To evaluate the presence of insulin resistance and its association with other metabolic abnormalities in obese children and adolescents.
MethodsRetrospective study of 220 children and adolescents aged 5-14 years. Anthropometric measurements were performed (weight, height, and waist circumference) and clinical (gender, age, pubertal stage, and degree of obesity) and biochemical (glucose, insulin, total cholesterol, and fractions, triglycerides) data were analyzed. Insulin resistance was identified by the homeostasis model assessment for insulin resistance (HOMA-IR) index. The analysis of the differences between the variables of interest and the HOMA-IR quartiles was performed by ANOVA or Kruskal-Wallis tests.
ResultsInsulin resistance was diagnosed in 33.20% of the sample. It was associated with low levels of high-density lipoprotein cholesterol (HDL-C; p=0.044), waist circumference measurement (p=0.030), and the set of clinical and metabolic (p=0.000) alterations. Insulin-resistant individuals had higher mean age (p=0.000), body mass index (BMI; p=0.000), abdominal circumference (p=0.000), median triglycerides (p=0.001), total cholesterol (p≤0.042), and low-density lipoprotein cholesterol (LDL–C; p≤0.027); and lower HDL-C levels (p=0.005). There was an increase in mean BMI (p=0.000), abdominal circumference (p=0.000), and median triglycerides (p=0.002) as the values of HOMA -IR increased, with the exception of HDL-C, which decreased (p=0.001). Those with the highest number of simultaneous alterations were between the second and third quartiles of the HOMA–IR index (p=0.000).
ConclusionThe results confirmed that insulin resistance is present in many obese children and adolescents, and that this condition is associated with alterations that represent an increased risk for developing metabolic disorders in adulthood.
Avaliar a presença de resistência à insulina e sua relação com outras alterações metabólicas, em crianças e adolescentes obesos.
MétodosEstudo retrospectivo de 220 crianças e adolescentes de 5 a 14 anos. Foram realizadas avaliações antropométricas (peso, estatura e circunferência abdominal), clínicas (sexo, idade, estágio puberal e grau de obesidade) e bioquímicas (glicemia, insulina, colesterol total e frações, triglicerídeos). A resistência à insulina foi identificada pelo índice HOMA-IR. A análise das diferenças entre as variáveis de interesse e os quartis do HOMA-IR foi realizada pelos testes ANOVA ou Kruskal-Wallis.
ResultadosA resistência à insulina foi diagnosticada em 33,20% da amostra. Associou-se a níveis baixos de HDL-C (p=0,044), medida da circunferência abdominal aumentada (p=0,030) e ao conjunto de alterações clínicas e metabólicas (p=0,000). Os indivíduos resistentes apresentaram maiores médias de idade (p=0,000), IMC (p=0,000), medida da circunferência abdominal (p=0,000) e maiores medianas de triglicerídeos (p=0,001), colesterol total (p≤0,042), LDL-C (p≤0,027) e menores de HDL-C (p=0,005). Houve aumento das médias de IMC (p=0,000), medida da circunferência abdominal (p=0,000) e mediana de triglicerídeos (p=0,002) à medida que os valores do HOMA-IR se elevavam, com exceção dos níveis de HDL-C que diminuíram (p=0,001). Aqueles que apresentaram o maior conjunto de alterações simultâneas estavam entre o segundo e terceiro quartis do HOMA-IR (p=0,000).
ConclusãoOs resultados confirmaram que a resistência à insulina está presente em muitas crianças e em muitos adolescentes obesos, e que esta condição está associada a alterações que representam aumento do risco para o desenvolvimento de distúrbios metabólicos na maturidade.
Obesity is a chronic disease with multifactorial etiology. Its occurrence is associated with increased morbidity and mortality and reduced life expectancy. In childhood and adolescence, it often leads to important metabolic alterations, which, depending on the duration and severity, may determine the development of chronic diseases in adulthood.1,2 In this context, insulin resistance (IR) is emerging as an important disorder among young individuals. Studies have emphasized that patients with IR have a higher predisposition to the future development of metabolic syndrome (MS), type II diabetes mellitus (DM2), and cardiovascular disease. Correlations were identified between IR and clinical and metabolic alterations, especially among obese children and adolescents, indicating that obesity is a major trigger for their development.3–7
The mechanisms by which IR occurs are not entirely clear. It is essentially characterized by the decrease in the capacity of attaining normal plasma insulin concentrations, promoting adequate peripheral glucose uptake, maintaining liver glycogenesis in balance, and inhibiting the production of very-low-density lipoprotein.8 The diagnosis of IR is not easy, due to the lack of a single method capable of estimating the degree of individual sensitivity to insulin.
