This study aimed at evaluating the predictors and outcomes associated with multidrug-resistant gram-negative bacterial (MDR-GNB) infections in an oncology pediatric intensive care unit (PICU).
MethodsData were collected relating to all episodes of GNB infection that occurred in a PICU between January of 2009 and December of 2012. GNB infections were divided into two groups for comparison: (1) infections attributed to MDR-GNB and (2) infections attributed to non-MDR-GNB. Variables of interest included age, gender, presence of solid tumor or hematologic disease, cancer status, central venous catheter use, previous Pseudomonas aeruginosa infection, healthcare-associated infection, neutropenia in the preceding 7 days, duration of neutropenia, length of hospital stay before ICU admission, length of ICU stay, and the use of any of the following in the previous 30 days: antimicrobial agents, corticosteroids, chemotherapy, or radiation therapy. Other variables included initial appropriate antimicrobial treatment, definitive inadequate antimicrobial treatment, duration of appropriate antibiotic use, time to initiate adequate antibiotic therapy, and the 7- and 30-day mortality.
ResultsMultivariate logistic regression analyses showed significant relationships between MDR-GNB and hematologic diseases (odds ratio [OR] 5.262; 95% confidence interval [95% CI] 1.282–21.594; p=0.021) and healthcare-associated infection (OR 18.360; 95% CI 1.778–189.560; p=0.015). There were significant differences between MDR-GNB and non-MDR-GNB patients for the following variables: inadequate initial empirical antibiotic therapy, time to initiate adequate antibiotic treatment, and inappropriate antibiotic therapy.
ConclusionsHematologic malignancy and healthcare-associated infection were significantly associated with MDR-GNB infection in this sample of pediatric oncology patients.
Este estudo visou avaliar os preditores e resultados associados às infecções por bactérias gram-negativas multirresistentes (BGN-MR) em uma unidade de terapia intensiva pediátrica oncológica (UTIP).
MétodosForam coletados dados com relação a todos os episódios de infecção por BGN que ocorreram em uma UTIP entre janeiro de 2009 e dezembro de 2012. As infecções por BGN foram divididas em dois grupos para comparação: 1) infecções atribuídas a BGN-MR e 2) infecções atribuídas a BGN não multirresistente. As variáveis de interesse incluíram idade, sexo, presença de tumor sólido ou malignidade hematológica, câncer, uso de cateter venoso central, infecção anterior por Pseudomonas aeruginosa, infecção hospitalar, neutropenia nos 7 dias anteriores, duração da neutropenia, tempo de internação antes da UTI, duração da internação na UTI e uso de quaisquer dos seguintes nos 30 dias anteriores: agentes antimicrobianos, corticosteroides, quimioterapia ou radioterapia. Outras variáveis incluíram: tratamento antimicrobiano inicial adequado, tratamento antimicrobiano definitivo inadequado, duração do uso de antibióticos adequados, tempo de início da terapia antibiótica adequada, mortalidade em 7 dias e mortalidade em 30 dias.
ResultadosAs análises de regressão logística multivariada mostraram relações significativas entre as BGN-MR e as doenças hematológicas (razão de chance (RC) 5,262; intervalo de confiança de 95% (IC de 95%) 1,282–21,594; p=0,021) e infecções hospitalares (RC 18,360; IC de 95% 1,778–189,560; p=0,015). Houve diferenças significativas entre os pacientes com BGN-MR e BGN não MR com relação às seguintes variáveis: recebimento de terapia antibiótica empírica inicial inadequada, tempo para início do tratamento antibiótico adequado e recebimento de terapia antibiótica inadequada.
ConclusõesA malignidade hematológica e a infecção hospitalar foram significativamente associadas à infecção por BGN-MR nessa amostra de pacientes pediátricos oncológicos.
