The aim of our study was to evaluate the expression of MMP-2 and MMP-9 as a prognostic factor in patients diagnosed with Hodgkin Lymphoma (HL).
MethodsIn the present study, 45 paraffin biopsies from patients up to 19 years old diagnosed with HL were used in two referral hospitals in the state of Pernambuco, Brazil. Risk groups were classified into favorable and unfavorable, according to Ann Arbor. The expression of matrix metalloproteinases 2 and 9 and their inhibitors was performed by immunohistochemistry (IHC). Data were analyzed using the GraphPad Prism 5 program.
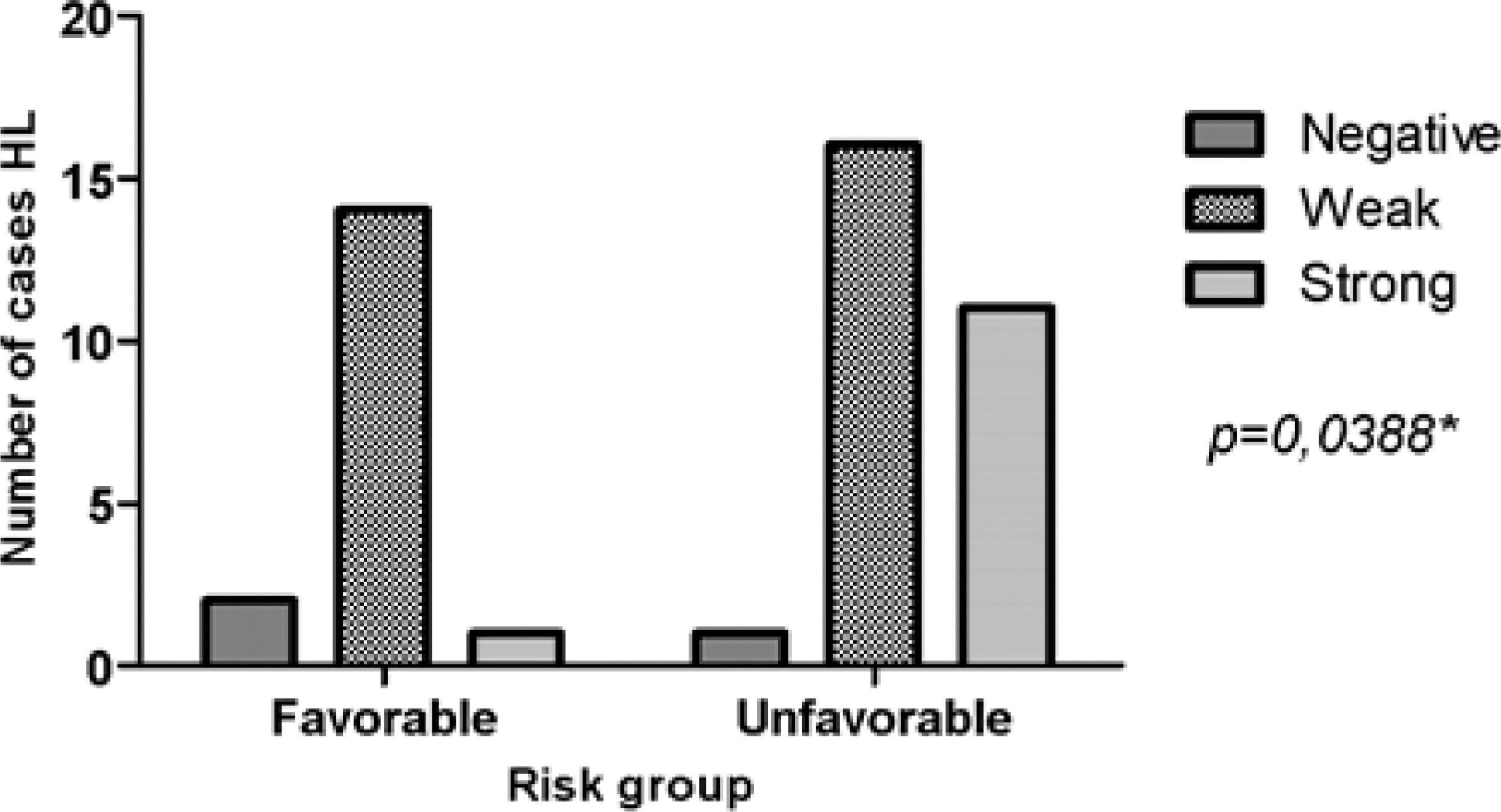
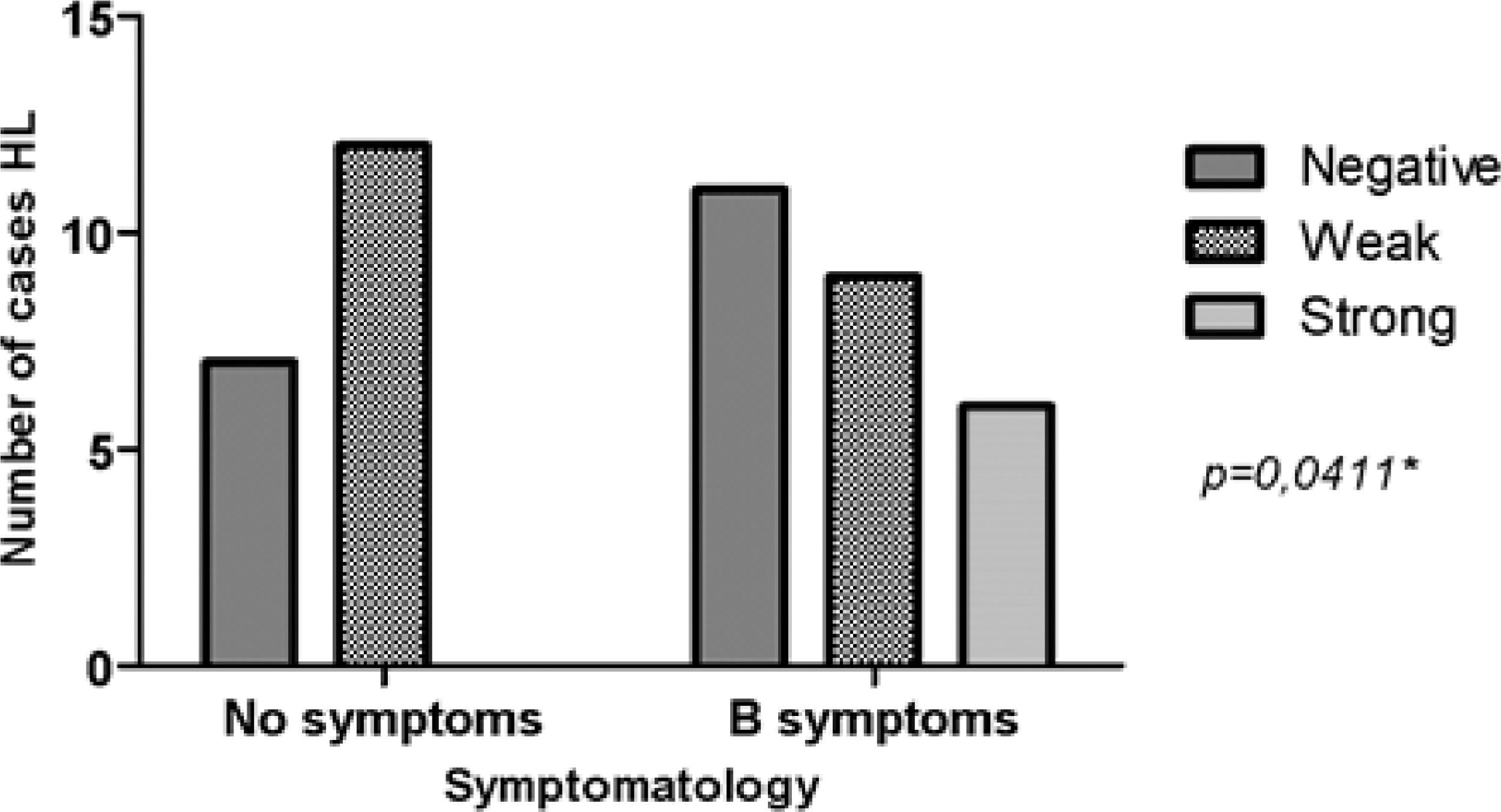
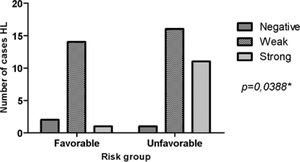
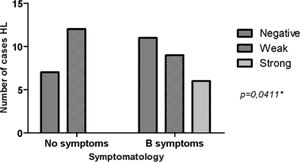
ResultsMMP-2 intensity pattern was stronger (>10% of the total field) in patients with stage III/IV and B symptoms. MMP-2 showed an association with the risk group (p = 0.0388). That is, the stronger the MMP-2 marking, the greater the unfavorable risk. However, for MMP-9 there was no difference in the stronger intensity pattern in relation to stages I/II and III/IV, only in the presence of B symptoms. MMP-9 showed an association with B Symptoms (p = 0.0411). Therefore, patients with B symptoms have higher MMP-9 expression.