Among the different methods are the direct tests, which seek to analyze the effects of a predetermined amount of administered insulin (insulin tolerance test, insulin suppression test, and clamping), and the indirect tests, which evaluate the effect of endogenous insulin (fasting insulin, homeostasis model assessment [HOMA], and the oral glucose tolerance test [OGTT]). The gold standard is the hyperinsulinemic euglycemic clamp method, but the complexity and high cost of the method prevent its use in daily clinical practice and in epidemiological studies.9 The HOMA for insulin resistance (HOMA-IR) index is a widely used method in adults and has been validated in children and adolescents, by comparing with rates based on the OGTT and the hyperinsulinemic euglycemic clamp.
Some authors recommend that cutoff values of approximately 3 are able to identify IR in this population.10–15 IR is one of the most important effects found in obese patients and it appears to be the factor that triggers other metabolic alterations. Thus, the present study aimed to evaluate the presence of IR and its associations with other metabolic abnormalities in obese children and adolescents.
MethodsThis was a retrospective cross-sectional study, with primary data collection performed in children and adolescents from the Obesity Outpatient Clinic in Osasco, São Paulo, from April of 2010 to January of 2012. A total of 220 patients were analyzed, aged 5 to 14 years old, who had not undergone any weight reduction intervention. The minimum sample size (201 children and adolescents) was calculated taking into account the outcome of IR in this population, a significance level of 5% (α=0.05), statistical power of 95% (1 - β=0.95), and 20% eventual losses.
Measurements of weight, height, and waist circumference (WC) were obtained at the anthropometric assessment. Weight was measured using a platform-type Filizola scale (Filizola, São Paulo, Brazil) placed on a smooth surface, with capacity up to 150kg and precision of 100g. The subjects were barefoot and wearing light clothing, standing on the center of the scale and in vertical position. Height was measured in the standing position, barefoot and heels in parallel, using a stadiometer with a resolution of 1mm. To evaluate the nutritional status of children and adolescents, the body mass index (BMI) Z-score was used, according to the criteria proposed by the World Health Organization,16 and individuals were categorized as obese (BMI z >+2 ≤+3) or severely obese (BMI z >+3).
WC was measured with the individual in the standing position, at midpoint between the lower border of the last rib and the upper border of the iliac crest on the horizontal plane, using an inextensible tape graduated in millimeters. WC was considered to be increased when its value was at or above the 90th percentile for gender and age.17
The degree of sexual maturity of individuals was assessed by a pediatric endocrinologist and classified according to Tanner.18 Therefore, individuals were considered prepubertal when they were at stage 1l pubertal at stages 2, 3, and 4; and post-pubertal at stage 5. Laboratory tests were performed according to the routine of the Obesity Outpatient Clinic and included serum total cholesterol and fractions, triglycerides, insulin, and fasting glucose levels.
