To compare mortality and morbidity in very low birth weight infants (VLBWI) born to women with and without diabetes mellitus (DM).
MethodsThis was a cohort study with retrospective data collection (2001–2010, n=11.991) from the NEOCOSUR network. Adjusted odds ratios and 95% confidence intervals were calculated for the outcome of neonatal mortality and morbidity as a function of maternal DM. Women with no DM served as the reference group.
ResultsThe rate of maternal DM was 2.8% (95% CI: 2.5-3.1), but a significant (p=0.019) increase was observed between 2001-2005 (2.4%, 2.1-2.8) and 2006-2010 (3.2%, 2.8-3.6). Mothers with DM were more likely to have received a complete course of prenatal steroids than those without DM. Infants of diabetic mothers had a slightly higher gestational age and birth weight than infants of born to non-DM mothers. Distribution of mean birth weight Z-scores, small for gestational age status, and Apgar scores were similar. There were no significant differences between the two groups regarding respiratory distress syndrome, bronchopulmonary dysplasia, intraventricular hemorrhage, periventricular leukomalacia, and patent ductus arteriosus. Delivery room mortality, total mortality, need for mechanical ventilation, and early-onset sepsis rates were significantly lower in the diabetic group, whereas necrotizing enterocolitis (NEC) was significantly higher in infants born to DM mothers. In the logistic regression analysis, NEC grades 2-3 was the only condition independently associated with DM (adjusted OR: 1.65 [95% CI: 1.2 -2.27]).
ConclusionsVLBWI born to DM mothers do not appear to be at an excess risk of mortality or early morbidity, except for NEC.
Comparar mortalidade e morbidade em crianças de muito baixo peso (MBP) filhas de mães com e sem diabetes mellitus (DM).
MétodosEstudo de coorte com coleta retrospectiva de dados (2001 - 2010, n=11.991)da rede NEOCOSUR. Odds ratios ajustados foram calculados para mortalidade e morbilidade neonatal em função da DM materna. Mulheres sem DM serviram como grupo de referência.
ResultadosA taxa de DM materna foi 2,8% (IC 95% 2,5-3,1), mas um aumento significativo (p=0,019) entre 2001-2005 (2,4%) e 2006-2010 (3,2%) foi observado. As mães com DM eram mais propensas a terem recebido um curso completo de esteróides pré-natais que as sem DM. Os bebês de mães diabéticas tinham uma idade gestacional e peso ao nascer um pouco maior do que crianças filhas de não DM. Distribuição dos z escores de peso ao nascer, pequeno para idade gestacional e escores de Apgar foram semelhantes. Não houve diferenças significativas entre os dois grupos em termos de síndrome do desconforto respiratório, displasia broncopulmonar, hemorragia intraventricular, leucomalácia periventricular e persistência do ductus arteriosus. Mortalidade na sala de parto, mortalidade total, necessidade de ventilação mecânica e as taxas de sepse neonatal precoce foram significativamente menores no grupo diabético, enquanto enterocolite necrosante (NEC) foi significativamente maior em recém-nascidos de mães diabéticas. Em análises de regressão logística NEC foi a única condição independentemente associada com DM (OR ajustado 1,65 [IC 95% 1,21 -2,27]).
ConclusõesCrianças de MBP de mães com DM não têm aumento do risco de mortalidade ou morbidade precoce, exceto NEC.
Diabetes mellitus (DM) is the most common medical condition causing complications during pregnancy. It is estimated that 0.2% to 0.3% of all pregnancies are complicated by pregestational DM, and another 1% to 5% by gestational DM.1
Numerous studies indicate that the rates of perinatal complications among diabetic women are still substantially higher than those of the general population.2
Although there has been considerable progress in the care of diabetic pregnant women, the risk of premature delivery remains high.3 The exact incidence of prematurity in the pregnant diabetic women is controversial. A large series reported that 36% of infants born to mothers with gestational DM or those with pre-existing insulin-dependent DM were born before term, compared to 9.7% in the general population.4
Adequate pre-conceptional and gestational care reduces the frequency of congenital malformations and improves pregnancy outcomes.5
Despite substantial reductions in morbidity and mortality rates achieved with recent advances in neonatal care, prematurity remains the single most important determinant of neonatal morbidity in diabetic pregnancies.6 Although a large number of investigators have examined the influence of various perinatal risk factors on the outcome of very low birth weight infants (VLBWI), studies that specifically has focused on the outcome of VLBWI born to diabetic mothers are scarce.7–9 Furthermore, most of these data were from centers with a special interest in diabetes and pregnancy and no difference was observed between pre-gestational and gestational DM.
The present study aimed to compare mortality rates and early and late morbidity rates in VLBWI born to women with and without DM in a regional birth cohort over a ten-year period.
MethodsData collectionThe NEOCOSUR South American Network (http://www.neocosur.org/neocosur/) is a voluntary nonprofits association of neonatal intensive care units (NICUs) from six South American countries (Argentina, Brazil, Chile, Paraguay, Peru, and Uruguay), whose primary objective is to improve neonatal health. Briefly, this network provides a continuously updated database that prospectively gathers information from all live-born VLBWI (birth weight ranging from 500g to 1,500g) from the participating centers.