Patients with cancer and hematologic malignancy are at high risk of infections. A number of factors contribute to this risk, including immunosuppression related to the disease and aggressive treatments, such as chemotherapy, radiation therapy, steroid use, and hematopoietic stem cell transplantation.1 As a result, infection remains a frequent complication in patients with cancer and is responsible for intensive care unit (ICU) admissions.1 However, with recent advances in cancer treatments and improvements in critical care, an increasing number of patients with hematologic malignancies are being admitted to the ICU.2 Despite the improvements in outcomes associated with improved care, mortality remains high in critically ill patients with cancer or hematologic malignancies, particularly in the presence of ICU-acquired nosocomial infections.2
Specifically, the rate of infections related to multidrug-resistant gram-negative bacteria (MDR-GNB) in patients with cancer is increasing globally.3 However, treatment options for MDR-GNB infections are often limited. Carbapenems are the drugs of choice for infections caused by extended-spectrum β-lactamase (ESBL)-producing microorganisms, but their use may not be appropriate in infections caused by Pseudomonas aeruginosa, Acinetobacter baumannii, or Stenotrophomonas maltophilia, for which resistance to carbapenems is increasing. There are very few new antimicrobial agents available for the treatment of MDR-GNB.4 Owing to the lack of novel agents to treat resistant infections, clinicians must use antibiotics judiciously and appropriately to limit the development of resistance.5
The presence of cancer, hematologic malignancy, and a prior or current ICU stay increases the risk of mortality in patients with infections due to MDR-GNB.6 In addition, GNB infection is associated with MDR in febrile neutropenic pediatric cancer patients.7 Previous studies have provided information about the risk factors for colonization or infection with MDR-GNB in select patient populations, such as transplant patients and those in intensive or long-term care.8,9 Studies involving cancer patients have focused on bloodstream infections. Limited information is available regarding the spectrum and microbiology of these infections in other sites, such as the urinary tract, respiratory tract, gastrointestinal tract, and skin. This is despite the fact that such infections are not rare.10
Little is known, in particular, regarding the risk factors and outcomes of MDR-GNB infections in an oncology pediatric ICU (PICU). Therefore, this study aimed at evaluating the risk factors and outcomes associated with MDR-GNB infections in children with cancer and/or hematologic diseases.
Materials and methodsStudy designA case–control study was performed in the National Cancer Institute (Instituto Nacional do Câncer [INCA]) PICU, Rio de Janeiro, Brazil. INCA is a tertiary oncology public hospital, and the six-bed PICU admits only patients with solid tumors and hematologic malignancies. The principal reasons for admission to the PICU are postoperative care and infectious complications in oncology patients.
Data were collected from all infection episodes related to GNB that occurred between January 1, 2009 and December 31, 2012 in PICU patients, aged between 0 months and 18 years, who were hospitalized for more than 24h. The Ethics Committee of Fluminense Federal University approved the study.
Variables included age, gender, the presence of a solid tumor or hematologic malignancy, cancer status, and the presence of a central venous catheter. Information prior to the date of GNB infection was also collected: previous infection related to P. aeruginosa, neutropenia in the preceding 7 days, length of neutropenia ≥3 days, duration of ICU stay >3 days, length of hospitalization before ICU admission, and healthcare-associated infection, in addition to the use of antimicrobial agents, corticosteroids, and chemotherapy and/or radiation therapy in the previous 30 days.
Infections caused by GNB were divided into 2 groups: (1) infections attributed to MDR-GNB and (2) infections attributed to susceptible GNB (non-MDR-GNB). The groups were similar with respect to the infection site. Patients were included more than once in the analysis for separate episodes of infection. Others variables included: appropriate initial antimicrobial treatment, definitive inadequate antimicrobial treatment, duration of appropriate antibiotic use, length of time to initiate adequate antibiotic therapy, 7-day mortality, and 30-day mortality.
Definition of termsAn episode of infection was defined as the isolation of GNB in the presence of compatible clinical signs or symptoms from 3 days before the date of PICU admission to the last day of hospitalization in the PICU. Healthcare-associated infections and those present on admission were included.
The diagnostic criteria of the Centers for Disease Control and Prevention were used. GNB were isolated from cultures of the blood, urine, stool, bronchoalveolar lavage, tracheal aspirate, liquor, or catheter tip during this time. The tracheal aspirate was collected from endotracheal tubes and the tracheostomy of patients who underwent mechanical ventilation.
The diagnostic criteria for pneumonia included purulent tracheobronchial secretion or new pathogenic bacteria isolated from tracheal aspirate and at least two of the following criteria: fever (temperature >38°C); leukocytes >12,000cells/mL or <4000cells/mL increase; a new and persistent (>48h) infiltrate detected on a chest radiograph; new onset or worsening cough or dyspnea or tachypnea; or worsening gas exchange. Threshold values for cultured specimens used in the pneumonia criteria were ≥104CFU/mL in tracheal aspirate or bronchoalveolar lavage.