Blood samples were collected by venipuncture after fasting for 12hours. The samples were collected in vacuum tubes containing separator gel, without anticoagulant. After collection, the blood was centrifuged for ten minutes at 3,000rpm to separate the serum from the remaining components, and the serum was then used to perform the analyses. The levels of total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), and glucose were determined using enzymatic colorimetric kits processed in an Autohumalyzer A5 (Human GMBH, Kaiserslautern, Germany). Insulin was measured in an ACS-180 Automated Chemiluminescense System (Ciba Corning, Diagnostics Corp., Medifield, USA), and low-density lipoprotein cholesterol (LDL-C) was calculated using the equation of Friedewald et al.19
The results were compared with reference values for children and adolescents of the I Guideline for Prevention of Atherosclerosis in Childhood and Adolescence.20 The HOMA-IR index was used to evaluate IR and obtained by calculating the product of fasting plasma insulin (μU/mL) and fasting plasma glucose (mmol/L) divided by 22.5. The cutoff used was greater than or equal to 3.43 for both genders, according to Garcia Cuartero et al.15
The following were considered clinical and metabolic abnormalities: fasting glucose ≥ 100mg/dL, fasting insulin ≥ 15 microU/mL, total cholesterol ≥ 170mg/dL, LDL-C ≥ 130mg/dL, HDL-C ≤ 45mg/dL, triglycerides ≥ 130mg/dL, and waist circumference ≥ 90th percentile. A set of clinical and metabolic alterations was defined for each individual according to the number of prevalent conditions that ranged from 0 (no alteration) to 7 (presence of all alterations). The project was approved by the Research Ethics Committee of Universidade Federal de São Paulo - UNIFESP. Data collection was performed after written informed consent was obtained from the institution where the study was carried out. Data were entered into Excel 2010 (Microsoft, Washington, USA) spreadsheets and analyzed using SPSS version 19.0 (IBM Company, New York, USA). Continuous variables were tested for normality of distribution by Kolmogorov-Smirnov test. The differences for these variables were analyzed using the Mann-Whitney U-test or Student's t-test, according to the distribution. Categorical variables were compared by chi-squared test (with correction by Fisher's exact test). To evaluate IR and its association with clinical and metabolic variables, the HOMA-IR index was distributed into quartiles and the differences between the values of these variables were evaluated by ANOVA or the Kruskal-Wallis test, according to the distribution. For all analyses, statistical significance was considered as p<0.05.
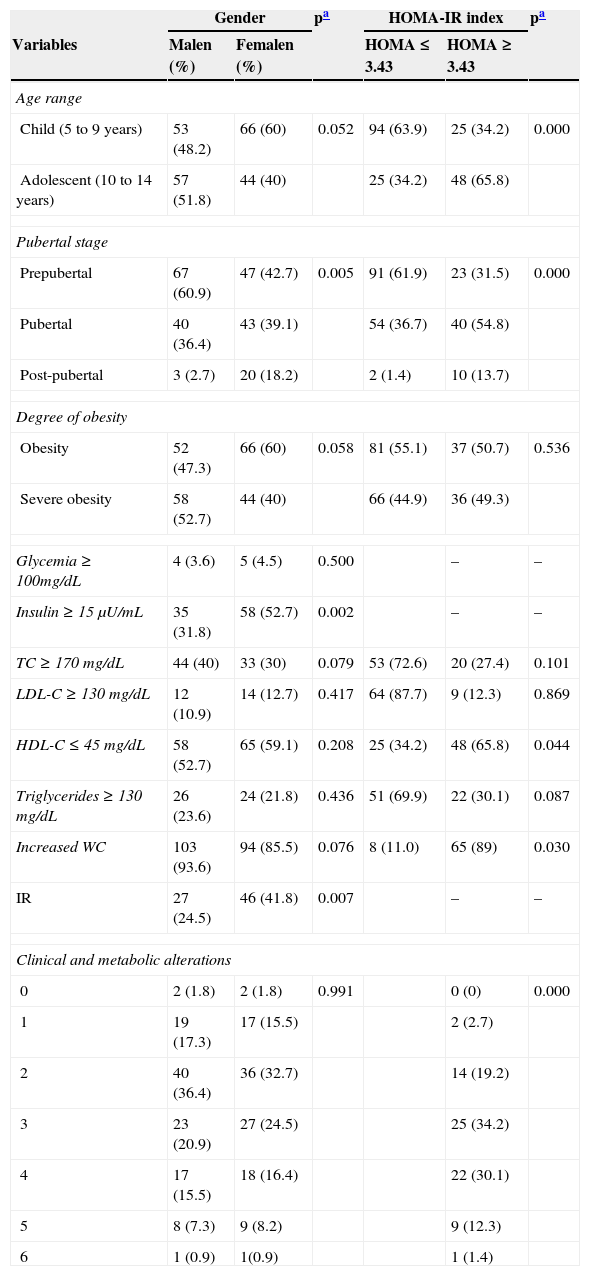
ResultsA total of 220 obese children and adolescents (50% girls, 54.1% children, 46.4% severely obese, and 51.8% prepubertal individuals) with a mean age of 9.13±2.11 years were included in the study. Significant percentages of clinical and metabolic alterations were found: increased WC measurements (89.5%), hyperinsulinemia (42.3%), hypercholesterolemia (35%), elevated LDL-C (23%), and low HDL-C (55.9%). Fasting glucose alteration was observed in one child and in eight adolescents. Table 1 presents the absolute values and percentages of clinical and metabolic characteristics of the children and adolescents. The highest frequencies of post-pubertal individuals (18.2% vs. 2.7% p=0.005), alterations in fasting insulin (52.7% vs. 31.8% p=0.002), and IR (41.8% vs. 24.5% p=0.007) were observed among females. Fasting glucose was abnormal in 3.6% of males and 4.5% of females, although there was no significant difference between genders (p=0.500).
