To evaluate the prognostic performance of the Pediatric Index of Mortality 2 (PIM2), ferritin, lactate, C-reactive protein (CRP), and leukocytes, alone and in combination, in pediatric patients with sepsis admitted to the pediatric intensive care unit (PICU).
MethodsA retrospective study was conducted in a PICU in Brazil. All patients aged 6 months to 18 years admitted with a diagnosis of sepsis were eligible for inclusion. Those with ferritin and C-reactive protein measured within 48h and lactate and leukocytes within 24h of admission were included in the prognostic performance analysis.
ResultsOf 350 eligible patients with sepsis, 294 had undergone all measurements required for analysis and were included in the study. PIM2, ferritin, lactate, and CRP had good discriminatory power for mortality, with PIM2 and ferritin being superior to CRP. The cutoff values for PIM2 (> 14%), ferritin (> 135ng/mL), lactate (> 1.7mmol/L), and CRP (> 6.7mg/mL) were associated with mortality. The combination of ferritin, lactate, and CRP had a positive predictive value of 43% for mortality, similar to that of PIM2 alone (38.6%). The combined use of the three biomarkers plus PIM2 increased the positive predictive value to 76% and accuracy to 0.945.
ConclusionsPIM2, ferritin, lactate, and CRP alone showed good prognostic performance for mortality in pediatric patients older than 6 months with sepsis. When combined, they were able to predict death in three-fourths of the patients with sepsis. Total leukocyte count was not useful as a prognostic marker.
Sepsis remains a major cause of mortality in low- and middle-income countries.1 Good practice recommends early recognition of sepsis, with airway stabilization, crystalloid fluid resuscitation, and antibiotic administration within the first hour of presentation; other first-hour recommendations include vasoactive drug infusion in cases of poor response to initial fluid infusion.2 Especially in these cases, clinical examination alone is insufficient to differentiate patients at increased risk of death within the multiple phenotypes of sepsis.
Prognostic scores and biomarkers are commonly used in patients admitted to intensive care units to direct resources, to suggest a more rigorous monitoring, or to predict the risk of early deterioration. The Pediatric Index of Mortality 2 (PIM2) is a widely used prognostic score that has been properly validated in the pediatric population.3,4 By means of ten clinical and laboratory variables, PIM2 provides a percentage result that indicates the probability of death. Other laboratory tests that are not part of the PIM2 formula have also been studied as prognostic biomarkers, whether alone or in combination.5–9 Biomarkers such as lactate, ferritin, C-reactive protein (CRP), and leukocytes have attracted attention for being inexpensive, widely-available tests already used for other purposes in patients admitted to pediatric intensive care units (PICUs) in low- and middle-income countries. However, they have never been evaluated together for the purpose of estimating the risk of death in pediatric patients with sepsis.
The main objective of this study was to evaluate the prognostic performance of PIM2, ferritin, lactate, CRP, and leukocytes in patients with sepsis admitted to the PICU in a middle-income country. The authors also evaluated whether a combination of these prognostic markers would improve the ability to predict in-hospital mortality.
MethodsThis retrospective study was conducted in the PICU of Hospital São Lucas, a tertiary care hospital affiliated with School of Medicine, Pontifical Catholic University of Rio Grande do Sul (PUCRS), Porto Alegre, Brazil. The study was approved by the research ethics committee of the institution (approval No. 04621518.0.0000.5336). The study setting was a 12-bed medical-surgical PICU providing care to patients aged 1 month to 18 years. Hospital São Lucas is a private hospital linked to the Brazilian Unified Health System (Sistema Único de Saúde [SUS]), and approximately 70% of patients are admitted through this system, with a mean of 400 PICU admissions per year. The SUS provides coverage for the entire population of the country, but is mainly used by low-income families. Many patients are admitted through the hospital’s emergency department, which has eight observation beds. The hospital also has a medical residency program in Pediatrics and Pediatric Intensive Care Medicine, in addition to master’s and doctoral programs in Pediatrics and Child Health.
All patients aged 6 months to 18 years admitted to the PICU between July 2013 and January 2017 with a diagnosis of sepsis made 24h before or immediately after admission were included in the study. To define sepsis, the authors reviewed the patients’ medical and nursing records, vital signs, and laboratory test results in electronic medical records, using the 2005 classification of Goldstein et al.10 In this classification, sepsis is characterized by the presence of two or more criteria for systemic inflammatory response syndrome (SIRS) (body temperature > 38.5°C or < 36°C, tachycardia, tachypnea, leukocytosis, leukopenia, or > 10% immature forms for age), one of which must be abnormal temperature or leukocyte count, associated with suspected or proven infection. Severe sepsis was defined as sepsis associated with cardiovascular organ dysfunction or acute respiratory distress syndrome, or two or more other organ dysfunctions. Septic shock was defined as sepsis and cardiovascular organ dysfunction. This study only included patients older than 6 months because ferritin levels are influenced by maternal stores and by the switch from fetal to adult hemoglobin in the first 6 months of life. Exclusion criteria were congenital disorders of iron metabolism, liver disorders, and immunosuppression that could interfere with ferritin, lactate, CRP, and leukocyte levels; length of PICU stay < 8h; and admission for terminal palliative care.