Catheter cultures were performed when a catheter was removed for suspected intravascular catheter-related infection, unexplained sepsis or erythema overlying the catheter insertion site, or purulence at the catheter insertion site. At least one of the following signs or symptoms was required for the diagnosis of catheter-associated infection: fever (>38°C), pain, erythema, heat at the vascular site with no other recognized cause and >15 colonies cultured from an intravascular cannula tip using a semiquantitative culture method, and lack of a blood culture or no organisms cultured from blood.
The diagnostic criteria for urinary tract infection included a positive urine culture of ≥105CFU/mL and with no more than two species of microorganisms, and at least one of following signs or symptoms was required for diagnosis: fever (>38°C); dysuria; suprapubic tenderness; costovertebral angle pain or tenderness with no other recognized cause.
The diagnostic criteria for gastroenteritis included acute onset of diarrhea (liquid stools for >12h) with or without vomiting or fever (>38°C), no likely non-infectious cause (e.g., diagnostic tests, therapeutic regimen other than antimicrobial agents, acute exacerbation of a chronic condition, or psychological stress), and an enteric pathogen detected in a stool culture.
MDR-GNB infection was considered in the presence of ESBL-producing Enterobacteriaceae, microorganisms with intrinsic resistance mechanisms such as S. maltophilia and Elizabethkingia meningoseptica, or carbapenem-resistant GNB. Carbapenem-resistant P. aeruginosa and A. baumannii were considered MDR-GNB. Polymicrobial infection involved the isolation of more than one pathogen from a culture sample. Infection was classified as healthcare acquired when onset occurred >48h after admission to the study hospital (ward or PICU). Neutropenia was defined as an absolute neutrophil count <500/mm3 in the blood sample.
Hematologic malignancy included leukemias and lymphoma. Cancer status was classified into three categories: remission/controlled (patients in cancer remission or control who had undergone previous treatments, without evidence of recurrence according to the attending oncologist/hematologist), recent diagnosis (patients with active disease diagnosed within the previous three months with need for first-line anticancer treatment), and relapse/recurrence (patients with active disease with relapse or recurrence).
Initial antimicrobial treatment was considered inappropriate if the initial treatment regimen did not include at least one antibiotic active against the microorganism in vitro and when the antibiotic treatment did not commence on the date of the positive culture. In patients with ESBL-producing bacteria cultures, treatment with penicillin and cephalosporin was considered inappropriate. Definitive inadequate antimicrobial treatment occurred when the patient did not receive any antibiotic active against the microorganism in vitro during the period of hospitalization.
Death within 7 days or 30 days after the date of the positive culture was considered as 7-day or 30-day mortality, respectively.
Microbiological proceduresBacterial classification was performed by the INCA microbiology laboratory using the Vitek automated method (Bio-Merieux Inc., Marcy l’Etoile, France) and manually confirmed for bacterial isolate identification and antimicrobial resistance, as per the Clinical Laboratory Standard Institute (CLSI) standards.
Statistical analysisStatistical analysis was conducted using SPSS v.17 (SPSS Inc., Chicago, Illinois, USA). A value of p<0.05 was considered statistically significant. Continuous variables were presented as medians, and comparative analysis was conducted using independent t-tests. Chi-squared and Fisher's exact tests were used to compare categorical variables, and odds ratios (OR) with 95% confidence intervals (95% CI) were calculated. Univariate logistic regression analyses identified statistically significant variables (p<0.05) related to MDR-GNB infection for inclusion in the multivariate logistic regression analysis, which was performed using a stepwise model.
ResultsThere were 765 admissions to the PICU between January 1, 2009 and December 31, 2012. There were 101 episodes of GNB infection in 76 patients in 86 admissions, and 47 (46.5%) of these episodes were related to MDR-GNB infection.
The most frequently occurring pathogens among the MDR-GNB were as follows: A. baumannii, seven (17%); S. maltophilia, seven (15%); Enterobacter spp., seven (15%); and Klebsiella pneumoniae, seven (15%). The most frequently occurring pathogens among the non-MDR-GNB were as follows: P. aeruginosa, 22 (41%); A. baumannii, 14 (26%); and K. pneumoniae, eight (15%). Polymicrobial infection was present in one MDR-GNB patient (2.0%, n=1/47) and two non-MDR-GNB patients (3.8%, n=2/54).