ConclusionOur results suggest that MMP-2 expression is associated with HL progression. While MMP-9 expression is related to the clinical worsening of these patients. However, further studies are needed to evaluate the exact role of these proteins in hematologic malignancies.
Hodgkin's lymphoma (HL) is a rare malignant neoplasm that affects the lymphatic system,1,2 characterized by clonal proliferation of T or B lymphocytes in different stages of differentiation.3 Based on the characteristics of tumor cells, HL is divided into two distinct disease groups: classic Hodgkin's lymphoma (CHL) consisting of the subtypes: nodular sclerosis, mixed cellularity, lymphocyte rich, and lymphocyte depleted; and nodular lymphocytic predominance Hodgkin's lymphoma (NLPHL).2,4 NLPHL is the rarest form of the disease, with approximately 5% of all cases of HL, while CHL affects most cases.5 When diagnosed, 80% of NLPHL cases present with early or localized disease (I or II) without B symptoms,6 whereas CHL mostly includes patients in the more advanced stages (III or IV), presence of B symptoms, associated with the presence of Epstein-Barr virus (EBV) and unfavorable prognosis.6,7 The determination of HL dissemination capacity is extremely important in the clinical management, treatment, and prognosis of the patient.3 In an attempt to establish risk-adapted therapies, HL is divided into clinical risk groups taking into account staging and presence or absence of B symptoms, which are recognized by most treatment protocols: favorable (I, IIA and IIIA) and unfavorable (IIB, IIIB, and IV).8
Matrix metalloproteinases (MMPs) and their inhibitors (tissue matrix metalloproteinase inhibitors, TIMPs) play essential roles in extracellular matrix remodeling.9 However, the expression of metalloproteinases (MMP-2 and MMP-9) in malignant tumors is commonly associated with processes of cell differentiation, invasion, and metastasis.10,11 In addition to being directly involved in the prognosis of different neoplastic processes.10,12-14 MMP-9 expression can also be mediated by Epstein-Barr virus (EBV) infection,15 which is associated with classic Hodgkin's lymphoma (CHL) in about 40% of cases.7
TIMPs have a protective factor against tumor progression due to their ability to inhibit metalloproteinases.16,17 In lower tumor stages, the number of TIMPs is greater than that of MMPs, and the reverse has been observed in more advanced staging, promoting exacerbated degradation of the extracellular matrix by increased tumor cell proliferation and metastasis.14
The aim of this study was to determine the expression of MMP-2 and MMP-9, in Hodgkin's lymphoma patients and to evaluate their value as prognostic factors.