Patients who had ferritin and CRP measured within 48h, and lactate and leukocytes within 24h of admission to the PICU were considered for the prognostic performance analysis. When patients had more than one measurement, the one with the highest level was included in the analysis. This strategy was used because the authors believe that these levels may take some time to rise after the inflammatory insult. All measurements were performed as a routine practice for septic patients in the unit.
The following data were collected for all patients included in the study: demographic characteristics, such as age, sex, weight, type of patient (medical or surgical), body mass index (BMI) Z-score, PIM2,3 and PICU readmission within 72h of discharge; laboratory tests, such as ferritin, CRP, lactate, and complete blood count; clinical characteristics and outcomes, such as definition of severe sepsis and septic shock, presence and type of the identified etiologic agent, primary site of infection, length of hospital and PICU stay, need for blood transfusion, need for mechanical ventilation and vasoactive drugs, ventilator-free days and vasoactive drug-free days calculated according to Schoenfeld et al.,11 presence of a complicated course (defined as need for mechanical ventilation, vasoactive drug use, or presence of two organ dysfunctions on day seven of PICU admission, based on the criteria of Goldstein et al.,10 or death), anemia (defined as hemoglobin < 11g/dL), iron-deficiency anemia (defined as hemoglobin < 11g/dL and mean corpuscular volume < 80fL), presence of complex chronic condition according to Feudtner et al.,12 and death.
For statistical analysis, categorical variables were expressed as number and percentage and analyzed by Fisher’s exact test or Pearson’s chi-squared test. Bonferroni correction was applied for comparisons of more than two groups. Continuous variables were expressed as median and interquartile range (IQR) and analyzed by the nonparametric Mann-Whitney U test The five test variables (PIM2, ferritin, lactate, CRP, and leukocytes) were Log10-transformed, and it was decided to use only ferritin in logarithmic form because of the resultant log-rank and p-values. Because this is a prediction and association study, it was also decided to perform only univariate analysis by testing the variables one by one, making no attempt to define causality. Sensitivity, specificity, accuracy (proportion of all correct tests to the total number of results obtained), positive predictive value, negative predictive value, and positive likelihood ratio were calculated to determine the prognostic accuracy of the five variables for mortality. Areas under the receiver operating characteristic (ROC) curve were calculated and compared using the method of DeLong et al.13 Cutoff values for the five variables were determined by Youden’s index.14 Kaplan-Meier survival curves were generated taking into account death or hospital discharge. Different combinations between the five variables were tested to achieve the best prognostic performance. A p-value < 0.05 was considered significant for all analyses. Data analysis was performed in SPSS, v. 17.0 (IBM SPSS Statistics – Armonk, NY, United States) and MedCalc, v. 15.8 (MedCalc Software BVBA – Ostend, Belgium).
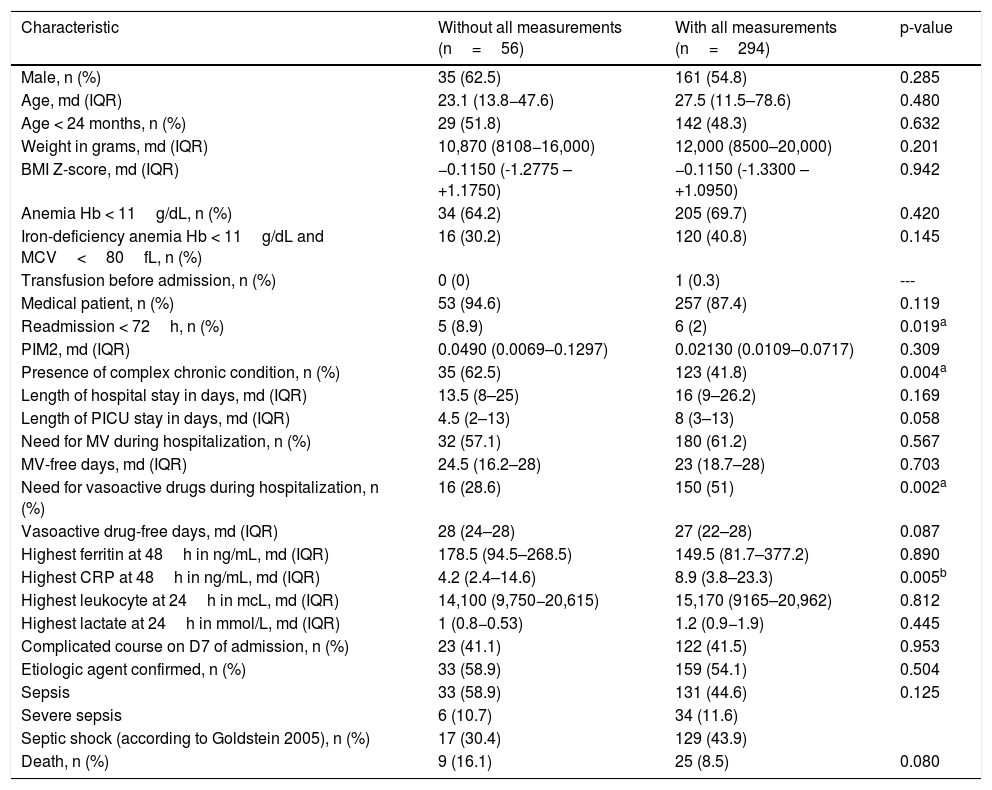
ResultsOf 1407 patients admitted during the study period, 552 were diagnosed with sepsis and 350 patients older than 6 months with sepsis were eligible for inclusion. Of these, 294 had undergone all measurements required for the analysis of prognostic markers and were included. Among the 56 excluded patients, ferritin was not measured in 38, CRP in 19, lactate in 18, and leukocytes in three. No patient was excluded due to immunosuppression that interfered with the studied biomarkers. Table 1 shows the clinical and demographic characteristics and outcomes of patients with and without measurements of all four prognostic biomarkers during PICU stay.