Absolute and percentage values of clinical and metabolic characteristics of obese children and adolescents, according to gender and HOMA-IR index.
| Gender | pa | HOMA-IR index | pa | |||
|---|---|---|---|---|---|---|
| Variables | Malen (%) | Femalen (%) | HOMA ≤ 3.43 | HOMA ≥ 3.43 | ||
| Age range | ||||||
| Child (5 to 9 years) | 53 (48.2) | 66 (60) | 0.052 | 94 (63.9) | 25 (34.2) | 0.000 |
| Adolescent (10 to 14 years) | 57 (51.8) | 44 (40) | 25 (34.2) | 48 (65.8) | ||
| Pubertal stage | ||||||
| Prepubertal | 67 (60.9) | 47 (42.7) | 0.005 | 91 (61.9) | 23 (31.5) | 0.000 |
| Pubertal | 40 (36.4) | 43 (39.1) | 54 (36.7) | 40 (54.8) | ||
| Post-pubertal | 3 (2.7) | 20 (18.2) | 2 (1.4) | 10 (13.7) | ||
| Degree of obesity | ||||||
| Obesity | 52 (47.3) | 66 (60) | 0.058 | 81 (55.1) | 37 (50.7) | 0.536 |
| Severe obesity | 58 (52.7) | 44 (40) | 66 (44.9) | 36 (49.3) | ||
| Glycemia ≥ 100mg/dL | 4 (3.6) | 5 (4.5) | 0.500 | – | – | |
| Insulin ≥ 15μU/mL | 35 (31.8) | 58 (52.7) | 0.002 | – | – | |
| TC ≥ 170 mg/dL | 44 (40) | 33 (30) | 0.079 | 53 (72.6) | 20 (27.4) | 0.101 |
| LDL-C ≥ 130 mg/dL | 12 (10.9) | 14 (12.7) | 0.417 | 64 (87.7) | 9 (12.3) | 0.869 |
| HDL-C ≤ 45 mg/dL | 58 (52.7) | 65 (59.1) | 0.208 | 25 (34.2) | 48 (65.8) | 0.044 |
| Triglycerides ≥ 130 mg/dL | 26 (23.6) | 24 (21.8) | 0.436 | 51 (69.9) | 22 (30.1) | 0.087 |
| Increased WC | 103 (93.6) | 94 (85.5) | 0.076 | 8 (11.0) | 65 (89) | 0.030 |
| IR | 27 (24.5) | 46 (41.8) | 0.007 | – | – | |
| Clinical and metabolic alterations | ||||||
| 0 | 2 (1.8) | 2 (1.8) | 0.991 | 0 (0) | 0.000 | |
| 1 | 19 (17.3) | 17 (15.5) | 2 (2.7) | |||
| 2 | 40 (36.4) | 36 (32.7) | 14 (19.2) | |||
| 3 | 23 (20.9) | 27 (24.5) | 25 (34.2) | |||
| 4 | 17 (15.5) | 18 (16.4) | 22 (30.1) | |||
| 5 | 8 (7.3) | 9 (8.2) | 9 (12.3) | |||
| 6 | 1 (0.9) | 1(0.9) | 1 (1.4) | |||
HOMA-IR, homeostasis model assessment for insulin resistance; TC, total cholesterol; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol; WC, waist circumference; IR, insulin resistance.
Males and females were similar regarding age (p=0.052), degree of obesity (p=0.058), WC increase (p=0.076), and alterations in levels of total cholesterol (p=0.079), LDL-C (p=0.417), HDL-C (p=0.208), and triglycerides (p=0.436). It was found that 15.5% of the males and 16.4% of the females had four clinical or metabolic alterations simultaneously, although no difference was observed between genders (p=0.991). IR was diagnosed in 33.20% of the sample (mean value of HOMA-IR index=3.26±2.67). The highest frequencies of IR were observed among adolescents (65.8%) and pubertal subjects (54.8%). There were associations between IR and low levels of HDL-C (p=0.044), increased WC measurement (p=0.030), and the number of clinical and metabolic alterations (p=0.000) (Table 1).