Among the GNB infection episodes, MDR was found at the following frequencies for each of the bacterial strains: 100% (n=2/2) of Morganella morganii, 100% (n=1/1) of Citrobacter spp., 87.5% (n=7/8) of Enterobacter spp., 50% (n=5/10) of Escherichia coli, 46.6% (n=7/15) of K. pneumoniae, 36.4% (n=8/22) of A. baumannii, 18.5% (n=5/27) of P. aeruginosa, and 0% (n=0/2) of Proteus mirabilis. There were seven episodes of infection by S. maltophilia and four episodes of infection by E. meningoseptica, which are microorganisms with intrinsic resistance mechanisms. There were three episodes of polymicrobial infection: one of K. pneumoniae and non-MDR-GNB E. coli, one of Klebsiella oxytoca and non-MDR-GNB E. coli, and one of MDR P. aeruginosa and S. maltophilia.
In the MDR-GNB group, bacteria were most frequently isolated from the tracheal aspirate (n=17, 36.2%), followed by blood culture (n=8, 17%), urine culture (n=4, 8.5%), and catheter tip culture (n=3, 6.4%). In the non-MDR-GNB group, bacteria were most frequently isolated from the tracheal aspirate (n=22, 40.7%), urine culture (n=10, 18.5%), blood culture (n=10, 18.5%), and a combination of blood culture and catheter tip culture (n=4, 7.4%). There was concordance between isolate microorganisms, when GNB were isolated from a combination of different culture. 63.8% (30/47) of the MDR-GNB group and 70.3% (38/54) of the non-MDR-GNB group were mechanically ventilated patients.
The most frequent diseases in the group with MDR-GNB infection were central nervous system tumor (19%), neuroblastoma (15%), non-Hodgkin lymphoma (15%), and acute lymphoblastic leukemia (15%). In the group with non-MDR-GNB, the most frequent diseases were central nervous system tumor (52%), neuroblastoma (11%), rhabdomyosarcoma (6%), and Wilms tumor (6%). Only one patient in the study group had undergone a bone marrow transplant.
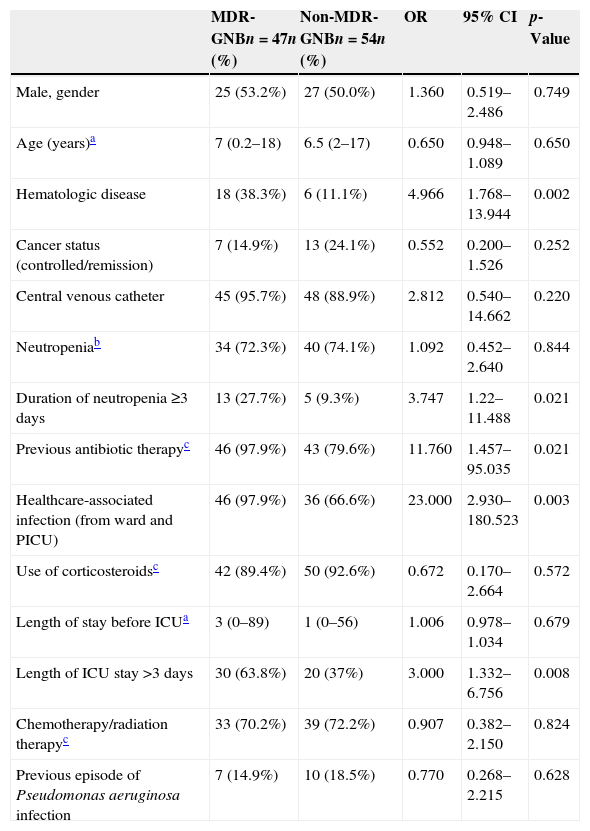
Demographic and clinical characteristics are provided in Table 1.
