation is significantly higher than in the group of non-diabetic pregnant women, probably due to abnormal myocardial performance at the end of pregnancy, changes in maturation, and myocardial development.19,20
The control of diabetes during pregnancy is essential in the development and maturation of the cardiovascular system of these fetuses and newborns. Early nutritional adaptations in intrauterine life can leave permanent changes in carbohydrate metabolism, resulting in disorders in adults, such as obesity, diabetes and cardiovascular diseases.10
Our study was conducted with a small sample and a significant time ago; in addition, the pregnant women were only treated with insulin therapy. However, it includes a little studied area, mainly with regard to the analysis of patient’s profile, with prenatal and postnatal follow-up, focusing on the echocardiographic evaluation of several cardiac parameters.
We conclude that even in diabetic pregnant women considered to be well-controlled, changes in cardiac function at birth increase. The same does not happen in relation to hypertrophic cardiomyopathy, which had a lower incidence at birth, demonstrating that the adequate clinical control of the pregnant woman leads to improvement in myocardial hypertrophy. Fetal and neonatal cardiac changes are frequent, but prospective studies to assess these changes in childhood, youth and adulthood deserve consideration.
Conflicts of interestThe authors declare no conflicts of interest.
Study conducted at Universidade da Região de Joinville (UNIVILLE), Joinville, SC, Brazil.