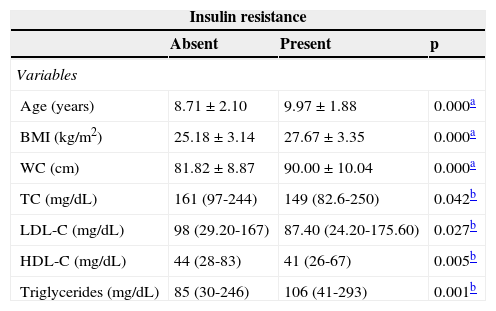
Table 2 demonstrates that the insulin resistant individuals had higher mean age (9.97±1.88 versus 8.71±2.10, p=0.000), BMI (27.67±3.14 versus 25.18±p=0.000), WC measurement (90±10.04cm versus 81.82±8.87cm p =0.000) and higher median triglyceride levels (85mg/dL (30-246mg/dL) versus 106mg/dL (41-293mg/dL) p=0.001) when compared with those who did not have IR. Exceptions were observed in relation to median total cholesterol (153.35±32.64mg/dL versus 162.70±29.99mg/dL, p ≤ 0.042); LDL-C (87.40mg/dL (24.20-175.60mg/dL) versus 98mg/dL (29.20-167mg/dL), p ≤ 0.027); and HDL-C (41mg/dL (26-67mg/dL) versus 44mg/dL (28-83mg/dL), p=0.005), which decreased in the presence of IR. In the distribution of clinical and metabolic variables of children and adolescents according to HOMA-IR quartiles, increases in mean BMI (p=0.000), WC measurement (p=0.000), and median triglycerides (p=0.002) were also observed as the values of HOMA-IR increased, except for HDL-C, which decreased (p=0.001).
Central tendency and dispersion values of the clinical and metabolic variables tendency of children and adolescents, according to the absence or presence of insulin resistance.
| Insulin resistance | |||
|---|---|---|---|
| Absent | Present | p | |
| Variables | |||
| Age (years) | 8.71±2.10 | 9.97±1.88 | 0.000a |
| BMI (kg/m2) | 25.18±3.14 | 27.67±3.35 | 0.000a |
| WC (cm) | 81.82±8.87 | 90.00±10.04 | 0.000a |
| TC (mg/dL) | 161 (97-244) | 149 (82.6-250) | 0.042b |
| LDL-C (mg/dL) | 98 (29.20-167) | 87.40 (24.20-175.60) | 0.027b |
| HDL-C (mg/dL) | 44 (28-83) | 41 (26-67) | 0.005b |
| Triglycerides (mg/dL) | 85 (30-246) | 106 (41-293) | 0.001b |
BMI, body mass index; WC, waist circumference; TC, total cholesterol; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol.