Comparison of clinical and demographic characteristics and outcomes between patients with and without measurements of four prognostic markers.
| Characteristic | Without all measurements (n=56) | With all measurements (n=294) | p-value |
|---|---|---|---|
| Male, n (%) | 35 (62.5) | 161 (54.8) | 0.285 |
| Age, md (IQR) | 23.1 (13.8−47.6) | 27.5 (11.5–78.6) | 0.480 |
| Age < 24 months, n (%) | 29 (51.8) | 142 (48.3) | 0.632 |
| Weight in grams, md (IQR) | 10,870 (8108−16,000) | 12,000 (8500–20,000) | 0.201 |
| BMI Z-score, md (IQR) | −0.1150 (-1.2775 – +1.1750) | −0.1150 (-1.3300 – +1.0950) | 0.942 |
| Anemia Hb < 11g/dL, n (%) | 34 (64.2) | 205 (69.7) | 0.420 |
| Iron-deficiency anemia Hb < 11g/dL and MCV<80fL, n (%) | 16 (30.2) | 120 (40.8) | 0.145 |
| Transfusion before admission, n (%) | 0 (0) | 1 (0.3) | --- |
| Medical patient, n (%) | 53 (94.6) | 257 (87.4) | 0.119 |
| Readmission < 72h, n (%) | 5 (8.9) | 6 (2) | 0.019a |
| PIM2, md (IQR) | 0.0490 (0.0069–0.1297) | 0.02130 (0.0109–0.0717) | 0.309 |
| Presence of complex chronic condition, n (%) | 35 (62.5) | 123 (41.8) | 0.004a |
| Length of hospital stay in days, md (IQR) | 13.5 (8–25) | 16 (9–26.2) | 0.169 |
| Length of PICU stay in days, md (IQR) | 4.5 (2–13) | 8 (3–13) | 0.058 |
| Need for MV during hospitalization, n (%) | 32 (57.1) | 180 (61.2) | 0.567 |
| MV-free days, md (IQR) | 24.5 (16.2–28) | 23 (18.7–28) | 0.703 |
| Need for vasoactive drugs during hospitalization, n (%) | 16 (28.6) | 150 (51) | 0.002a |
| Vasoactive drug-free days, md (IQR) | 28 (24–28) | 27 (22–28) | 0.087 |
| Highest ferritin at 48h in ng/mL, md (IQR) | 178.5 (94.5–268.5) | 149.5 (81.7–377.2) | 0.890 |
| Highest CRP at 48h in ng/mL, md (IQR) | 4.2 (2.4–14.6) | 8.9 (3.8–23.3) | 0.005b |
| Highest leukocyte at 24h in mcL, md (IQR) | 14,100 (9,750−20,615) | 15,170 (9165–20,962) | 0.812 |
| Highest lactate at 24h in mmol/L, md (IQR) | 1 (0.8−0.53) | 1.2 (0.9−1.9) | 0.445 |
| Complicated course on D7 of admission, n (%) | 23 (41.1) | 122 (41.5) | 0.953 |
| Etiologic agent confirmed, n (%) | 33 (58.9) | 159 (54.1) | 0.504 |
| Sepsis | 33 (58.9) | 131 (44.6) | 0.125 |
| Severe sepsis | 6 (10.7) | 34 (11.6) | |
| Septic shock (according to Goldstein 2005), n (%) | 17 (30.4) | 129 (43.9) | |
| Death, n (%) | 9 (16.1) | 25 (8.5) | 0.080 |
md (IQR), median (interquartile range); BMI, body mass index; PIM2, Pediatric Index of Mortality 2; PICU, pediatric intensive care unit; MV, mechanical ventilation; D7, day seven of admission; Hb, hemoglobin; MCV, mean corpuscular volume; CRP, C-reactive protein.