Materials and methodsSamples and data collectionIn the present study, 50 paraffin blocks were used in patients up to 19 years old, diagnosed with LH from November 1986 to December 2016 at both the Oswaldo Cruz University Hospital and the Pernambuco Cancer Hospital, Brazil. The clinical and biological data of these patients were collected by analysis of outpatient records and used in the present study. The clinical variables analyzed were: age, gender, histopathological diagnosis, staging, presence of B symptoms, clinical risk group, EBV detection, and response to treatment.
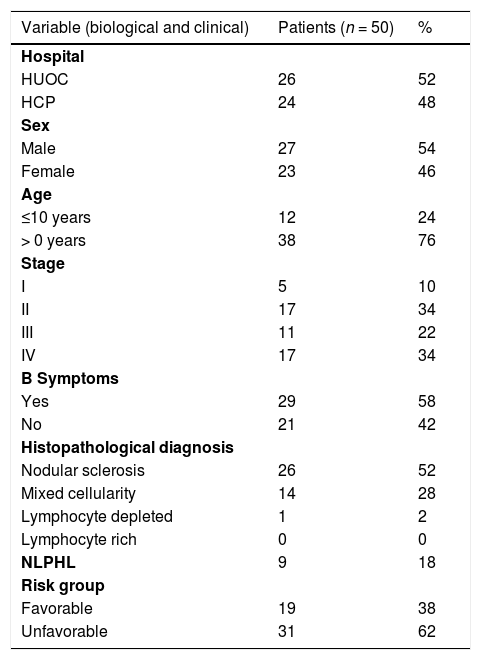
Stratification of risk groupsPatients were stratified according to the Ann Arbor system.11 Regarding the disease risk group, patients were classified as favorable (I, IIA and IIIA, without B symptoms) and unfavorable (IIB, IIIB and IV, with B symptoms. These parameters were also used in Table 1.
Clinical, biological characteristics of Hodgkin's lymphoma patients.
B symptoms: fever greater than 38°C intermittent, night sweats, and loss of at least 10% of body weight in the last 06 months. NLPHL: Hodgkin's lymphoma nodular lymphocytic predominance.
All 10% formalin-fixed, paraffin-embedded HL specimens from the cases selected for this study were submitted to 4µm histological sections placed on silanized glass slides for immunohistochemistry (IHC), following the protocol proposed by Lopes et al.18 and Pedroso et al.,19 with minor modifications according to the standards of the Laboratory of Cytological and Molecular Research of the Federal University of Pernambuco, Brazil.
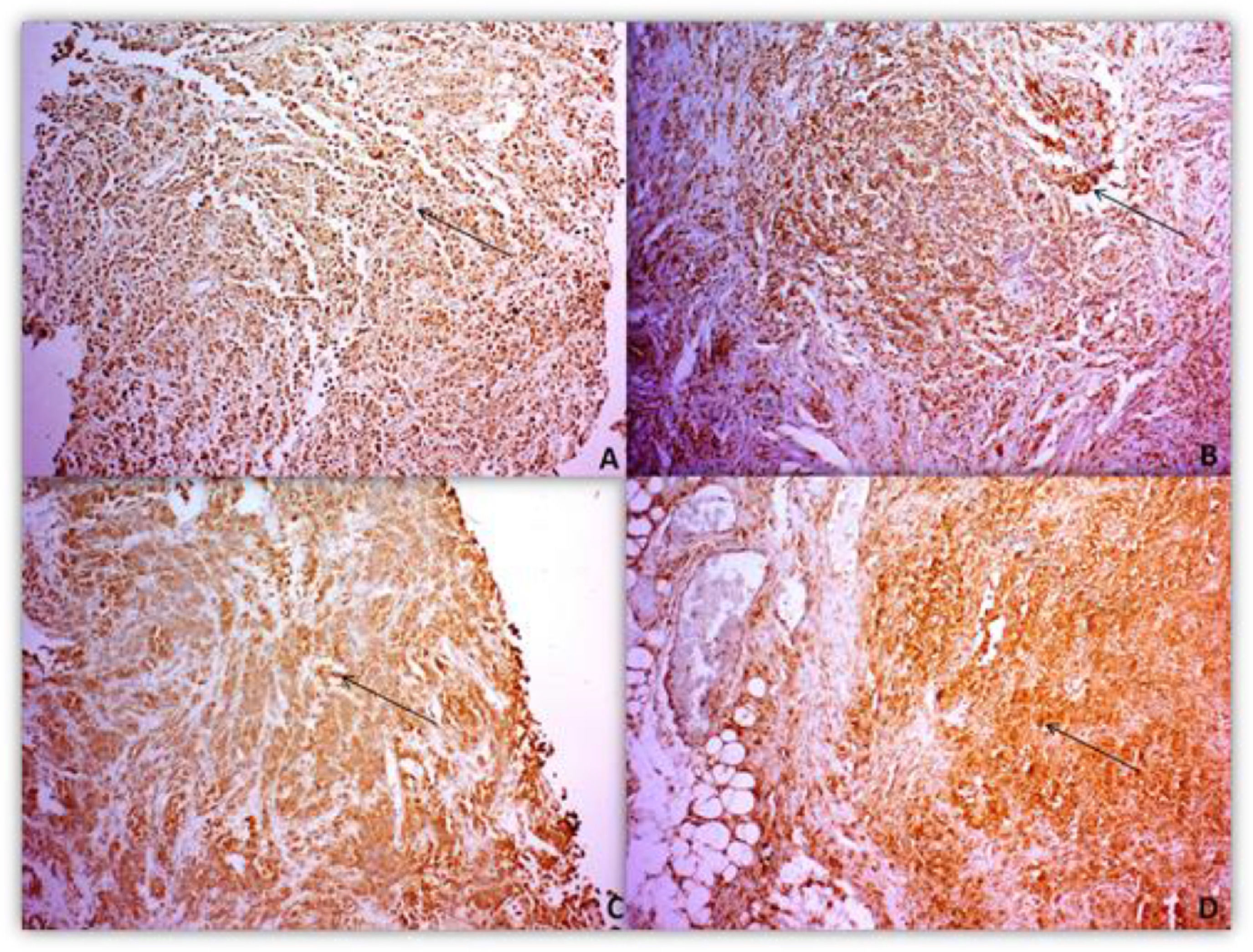
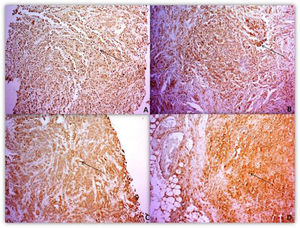