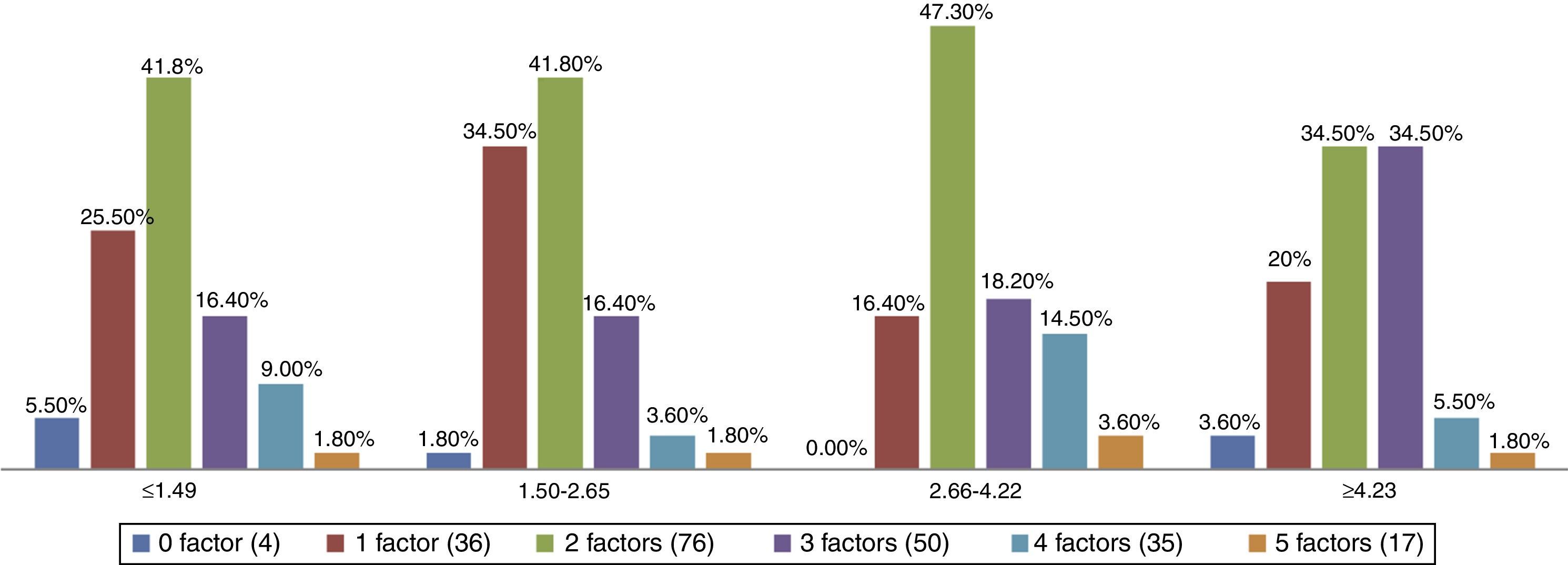
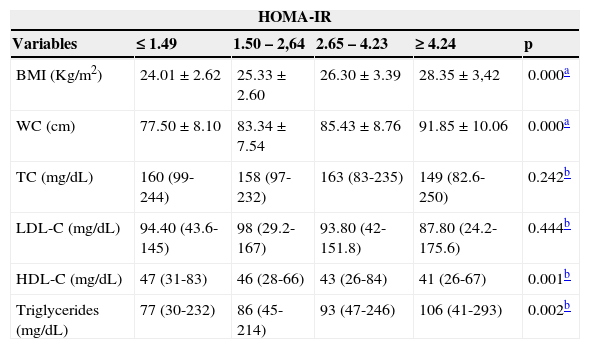
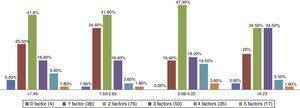
There were no significant differences between the values of total cholesterol (p=0.242) and the LDL-C and the HOMA-IR quartiles (p=0.444; Table 3). Those with the highest number of simultaneous clinical and metabolic alterations were between the second and third quartiles of the HOMA-IR index (p=0.000) (Fig. 1).
Clinical and metabolic profile of obese children and adolescents, according to quartiles of the HOMA-IR index.
| HOMA-IR | |||||
|---|---|---|---|---|---|
| Variables | ≤ 1.49 | 1.50 – 2,64 | 2.65 – 4.23 | ≥ 4.24 | p |
| BMI (Kg/m2) | 24.01±2.62 | 25.33±2.60 | 26.30±3.39 | 28.35±3,42 | 0.000a |
| WC (cm) | 77.50±8.10 | 83.34±7.54 | 85.43±8.76 | 91.85±10.06 | 0.000a |
| TC (mg/dL) | 160 (99-244) | 158 (97-232) | 163 (83-235) | 149 (82.6-250) | 0.242b |
| LDL-C (mg/dL) | 94.40 (43.6-145) | 98 (29.2-167) | 93.80 (42-151.8) | 87.80 (24.2-175.6) | 0.444b |
| HDL-C (mg/dL) | 47 (31-83) | 46 (28-66) | 43 (26-84) | 41 (26-67) | 0.001b |
| Triglycerides (mg/dL) | 77 (30-232) | 86 (45-214) | 93 (47-246) | 106 (41-293) | 0.002b |
HOMA-IR, homeostasis model assessment for insulin resistance; BMI, body mass index; WC, waist circumference; TC, total cholesterol; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol.