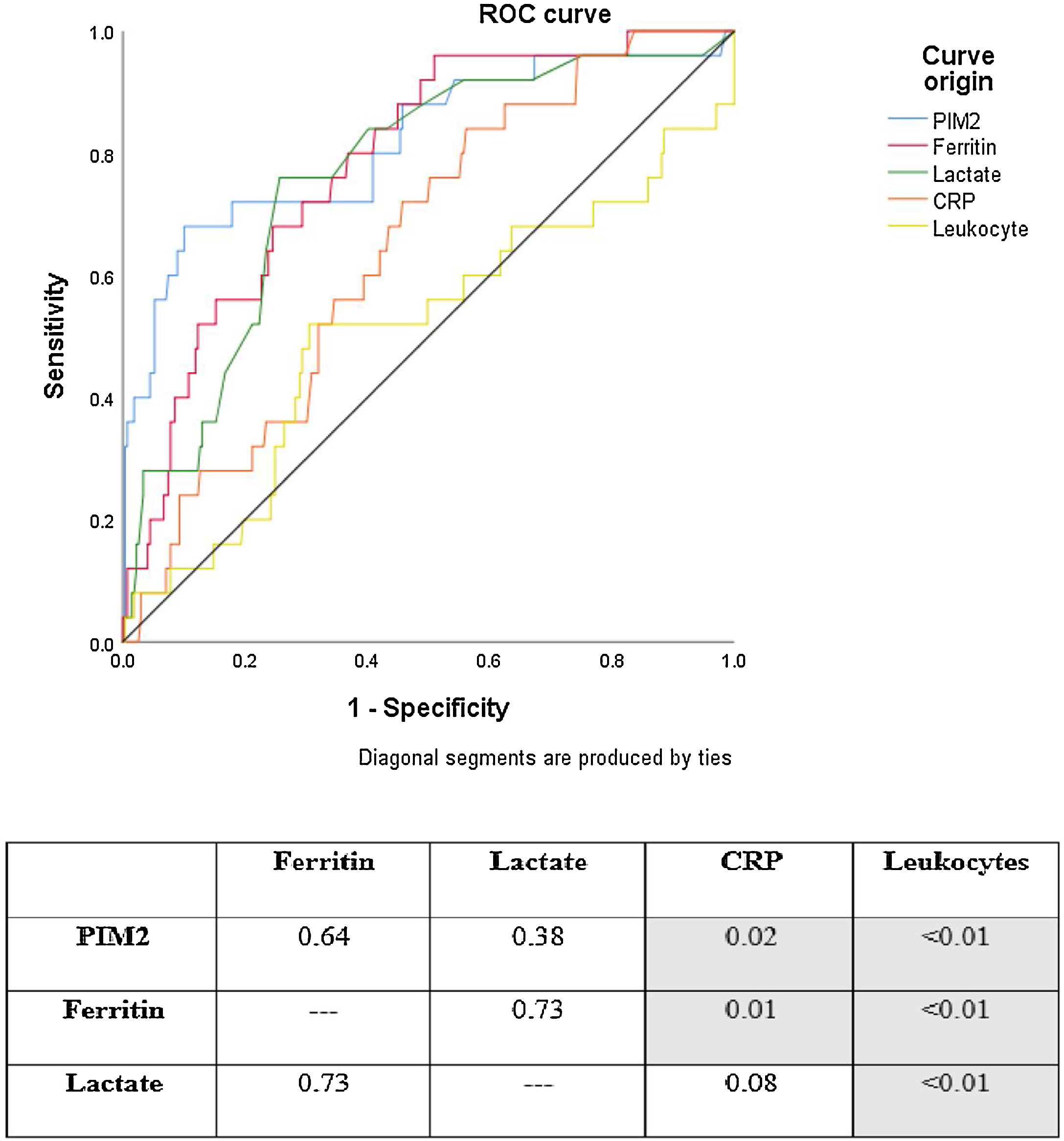
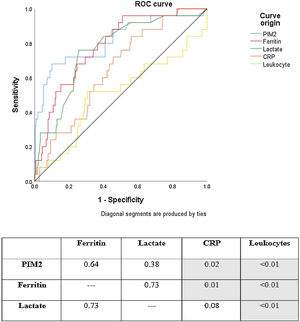
ROC curve analysis showed that PIM2, ferritin, lactate, and CRP had good discriminatory power for mortality in the study sample. Leukocytes were not useful for this purpose. The ROC curves for PIM2, ferritin, and lactate were similar. CRP, however, showed poorer performance than PIM2 and ferritin. Fig. 1 shows a comparison of ROC curves and the respective p-values for each cross-tabulation. In descending order, area under the curve (AUC) values are as follows: PIM2 0.815 (95% confidence interval [CI] 0.766–0.858); ferritin 0.785 (95% CI 0.733–0.830); lactate 0.762 (95% CI 0.709–0.810); CRP 0.648 (95% CI 0.590–0.702); and leukocytes 0.508 (95% CI 0.450–0.567).