Primary monoclonal antibodies directed to matrix metalloproteinases (MMP-2 and -9) and their inhibitors (TIMP-1 and -2) were used to detect such proteins in the tumor microenvironment. IHC reactivity was based on location and intensity pattern. In the intensity parameter, a gradation was established consisting of: (-) negative (no signs of reactivity), (+) weak (with reactivity in up to 10% of the field and in up to 50% of the field, and (++/+++) strong with reactivity above 50%. While localization was performed by detecting the protein at one or more sites, either in the nucleus, nuclear membrane, cytoplasm and/or cytoplasmic membrane of the cells under study. Analyzes were recorded by the AxioVisio ZEISS® optical image capture system.
Statistical analysisThe data collected regarding the medical records and marking patterns of metalloproteinases and their inhibitors were tabulated using Excel® software for further analysis. Fisher's exact test was used to evaluate associations between categorical variables analyzed. To verify the association of the intensity pattern of MMP-2 and MMP-9 with the clinical risk group and the presence of B symptoms, the chi-square test was used. The confidence interval (CI) adopted was 95% with a significance level of p <0.05. GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA) was used for data analysis.
Ethical ConsiderationsThe ethical aspects are in accordance with Resolution 466/2012 of the National Health Council, where the bioethical principles were respected. The project was approved by the Ethics Committee of the Oswaldo Cruz Hospital Complex and Cardiac Emergency Room of Pernambuco (CAAE 43297515.6.000.5192, opinion 1.19857) and the Pernambuco Cancer Hospital (Opinion 10.894.988 / 0001-33).