Prevalence of simultaneity of clinical and metabolic alterations in obese children and adolescents, according to quartiles of the HOMA-IR index.
Note: p=0.00 - Chi-square test with correction by Fisher's exact test. The clinical and metabolic risk factors are: Total Cholesterol ≥170mg/dL, LDL-C ≥130mg/dL, HDL-C ≤45mg/dL, triglycerides ≥130mg/dL and increased waist circumference measurement.
Childhood can be considered a critical period for the onset or continuity of obesity, and the starting point for the emergence and development of important clinical and metabolic changes, which, depending on the duration and severity, can impair the health in adulthood.21 When assessing the nutritional status of children and adolescents, it is important not only to know the chronological age, but also the degree of sexual maturity, especially when assessing the risk of persistence of obesity and associated morbidities, as the probability of a obese child to remain obese as an adult ranges from 20% to 50% prior to sexual maturation and from 50% to 70% after puberty.22
The present study showed that 42.7% of individuals had already started puberty, and that most of these children and adolescents, in addition to obesity, had important clinical and metabolic alterations, similar to those found in other studies that showed that increased BMI potentiates the onset of metabolic risk factors in this population. The finding of nine cases of impaired fasting glucose (one case among the children and eight cases among the adolescents) is an object of concern due to its early onset, as this alteration is rarely seen in children and adolescents, even in samples consisting only of obese individuals.
The prevalence of alterations in fasting insulin, and HDL-C and LDL-C lipid fractions are relevant, as studies have shown that hyperinsulinemia is an independent risk factor for the development of cardiovascular disease, by potentiating the onset of dyslipidemia. In this situation, there is a decrease in the capacity of insulin to stimulate glucose utilization by muscle and adipose tissue, causing damage to lipolysis suppression, a condition that increases the circulation of free fatty acids and further alters glucose transport to target tissues, inhibiting insulin action. Insulin resistance leads to increased fatty acid oxidation, providing substrate for the synthesis of triglycerides and increasing LDL-C release to serum.23,24 A study that evaluated the levels of serum insulin for eight years in children aged 5-9 years and young adults aged 17-23 years demonstrated that, among those with hyperinsulinemia, dyslipidemia cases were three-fold higher.25
Serrano et al. observed that overweight adolescents were 4.5-fold more likely to have alterations in HOMA-IR index and that these values were higher in adolescents with higher percentage of body fat.26 Costa et al. investigated the clustering of cardiovascular risk factors in 118 children and adolescents according to BMI quartiles and found that being overweight was associated with increased blood pressure, triglycerides, HOMA-IR index, and low HDL-C, comprising a pro-atherosclerotic profile in this population.27 In the present study, the identified prevalence of IR is consistent with that found by other authors in national and international studies, which confirms the severity of the problem.4,6,7 This condition was associated with female gender, adolescents, pubertal individuals, decrease in serum HDL-C, increased waist circumference, and the number of clinical and metabolic alterations found in the group.
The literature demonstrates that there is still no consensus on the cutoff of HOMA-IR for the assessment of children and adolescents, as these values tend to vary during these life stages. To establish a correlation between the cutoff and the associated risk, it is necessary to develop prospective studies that would consider, among other things, that the values of fasting insulin vary during childhood and adolescence, which demand a long period of observation. However, there is an agreement that research on the association between obesity and IR in children and adolescents may promote the early identification of factors that influence the development of cardiovascular disease and DM2.11–15
In this study, to adjust for the physiological IR that occurs during adolescence, it was decided to use the HOMA-IR cutoff of 3.43 suggested by Garcia Cuartero et al.,15 who assessed children and adolescents aged 1 month to 18 years by taking into account variations of this index for age and gender, also noting the pubertal stage according to the Tanner criteria.18
In the present study, when evaluating the values of the clinical and metabolic variables of these children and adolescents according to the presence or absence of IR, it was observed that those who were insulin-resistant had higher values of BMI, WC, and triglycerides, and decreased HDL-C levels, in agreement with other studies that also highlighted the association between obesity, IR, and metabolic alterations in children and adolescents.