Mortality ROC curves for prognostic markers analyzed in the sample. The numbers in the table indicate p-values for comparisons between curves (ROC curves were compared using the method of DeLong et al.13) Area under the curve (AUC) values: PIM2 AUC 0.815 (95% CI 0.766–0.858); Ferritin AUC 0.785 (95% CI 0.733–0.830); Lactate AUC 0.762 (95% CI 0.709–0.810); CRP AUC 0.648 (95% CI 0.590–0.702); Leukocytes AUC 0.508 (95% CI 0.450–0.567).
PIM2, Pediatric Index of Mortality 2; CRP, C-reactive protein.
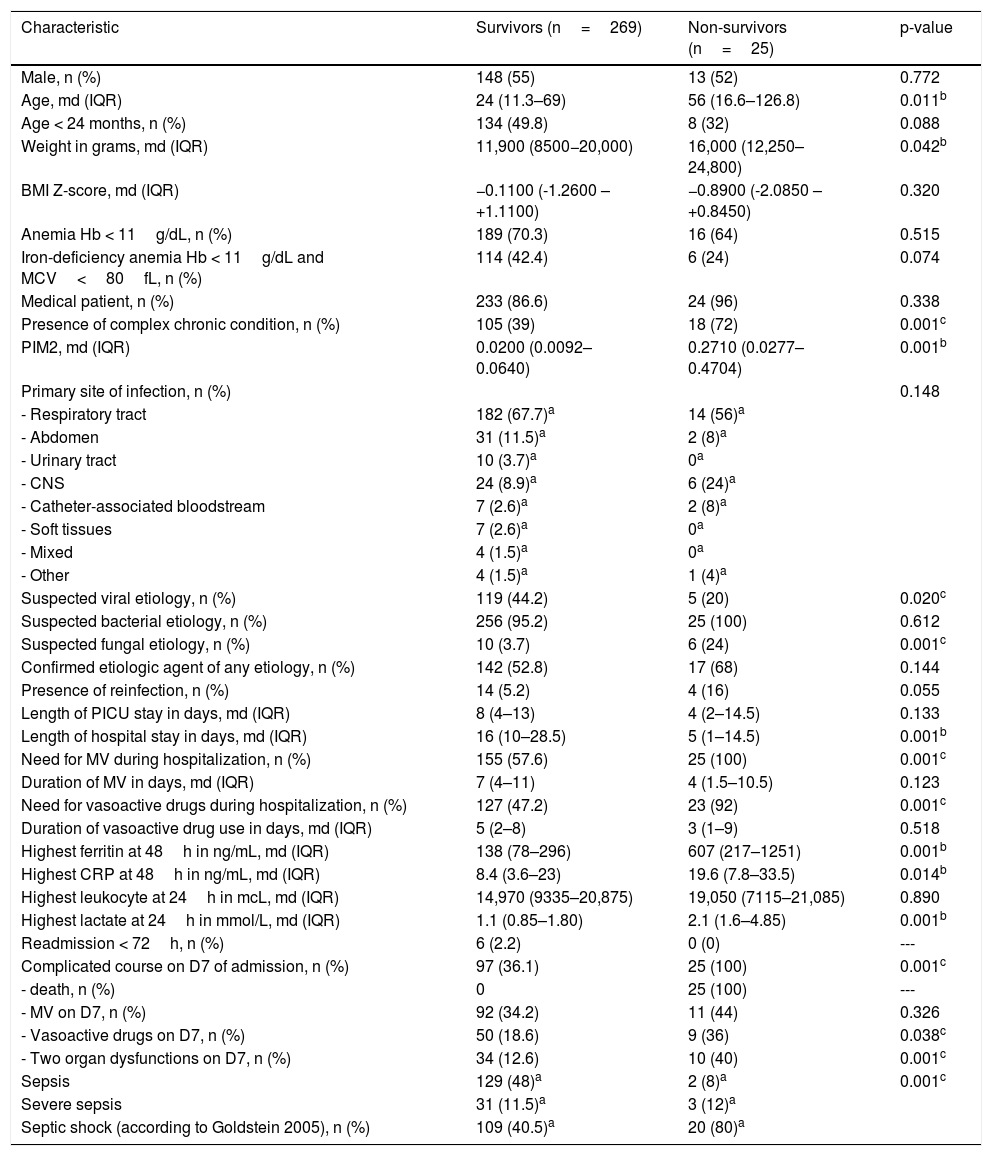
The clinical and demographic characteristics and outcomes of survivors vs. non-survivors are shown in Table 2. Non-survivors were older and had more severe sepsis on admission (represented by PIM2 score), in addition to a higher prevalence of complex chronic conditions and greater suspicion of fungal infections. Regarding the four prognostic biomarkers under analysis, the two groups differed in ferritin, lactate, and CRP levels. Univariate logistic regression analysis showed an association of these three biomarkers with mortality: Log10 ferritin (p<0.001, Exp(B) 5.075; 95% CI 2.536–10.155); CRP (p=0.029, Exp(B) 1.033; 95% CI 1.003–1.063); and lactate (p<0.001, Exp(B) 1.487; 95% CI 1.217–1.817).