Demographic and clinical variables relating to multidrug-resistant gram-negative bacterial (MDR-GNB) infection in a pediatric intensive care unit (PICU), analyzed using univariate logistic regression.
| MDR-GNBn=47n (%) | Non-MDR-GNBn=54n (%) | OR | 95% CI | p-Value | |
|---|---|---|---|---|---|
| Male, gender | 25 (53.2%) | 27 (50.0%) | 1.360 | 0.519–2.486 | 0.749 |
| Age (years)a | 7 (0.2–18) | 6.5 (2–17) | 0.650 | 0.948–1.089 | 0.650 |
| Hematologic disease | 18 (38.3%) | 6 (11.1%) | 4.966 | 1.768–13.944 | 0.002 |
| Cancer status (controlled/remission) | 7 (14.9%) | 13 (24.1%) | 0.552 | 0.200–1.526 | 0.252 |
| Central venous catheter | 45 (95.7%) | 48 (88.9%) | 2.812 | 0.540–14.662 | 0.220 |
| Neutropeniab | 34 (72.3%) | 40 (74.1%) | 1.092 | 0.452–2.640 | 0.844 |
| Duration of neutropenia ≥3 days | 13 (27.7%) | 5 (9.3%) | 3.747 | 1.22–11.488 | 0.021 |
| Previous antibiotic therapyc | 46 (97.9%) | 43 (79.6%) | 11.760 | 1.457–95.035 | 0.021 |
| Healthcare-associated infection (from ward and PICU) | 46 (97.9%) | 36 (66.6%) | 23.000 | 2.930–180.523 | 0.003 |
| Use of corticosteroidsc | 42 (89.4%) | 50 (92.6%) | 0.672 | 0.170–2.664 | 0.572 |
| Length of stay before ICUa | 3 (0–89) | 1 (0–56) | 1.006 | 0.978–1.034 | 0.679 |
| Length of ICU stay >3 days | 30 (63.8%) | 20 (37%) | 3.000 | 1.332–6.756 | 0.008 |
| Chemotherapy/radiation therapyc | 33 (70.2%) | 39 (72.2%) | 0.907 | 0.382–2.150 | 0.824 |
| Previous episode of Pseudomonas aeruginosa infection | 7 (14.9%) | 10 (18.5%) | 0.770 | 0.268–2.215 | 0.628 |
CI, confidence interval; OR, odds ratio; ICU, intensive care unit.
The median neutropenia duration was 0 days in both groups (MDR-GNB infection, range 0–36; non-MDR-GNB infection, range 0–6; OR 3.747; 95% CI 1.222–11.488; p=0.021). The median of length of ICU stay was 8 days (0–80) in the group with MDR-GNB infection and 2 days (0–45) in the non-MDR-GNB infection group (OR 2.296; 95% CI 0.001–0.015; p=0.024).
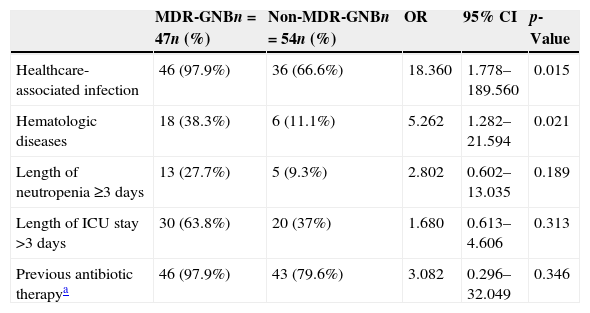
The results of the univariate logistic regression are described in Table 1. In the multivariate logistic regression analysis, the presence of hematologic diseases (OR 5.262; 95% CI 1.282–21.594; p=0.021) and healthcare-associated infection (OR 18.360; 95% CI 1.778–189.560; p=0.015) were significantly associated with MDR-GNB infection (Table 2).
Results of the multivariate logistic regression analysis for the outcome of multidrug-resistant gram-negative bacteria (MDR-GNB), including the variables that were statistically significant in the univariate logistic regression analysis.
| MDR-GNBn=47n (%) | Non-MDR-GNBn=54n (%) | OR | 95% CI | p-Value | |
|---|---|---|---|---|---|
| Healthcare-associated infection | 46 (97.9%) | 36 (66.6%) | 18.360 | 1.778–189.560 | 0.015 |
| Hematologic diseases | 18 (38.3%) | 6 (11.1%) | 5.262 | 1.282–21.594 | 0.021 |
| Length of neutropenia ≥3 days | 13 (27.7%) | 5 (9.3%) | 2.802 | 0.602–13.035 | 0.189 |
| Length of ICU stay >3 days | 30 (63.8%) | 20 (37%) | 1.680 | 0.613–4.606 | 0.313 |
| Previous antibiotic therapya | 46 (97.9%) | 43 (79.6%) | 3.082 | 0.296–32.049 | 0.346 |
CI, confidence interval; OR, odds ratio.