Mieldazis et al., when investigating the association between BMI, HOMA-IR, and insulin levels in a group of pre-pubertal children, concluded that there is a strong association between hyperinsulinemia and obesity, and that the higher the BMI, the higher the HOMA-IR index.3 Madeira et al., when assessing the impact of obesity on the components of metabolic syndrome (MS) in children, found that obese children showed differences in mean HDL-C, HOMA-IR, serum insulin, glucose/insulin ratio, and waist circumference, demonstrating that obesity had a significant influence on the metabolism.13
Lavrador et al. observed that in a sample of 80 post-pubertal obese adolescents divided into lower and higher degrees of obesity, those with a higher degree of obesity had higher frequencies of alterations in blood glucose, HOMA-IR, triglycerides, HDL-C, and blood pressure, demonstrating that the degree of obesity influences the onset of clinical and metabolic alterations,28 differently from the present study, in which no significant differences were found between the degree of obesity by gender and presence of insulin resistance. The method of distributing the sample according to the quartiles of the HOMA-IR index of the assessed children and adolescents confirmed that the higher the values of HOMA-IR, the higher the values of BMI, WC measurement, and triglycerides, and the lower the values of HDL-C, in agreement with other studies such as that of Ferreira et al., which evaluated the association of BMI and IR with MS in Brazilian children. The MS identified in the obese individuals and many of the risk factors that comprise it were above the third quartile of the HOMA-IR index. These findings reinforce the likely participation of obesity and IR in the development of cardiovascular risk factors, as these were higher in the higher percentiles of BMI and HOMA-IR.7 In a case-control study that evaluated the association of risk factors for cardiovascular disease in 52 children with IR, it was observed that the obese children had a higher prevalence of MS. Those with higher IR had more metabolic risk factors, and MS was present in 17.3% of the assessed children. In the same study, the mean HOMA-IR index was significantly different for females (3.8±2.2, 95% CI: 2.9-4.8) when compared to males (2.6±1.3, 95% CI: 2.1-3.1), p=0.016.6
In the present study, the frequency of simultaneous occurrence of clinical and metabolic alterations was more often observed between the second and third quartiles. However, the mean values of HOMA-IR in this study were higher than those found by Medeiros et al.5 (2.4), Ferreira et al.6 (3.2), Weiss et al.29 (3.12 to 8.69, according to the degree of obesity), and Hirschler et al.30 (2.76), differences that may be in part explained by obesity. Other factors that were not addressed in the present study, such as time of exposure to obesity and eating habits, can strongly contribute to IR. Although there is no consensus on the diagnosis of IR in children and adolescents, it is recommended that isolated or combined clinical and metabolic alterations are monitored especially in obese individuals, considering that excess body fat is the most important risk factor for the development of non-communicable chronic diseases in adulthood.
In fact, the present study confirms the literature data by showing that IR is present in obese children and adolescents, and that this condition is associated with clinical and metabolic alterations. Despite the limitations inherent to all cross-sectional studies regarding the difficulty in defining the temporal sequence of the line of causality, the present findings contribute to a better understanding of the association between IR and metabolic effects frequently observed in obese children and adolescents. It is noteworthy that the observed association between IR and the variables analyzed in this study indicate an increased risk for the development of cardiovascular disease, DM2, and MS in adulthood for this group.
The monitoring of this population, with periodic reassessments of the analyzed parameters, can provide greater consistency to the results. Obesity is a chronic disease with important consequences that tend to worsen with overweight persistence and severity. Therefore, treatment should be carried out adequately, by a multidisciplinary team and always with the inclusion of the family. Possible targets should be set according to the reality of each patient, aiming at achieving a progressive reduction in BMI and maintenance of the results achieved in relation to nutritional status and improvement of clinical and metabolic alterations.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the multidisciplinary team of the Childhood Obesity Program of the City Hall of Osasco-SP, for their invaluable contribution to this study: Dr. Bianca Souza Maas, Program Coordinator; Dr. Ana Paula Franco Paiano, Pediatrician; Ms. Célia Regina Mota G. Santos, Social Worker, and Ms. Silvana de Jesus Baptista Alegret, Psychologist.
Please cite this article as: Romualdo MC, de Nóbrega FJ, Escrivão MA. Insulin resistance in obese children and adolescents. J Pediatr (Rio J). 2014;90:600–7.
Study conducted at Post-Graduation Course in Nutrition, Universidade Federal de São Paulo (UNIFESP), Escola Paulista de Medicina (EPM), São Paulo, SP, Brazil.