Comparison of clinical and demographic characteristics and outcomes of survivors vs. non-survivors.
| Characteristic | Survivors (n=269) | Non-survivors (n=25) | p-value |
|---|---|---|---|
| Male, n (%) | 148 (55) | 13 (52) | 0.772 |
| Age, md (IQR) | 24 (11.3–69) | 56 (16.6–126.8) | 0.011b |
| Age < 24 months, n (%) | 134 (49.8) | 8 (32) | 0.088 |
| Weight in grams, md (IQR) | 11,900 (8500−20,000) | 16,000 (12,250–24,800) | 0.042b |
| BMI Z-score, md (IQR) | −0.1100 (-1.2600 – +1.1100) | −0.8900 (-2.0850 – +0.8450) | 0.320 |
| Anemia Hb < 11g/dL, n (%) | 189 (70.3) | 16 (64) | 0.515 |
| Iron-deficiency anemia Hb < 11g/dL and MCV<80fL, n (%) | 114 (42.4) | 6 (24) | 0.074 |
| Medical patient, n (%) | 233 (86.6) | 24 (96) | 0.338 |
| Presence of complex chronic condition, n (%) | 105 (39) | 18 (72) | 0.001c |
| PIM2, md (IQR) | 0.0200 (0.0092–0.0640) | 0.2710 (0.0277–0.4704) | 0.001b |
| Primary site of infection, n (%) | 0.148 | ||
| - Respiratory tract | 182 (67.7)a | 14 (56)a | |
| - Abdomen | 31 (11.5)a | 2 (8)a | |
| - Urinary tract | 10 (3.7)a | 0a | |
| - CNS | 24 (8.9)a | 6 (24)a | |
| - Catheter-associated bloodstream | 7 (2.6)a | 2 (8)a | |
| - Soft tissues | 7 (2.6)a | 0a | |
| - Mixed | 4 (1.5)a | 0a | |
| - Other | 4 (1.5)a | 1 (4)a | |
| Suspected viral etiology, n (%) | 119 (44.2) | 5 (20) | 0.020c |
| Suspected bacterial etiology, n (%) | 256 (95.2) | 25 (100) | 0.612 |
| Suspected fungal etiology, n (%) | 10 (3.7) | 6 (24) | 0.001c |
| Confirmed etiologic agent of any etiology, n (%) | 142 (52.8) | 17 (68) | 0.144 |
| Presence of reinfection, n (%) | 14 (5.2) | 4 (16) | 0.055 |
| Length of PICU stay in days, md (IQR) | 8 (4–13) | 4 (2–14.5) | 0.133 |
| Length of hospital stay in days, md (IQR) | 16 (10–28.5) | 5 (1–14.5) | 0.001b |
| Need for MV during hospitalization, n (%) | 155 (57.6) | 25 (100) | 0.001c |
| Duration of MV in days, md (IQR) | 7 (4–11) | 4 (1.5–10.5) | 0.123 |
| Need for vasoactive drugs during hospitalization, n (%) | 127 (47.2) | 23 (92) | 0.001c |
| Duration of vasoactive drug use in days, md (IQR) | 5 (2–8) | 3 (1–9) | 0.518 |
| Highest ferritin at 48h in ng/mL, md (IQR) | 138 (78–296) | 607 (217–1251) | 0.001b |
| Highest CRP at 48h in ng/mL, md (IQR) | 8.4 (3.6–23) | 19.6 (7.8–33.5) | 0.014b |
| Highest leukocyte at 24h in mcL, md (IQR) | 14,970 (9335–20,875) | 19,050 (7115–21,085) | 0.890 |
| Highest lactate at 24h in mmol/L, md (IQR) | 1.1 (0.85–1.80) | 2.1 (1.6–4.85) | 0.001b |
| Readmission < 72h, n (%) | 6 (2.2) | 0 (0) | --- |
| Complicated course on D7 of admission, n (%) | 97 (36.1) | 25 (100) | 0.001c |
| - death, n (%) | 0 | 25 (100) | --- |
| - MV on D7, n (%) | 92 (34.2) | 11 (44) | 0.326 |
| - Vasoactive drugs on D7, n (%) | 50 (18.6) | 9 (36) | 0.038c |
| - Two organ dysfunctions on D7, n (%) | 34 (12.6) | 10 (40) | 0.001c |
| Sepsis | 129 (48)a | 2 (8)a | 0.001c |
| Severe sepsis | 31 (11.5)a | 3 (12)a | |
| Septic shock (according to Goldstein 2005), n (%) | 109 (40.5)a | 20 (80)a |
md (IQR), median (interquartile range); BMI, body mass index; PIM2, Pediatric Index of Mortality 2; PICU, pediatric intensive care unit; MV, mechanical ventilation; D7, day seven of admission; Hb, hemoglobin; MCV, mean corpuscular volume; CNS, central nervous system; CRP, C-reactive protein.