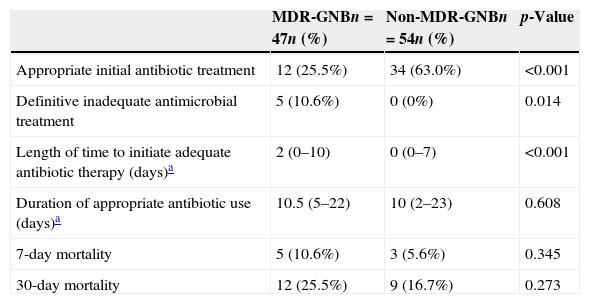
The others variables associated with MDR-GNB infection are detailed in Table 3. Patients with an MDR-GNB infection more frequently received inadequate initial empirical antibiotic therapy (74.5%) than those in the non-MDR-GNB group (37.0%, p<0.001), and the time to initiate adequate antibiotic therapy was longer for the MDR-GNB group (median: 2 days) than for the non-MDR-GNB group (median: 0 days, p<0.001). The MDR-GNB group was also significantly more likely to receive definitive inappropriate antibiotic therapy (10.6% vs. 0%, p=0.014).
Outcomes and others variables associated with the presence of multidrug-resistant gram-negative bacteria (MDR-GNB) in patients in a pediatric intensive care unit.
| MDR-GNBn=47n (%) | Non-MDR-GNBn=54n (%) | p-Value | |
|---|---|---|---|
| Appropriate initial antibiotic treatment | 12 (25.5%) | 34 (63.0%) | <0.001 |
| Definitive inadequate antimicrobial treatment | 5 (10.6%) | 0 (0%) | 0.014 |
| Length of time to initiate adequate antibiotic therapy (days)a | 2 (0–10) | 0 (0–7) | <0.001 |
| Duration of appropriate antibiotic use (days)a | 10.5 (5–22) | 10 (2–23) | 0.608 |
| 7-day mortality | 5 (10.6%) | 3 (5.6%) | 0.345 |
| 30-day mortality | 12 (25.5%) | 9 (16.7%) | 0.273 |
There is a lack of studies with pediatric oncology patients hospitalized in the ICU and with MDR-GNB infection. Hematologic malignancy and healthcare-associated infection emerged as important factors associated with MDR-GNB infection in this study.
Sepsis increases mortality in children with multiple organ dysfunction syndrome.11 There are wide regional variations in the incidence of each resistant pathogen, and these epidemiologic factors must be considered when making decisions about empiric antibiotics.5
Recent studies have reported high rates of MDR-GNB infection in the PICU or pediatric oncology patients. In children with hematological malignancies and febrile neutropenia, 51.6% of bacteremia episodes by K. pneumonia were resistant to ceftazidime.12 Moreover, in another study in pediatric oncology patients with febrile neutropenia, 50% of Acinetobacter spp., 44.4% of E. coli, 66.7% of K. pneumoniae, and 100% of P. aeruginosa were MDR.7 MDR cases were present in 57.1% of oncology pediatric patients with P. aeruginosa bacteremia13 and in 31.4% of P. aeruginosa bacteremia in children undergoing chemotherapy and hematopoietic stem cell transplantation.14
In the PICU, 30% of K. pneumonia15 and 23% of Enterobacteriaceae16 were ESBL-producing strains. Carbapenem resistance in pediatric patients in the ICU is also common, with resistance observed in 62% of A. baumannii.17
In the present study, MDR was detected in 50.0% of E. coli, 46.6% of K. pneumoniae, 36.4% of A. baumannii, and 18.5% of P. aeruginosa.
Recent studies in neutropenic children with cancer in different time periods have indicated an increase in the rate of infections related to A. baumannii.7 MDR A. baumannii are now considered one of the most challenging pathogens in critically ill patients,7 especially pediatric patients, due to the absence of new effective antimicrobial agents. In the present study, A. baumannii accounted for 21.8% (22/101) of all episodes of infection with GNB and were the most common MDR bacteria (17%, 8/47), confirming the importance of this etiologic agent in this population.
In this study, the most common types of infection in order of frequency were pneumonia, bloodstream infections, and urinary tract infections. Other studies have demonstrated that the main sites of healthcare-associated infections in PICU are pneumonia and bloodstream infection.18,19 Oncologic patients, when subjected to invasive procedures such as mechanical ventilation, are more susceptible to infectious complications than the general population.