ResultsClinical and biological characteristicsOf the 50 paraffin samples analyzed, 52% (26/50) were from the Oswaldo Cruz Universitário Hospital and 48% (24/50) from the Pathology Department of the Pernambuco Cancer Hospital, Brazil. According to the information present in the medical records, 54% of the patients (27/50) were male (M:F 1.17:1). Age at diagnosis ranged from 4 to 19 years (mean 13.5 years), with 76% of cases aged >10 years. Most patients had stage II or IV with 34% (17/50). The presence of B symptoms was observed in 58% (29/50) of the cases. Nodular sclerosis was the subtype of most patients with 52% (26/50), followed by mixed cellularity with 28% (14/50), while nodular lymphocytic predominance was observed in 18% (9/50). As for the risk group, 62% (31/50) were unfavorable (Table 1).
To evaluate the distribution between the clinical and biological characteristics of the patients, some variables were categorized into groups: age (≤10 and >10 years),20 risk group (favorable and unfavorable),20 histopathology (CHL and NLPHL)20 and staging (I/II and III/IV).8
The age variable was associated with gender (p = 0.0029). The authors observed that females were associated with the development of HL in the age group older than 10 years. B symptoms, with staging (p = 0.0442) and risk group (p < 0.0001). It was observed that patients with advanced staging (III/IV) were associated with the presence of B symptoms and unfavorable risk groups. Histopathology was associated with staging (p = 0.0065). Indicating that CHL was associated with advanced stages of the disease when compared to NLPHL. However, the risk group (p = 0.0670) and B symptoms (p = 0.4642) showed no association with histopathology.
Of the 50 medical records analyzed, 23 had information on the presence of EBV, of which 9 were positive and 14 negatives. This fact caused a limitation in our research, since it was not possible to determine the presence of EBV in the other patients included in the present study.
Regarding the response to treatment, 84% (42/50) of cases had complete remission of the disease during treatment, while 16% (8/50) had relapses. Forty-nine patients were alive and had a complete response (98%) and one (2%) died due to the evolution of the disease.
The intensity pattern of MMPs and TIMPsThe intensity pattern of immunohistochemistry (IHC) analyses ranged from negative to very intense. The staining reactivity indicates the presence of protein in the tissue (Fig. 1).
Of the 50 paraffin samples submitted to IHC, 5 were excluded because of damaged material. The clinical characteristics of the patients with HL and the MMP-2, MMP-9 protein intensity pattern, and their inhibitors were analyzed. The intensity parameter used for IHC assessment was: negative, weak and strong. The intensity pattern of MMP-2 was stronger in patients with advanced stages of the disease (III/IV) and the presence of B symptoms. However, for MMP-9, there was no difference in the strong intensity pattern associated with staging, but only in the presence of B symptoms. TIMP-1 presented a strong standard, differing from the standard found for MMP-9 in relation to staging. TIMP-2 presented a strong intensity pattern different from MMP-2 regarding staging and B symptoms.
MMP-2 showed significant relevance with an unfavorable risk group (p = 0.0388). However, it was not related to the presence of B symptoms (p = 0.2957). MMP-9 showed adverse results in relation to the same parameters, risk group (p = 0.8057) and B symptoms (p = 0.0411), as observed in Figs. 2 and 3, respectively. However, TIMP-1 and TIMP-2 showed no significant association for the risk group (p = 0.6880; p = 0.6496) and B symptoms (p = 0.1468; p = 0.9287), respectively.
DiscussionThis is the first study that proposed to evaluate the activity of MMP-2 and MMP-9 proteins and their inhibitors (TIMP-1 and TIMP-2) in children with Hodgkin's lymphoma in Brazil.