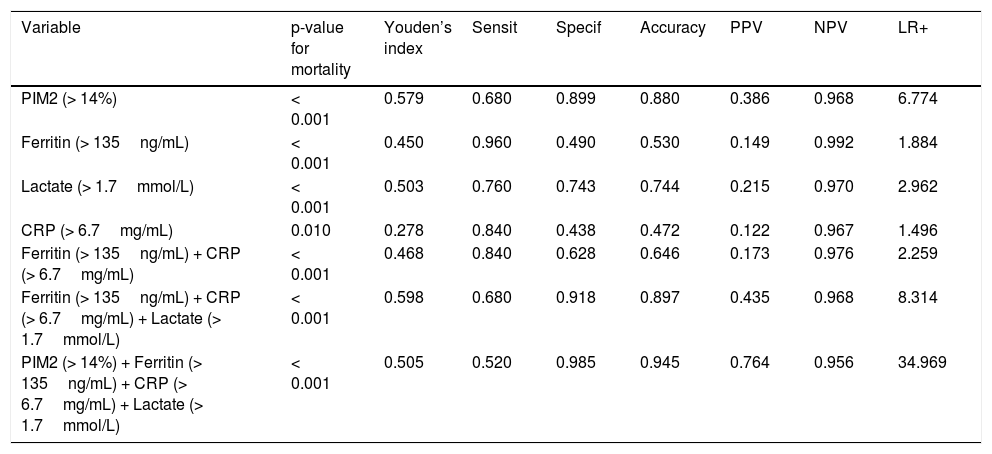
The cutoff values for PIM2 (> 14%), ferritin (> 135ng/mL), lactate (> 1.7mmol/L), and CRP (> 6.7mg/mL), as determined by Youden’s index, were associated with mortality. The combination of ferritin, lactate, and CRP had a positive predictive value of 43% for mortality, similar to that of PIM2 alone (38.6%). The combined use of the three biomarkers plus PIM2 increased the positive predictive value to 76% and accuracy to 0.945. The cutoff values and prognostic performance for mortality and the Kaplan-Meier survival curves of PIM2 and the three biomarkers, alone and in combination, are shown in Table 3 and in Supplementary material 1, respectively.
Cutoff values and diagnostic approach of PIM2, prognostic biomarkers, and their combinations.
| Variable | p-value for mortality | Youden’s index | Sensit | Specif | Accuracy | PPV | NPV | LR+ |
|---|---|---|---|---|---|---|---|---|
| PIM2 (> 14%) | < 0.001 | 0.579 | 0.680 | 0.899 | 0.880 | 0.386 | 0.968 | 6.774 |
| Ferritin (> 135ng/mL) | < 0.001 | 0.450 | 0.960 | 0.490 | 0.530 | 0.149 | 0.992 | 1.884 |
| Lactate (> 1.7mmol/L) | < 0.001 | 0.503 | 0.760 | 0.743 | 0.744 | 0.215 | 0.970 | 2.962 |
| CRP (> 6.7mg/mL) | 0.010 | 0.278 | 0.840 | 0.438 | 0.472 | 0.122 | 0.967 | 1.496 |
| Ferritin (> 135ng/mL) + CRP (> 6.7mg/mL) | < 0.001 | 0.468 | 0.840 | 0.628 | 0.646 | 0.173 | 0.976 | 2.259 |
| Ferritin (> 135ng/mL) + CRP (> 6.7mg/mL) + Lactate (> 1.7mmol/L) | < 0.001 | 0.598 | 0.680 | 0.918 | 0.897 | 0.435 | 0.968 | 8.314 |
| PIM2 (> 14%) + Ferritin (> 135ng/mL) + CRP (> 6.7mg/mL) + Lactate (> 1.7mmol/L) | < 0.001 | 0.505 | 0.520 | 0.985 | 0.945 | 0.764 | 0.956 | 34.969 |
PIM2, Pediatric Index of Mortality 2; CRP, C-reactive protein; PPV, positive predictive value; NPV, negative predictive value; LR+: positive likelihood ratio; Accuracy, proportion of all correct tests (true positives and true negatives) to the total number of results obtained.
This study demonstrated good prognostic performance for mortality using PIM2, ferritin, lactate, and CRP in pediatric patients older than 6 months with sepsis, in a middle-income setting with a high prevalence of iron-deficiency anemia. When compared to each other, ferritin and lactate were similar to PIM2, while CRP was slightly inferior. Leukocyte count was unable to discriminate patients at risk of death. Based on cutoff values determined by Youden’s index, the combination of ferritin, lactate, and CRP was able to predict death in approximately 43% of patients, and this rate increased to 76% when PIM2 was added to the combination. This is the first study to analyze, in this population profile, these five variables widely available in PICUs.