In patients with solid tumor, the primary treatment is often surgical. However, patients with hematological malignancy often receive more aggressive chemotherapy, resulting in longer periods of neutropenia, more febrile neutropenia episodes, and more frequent antibiotic use. Furthermore, because hematological patients often present with specific pathophysiological conditions that may alter the pharmacokinetic behavior of antimicrobials, differing administration schedules and antimicrobial dosing regimens from the standard schedules and regimens may be required. Specifically, increased doses (e.g., aminoglycosides, fluoroquinolones), prolonged infusion (e.g., beta-lactams), and therapeutic drug monitoring are important to optimize the antimicrobial use. Pharmacokinetic and pharmacodynamic principles must be considered when designing antimicrobial regimens, as it has become increasingly clear that suboptimal concentrations at the site of infection may contribute to increased microbial resistance.20
The present results regarding healthcare-associated infections also support those reported by previous studies in children with cancer.21 The rates of nosocomial infections and antibiotic resistance are much higher in the PICU compared with other hospital units. Nosocomial infections affect up to 30% of patients in the ICU, and they occur five to ten times more often than in non-ICU patients.5 In a study of pediatric patients with nosocomial infection, the overall incidence of nosocomial infection was 2.5%, ranging from 1% in general pediatric units to 23.6% in the PICU.18 Several factors may account for these differences, including the use of more invasive procedures, poor hand hygiene, lapses in aseptic techniques, selective pressure for antibiotics due to inappropriate use, patient transfers within the hospital, severe underlying disease, malnutrition, and immunosuppression.5 In the present study, the length of ICU stay, previous antibiotic use, and neutropenia duration were associated with MDR infection in the univariate analysis, but not in the multivariate analysis, likely owing to the fact that these variables were inter-related with healthcare-associated infections or hematologic diseases that were strongly associated with MDR-GNB infection in the multivariate analysis.
Relationships between resistant bacterial infections and poor outcomes have been reported.5 One determining factor in the outcome of patients with infections is the choice of appropriate empirical therapy within the first 48h of the initial symptoms and within the first 24h of a positive blood culture.4 In the current study, the group with MDR-GNB infections received inappropriate empirical antibiotic therapy more frequently than the group without MDR-GNB infections, and the time to receive adequate therapy was longer in the patients with MDR-GNB infections. There was also a trend for greater 7- and 30-day mortality in the patients with MDR-GNB; however, this was not statistically significant.
A previous study in pediatric cancer patients also reported no statistically significant differences in infection-related or all-cause mortality due to MDR-GNB infections.21
The relationship between the timeliness of adequate therapy and prognosis is complex. Physicians tend to prescribe early extended-spectrum antimicrobial therapy to patients with severe clinical presentations. In contrast, antimicrobial therapy is often delayed in patients who do not present with organ dysfunction or who have delayed positivity of blood cultures. Finally, patients with a rapid progression to death never receive antimicrobial therapy.22
In this study, the median time to initiate adequate antibiotic therapy was 2 days in children with MDR-GNB infection, with no impact on mortality. Studies regarding the effect of a delay to initiate adequate antibiotics on mortality in pediatric patients are lacking. However, in studies with adults, a delay of 2–3 days to initiate adequate antibiotics had little effect on patient mortality.23–26
The strengths of the current study include the population, which had a combination of risk factors for MDR-GNB infections: cancer and an ICU stay. Furthermore, the knowledge of risk factors for MDR-GNB might help clinicians identify patients who require more attention during the empirical prescription of antimicrobial therapy, special monitoring, and infection control measures.
This study had certain limitations, including the collection of retrospective data in a small sample. Despite this, there were no missing data, and significant differences were observed.
ConclusionPediatric patients with hematological cancer can experience healthcare-associated infection suspected to be associated with MDR-GNB. This reinforces the need for training about the transmission risks and preventive measures for health professionals (e.g., equipment handling techniques). Contact precautions for all patients with colonization or infection with MDR pathogens and an antibiotic protocol based on local epidemiology should be implemented.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: de Oliveira Costa P, Atta EH, da Silva AR. Infection with multidrug-resistant gram-negative bacteria in a pediatric oncology intensive care unit: risk factors and outcomes. J Pediatr (Rio J). 2015;91:435–41.