The strong intensity pattern was divergent in both metalloproteinases and their inhibitors regarding staging. The authors believe that TIMP-1 expression increases with disease progression, and therefore inhibits MMP-9 expression, preventing HL progression, as expected by the literature.21 However, as staging increases TIMP-2 expression decreases, resulting in increased MMP-2, and consequently disease progression.14,22 MMP-2 was associated with the unfavorable clinical risk group (p = 0.0388), and MMP-9 with B symptoms (p = 0.0411). HL carcinogenesis may be associated with increased expression of MMP genes and increased enzymatic activity of their products.14 MMP-2 and MMP-9 are proteins involved in angiogenesis and are able to contribute to tumor growth and invasion leading to rapid degradation of the extracellular matrix by increasing mRNA expression.14
However, studies report that strong MMP-2 expression correlates with a favorable prognosis, while MMP-9 expression showed adverse outcomes. The authors believe that the adverse role of MMP-9 may be associated with the control of immunological processes, but not the likelihood of tumor invasion or neovascularization.23 Other studies report that MMP-2 expression is also related to better disease prognosis MMP-9 to decreased survival, and TIMP-1 to tumor growth.23,24 However, these studies were performed in an adult population, where hormonal behavior may be influencing the expression of these molecules.23,24
Studies suggest that there is a relationship between MMP-2 expression and a worse prognosis for Non-Hodgkin's Lymphoma (NHL), but in others, this relationship is not observed. This relationship between MMP-2 expression and better or worse prognosis can be explained by the presence of polymorphisms located in the promoter region of the MMP-2 coding gene, which is associated with higher or lower protein production.25-27
Other authors discuss this discrepancy between higher MMP-2 expression in aggressive lymphomas and non-detection of tissue protein. They believe that perhaps the MMP-2 produced remains stored in the cell for a long time, and eventually is not secreted but captured directly from the producing cell by the tumor cells.23
Our results indicate that increased MMP-2 protein expression is associated with the unfavorable risk group. Increased MMP-9 expression is related to the risk of developing B symptoms, resulting in a worse prognosis for these patients.
Therefore, the authors believe that MMP-2 may be influencing extracellular matrix degradation leading to disease progression and that MMP-9 is related to the patient's clinical worsening due to the presence of B symptoms. The authors also suggest that these proteins may be used as a marker of poor prognosis for HL. The advantage is that these proteins can be dosed in serum, which could aid in the sensitivity and specificity of the biopsy as well as in evaluating treatment response.
Although TIMPs inhibit MMPs, their properties differ slightly. For example, while TIM-2 expression is constitutive, the other members of this family have inducible expressions and are often tissue-specific.28 Several molecules are capable of inducing TIMP expression including growth factors, cytokines, erythropoietin, and interleukins.29
A study by Cardeal et al.,30 analyzed the effect of HPV-16 oncoproteins on MMP-2, MMP-9, and TIMP-2 expression in keratinocyte culture, and found that E7 protein was associated with increased MMP-2 activity, while oncoproteins E6 and E7 participated in the decrease in TIMP-2 levels. The authors believe that TIMP-2 may be negatively regulated by the acute expression of HPV oncoproteins, which may favor unregulated MMP-2 activity in the context of HPV infection.30 Based on this theory, the authors believe that because EBV is also considered an oncogenic virus, its presence may be negatively regulating TIMP-2 activity. Thus, TIMP-2 would not inhibit MMP-2 activity, and its strong expression could lead to a poor prognosis.
Our analysis regarding prognosis was performed encompassing all subtypes of Hodgkin's Lymphoma. The main objective was to evaluate the expression of matrix metalloproteinases 2 and 9 in patients with HL. However, the comparison between the main groups within the disease (Classic Hodgkin's Lymphoma and Nodular Lymphocytic Predominance Hodgkin's Lymphoma) was performed to observe the expression levels of the studied metallo in relation to the two main groups of Hodgkin's Lymphoma.
ConclusionOur results suggest that MMP-2 expression is associated with Hodgkin's lymphoma progression. While MMP-9 expression is related to the clinical worsening of these patients. However, further studies are needed to evaluate the exact role of these proteins in hematologic malignancies.
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil – (CAPES) – Cod. 001.
Study conducted at the Universidade de Pernambuco, Recife, PE, Brazil.