The use of ferritin as a prognostic marker is not a novel concept. The present group has been studying this biomarker since 2007, an independent association of ferritin with mortality in pediatric patients was described.6 Its combined use with CRP has been investigated in PICUs, mainly because both are inexpensive, widely-available tests already used for other purposes in hospitals in low- and middle-income countries. Examples of such uses include ferritin for the diagnosis of iron-deficiency anemia and CRP as a complementary tool in the diagnosis of bacterial infection and as a marker of therapeutic response in sepsis.15,16 In a recent study, Horvat et al. reported an association of these two tests with mortality in a relevant sample of patients admitted to a general PICU.8 In their study, the combined use of maximum ferritin with CRP during hospitalization was able to predict death in 21.7% of patients, a finding similar to that of the present study (17.3%). However, the major difference was the cutoff value for ferritin (373ng/mL vs. 135ng/mL). A possible explanation for this may be the high prevalence of iron-deficiency anemia in this sample (40.8%). This indicates that caution must be exercised when extrapolating these cutoff values, especially to populations with unknown prevalence of iron-deficiency anemia.
Unlike in adults where lactate is used for the diagnosis of sepsis, in children it has a well-defined role as a prognostic marker.5,17,18 This occurs because most pediatric patients with sepsis and septic shock have admission-lactate levels within the normal range, rendering lactate useless for diagnosis.19 Both lactate and ferritin in the present cohort showed good performance in discriminating patients at risk of death, comparable to that of PIM2. To the best of the authors’ knowledge, this is the first study to compare these two biomarkers for this purpose. Lactate combined with CRP and ferritin could predict death in almost half of the patients, a performance similar to that of PIM2 alone (43.5% vs. 38.6%).
Mortality risk scores provide valuable tools to comparatively assess quality-of-care standards between different intensive care units or to examine changes over time within the same unit. One of the most widely used mortality risk scores is PIM2.3 Its use to estimate mortality in an individual patient is limited, since this score is intended to calculate mortality prediction in large populations with wide variability in number of cases and disease severity. When applied to the present cohort, which included only patients with sepsis, PIM2 showed good performance. The rationale of the use of PIM2 as an individual prognostic marker with a defined cutoff point is that, by being calculated at the time of PICU admission and ideally from data collected within the first hour of presentation, in addition to being widely used worldwide, this score could assume an additional role: early prediction of patients at risk of deterioration or death. Its use in combination with three biomarkers predicted death in three-fourths of the patients. It is believed that this type of analysis will serve to allow ferritin, lactate, and CRP to be incorporated in the near future into the calculation of an updated PIM score or even of other prognostic scores.
This study provides some practical contributions, including indication of good performance, in the same population and same time frame, of four prognostic biomarkers of interest that are inexpensive and already widely used for other purposes in PICUs. In addition, low ferritin levels were found to be associated with mortality in a setting with high prevalence of iron-deficiency anemia. Ghosh et al. have already pointed to the need to review the threshold level for hyperferritinemic sepsis in this population.20 Another important point was the assertion of the uselessness of leukocyte count as a prognostic marker in pediatric sepsis. There is no consensus on the use of leukocytes as a prognostic marker. Unlike in previous studies where a higher or lower leukocyte count has been associated with mortality, in the present study such an association was not observed.21,22 A possible explanation is that leukocytes are altered by the use of medications or by other medical conditions in patients with sepsis, such as corticosteroid use and recent chemotherapy, which could influence the results in these cohorts.
This study has some limitations that need to be addressed. First, patients older than 6 months were analyzed. This age group was chosen because their ferritin levels are no longer influenced by maternal stores or by the switch from fetal to adult hemoglobin. Second, 16% of patients with sepsis in this cohort had not undergone all measurements required for analysis. This group had a higher prevalence of complex chronic conditions and readmission rates, and lower use of vasoactive drugs. This may reflect a suboptimal practice in this profile of chronic patients. Third, this study was conducted at a single center and the cutoff points in our sample were determined by Youden’s index. This is one of the possible methods for obtaining cutoff points and may not be ideal for all clinical situations. Finally, tests performed only at the time of PICU admission were analyzed. The reason for this was that the authors aimed to identify early prognostic markers, which could be highly useful in low-resource settings where human or financial resources are prioritized.
ConclusionPIM2, ferritin, lactate, and CRP alone showed good prognostic performance for mortality in pediatric patients older than 6 months with sepsis. When combined (at the following cutoff values: PIM2>14%, ferritin > 135ng/mL, CRP>6.7mg/mL, and lactate > 1.7mmol/L), they were able to predict death in three-fourths of the patients with sepsis. Total leukocyte count was not useful as a prognostic marker.
Ethical approvalThis study was approved by the institutional Research Ethics Committee. Due to its purely retrospective nature, the requirement to obtain informed consent was waived (ethics approval No. 04621518.0.0000.5336).
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the entire multidisciplinary team of the PICU at Hospital São Lucas da PUCRS, especially the on-call physicians, residents, nurses, and physical therapists who work in the unit.
Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Hospital São Lucas, Faculdade de Medicina e Medicina Intensiva Pediátrica, Departamento de Pediatria, Porto Alegre, RS, Brazil.