A structured form is completed for each infant using predefined diagnostic criteria on maternal demographic details, pregnancy history and antenatal care, delivery, infant's status at delivery, diagnoses, procedures and complications during hospitalization, and outcome at discharge. Since 2001, data is prospectively and routinely collected and entered online at the NEOCOSUR Network Center. The data have been extensively validated, and the analyses of subsets have been reported in many articles to date.10,11
This was a cohort study with retrospective data collection gathered at the NEOCOSUR Network between 2001 and 2010. To screen for and diagnose DM, the World Health Organization (WHO) protocol was employed, because it is more inclusive and simple, with an 75-g oral glucose tolerance test recommended at 24–28 weeks for all women with risk factors for gestational DM.12 As this was a study across 22 maternity units, this criteria was not universally followed, and some individual centers used local criteria to screen for and diagnose DM.
Outcome measuresThe gestational age (GA) in completed weeks was defined as the best estimate of GA on the basis of last menstrual period and early prenatal ultrasound examination. Prematurity was defined according to WHO13 and was classified into the following subgroups: extremely preterm (< 28 weeks), very preterm (28-31 weeks), and late or moderately preterm (32-36.6 weeks).
Because the entry cutoff in the database is determined by a birth weight of ≤ 1,500g, and since small for GA (SGA) infants might theoretically be overrepresented, the authors chose to include only those infants who were delivered before 36 completed weeks in this analysis, which resulted in the exclusion of 20 infants. Twenty-two infants below 22 weeks of gestation were also excluded, because most of them did not survive. Three additional infants were excluded due to unknown GA, and 110 for unknown DM status of the mother. Thus, 10,867 deliveries and 11,991 infants were included in the analysis.
The gender-specific birth weight Z-score was calculated in accordance with a local intrauterine growth chart.14 According to Gruenwald,15 SGA and large for GA (LGA) were defined when the weight for GA and sex were less or more than two Z scores apart from its expected median, respectively. Results for SGA (n=308) and LGA (n=1,055) are only presented for GA 22-32 weeks, in order to prevent overrepresentation of both conditions.
Respiratory distress syndrome (RDS) was diagnosed according to clinical and radiologic criteria. Necrotizing enterocolitis (NEC) was diagnosed by the presence of clinical and radiologic characteristics according to the criteria of Bell et al.16 Only definite NEC (Bell grades 2 and 3) cases were included. Intraventricular hemorrhage (IVH) and periventricular leukomalacia (PVL) were diagnosed using cranial ultrasonography, and IVH was graded using the classification of Papile et al.17 Bronchopulmonary dysplasia (BPD) was defined according to the criteria of Bancalari & Claure, including clinical and radiologic features together with the requirement of oxygen supplementation at 28 days of age or at 36 weeks of postmenstrual age.18 Patent ductus arteriosus (PDA) was diagnosed according to clinical and ultrasonographic criteria. Early-onset sepsis was defined in the presence of positive blood culture before 72hours of life. Delivery room resuscitation was defined as one or more of the following: oxygen treatment, Ambu bag ventilation, intubation for ventilation, cardiac massage, and epinephrine administration. Combined rate of mortality or major complications included death or BPD, IVH 3-4, and NEC 2-3.
The NEOCOSUR score is a neonatal mortality risk score developed for VLBWI based on variables present at birth, before NICU admission, and it is an important tool for comparison between NICUs in developing countries.10 Surfactant treatment for preterm infants was the standard of care in all the NEOCOSUR NICUs during the study period.
Statistical analysisA sample size calculation assuming 25% in hospital mortality rate for the children of nondiabetic mothers showed that at 80% power and 5% significance level, this study could detect a risk of 1.5 in 144 infants of diabetic mothers and 577 infants born to non-DM (NDM) mothers.
Maternal characteristics and neonatal outcomes between DM and NDM groups were compared using the chi-squared test for categorical variables and Student's t-test or Mann-Whitney's test for continuous variables. Bivariate analysis was applied to examine the effect of maternal diabetic status (DM or NDM) on mortality and several morbidities of VLBW infants. Multivariable logistic regression analysis was used to assess the independent effect of DM status on mortality and other complications of prematurity. A fixed set of clinically important variables was introduced into the models: maternal age, multiple pregnancy, maternal hypertensive disorders, prenatal steroid treatment, mode of delivery, need for delivery room resuscitation, gender, GA, and birth weight Z-score.
The results of the logistic models are presented as adjusted odds ratios with the 95% confidence intervals. Stata 9.2 software (College Station, Texas, USA) was used for all statistical calculations. A significance level of 5% was used but, because of the large numbers, many of the differences examined were extremely statistically significant so, for convenience, any p-value<0.001 has been truncated to this value.
Furthermore, because it is likely that, in the present population, public and private maternity units had differing outcomes for VLBWI during the study period, the authors also investigated rates of DM and compared perinatal outcomes between both groups in DM mothers. In addition, the periods between 2001-2005 and 2006-2010 were compared to explore whether there were any impact in diagnoses or treatment practices on perinatal outcomes of diabetic pregnancies across time.
The research was conducted in accord with prevailing ethical principles and was approved by the Institutional Review Board of the Catholic University.
ResultsFrom January of 2001 to December of 2010, 12,146 VLBWI were registered in the database, accounting for>93% of all live-born VLBWI of NEOCOSUR centers.
A total of 304 pregnancies in women with pre-existing DM (type 1 autoimmune disease, insulin-resistant type 2, but controlled by medication or insulin) or gestational DM (whether requiring insulin or not) that ended during the ten-year study period were identified. The remainder (n= 10,563) were pregnancies without DM.
The rate of ‘DM pregnancy’ was 2.8% (95% CI 2.5–3.1) or one in every 35 deliveries. The corresponding figures for public and private centers were 2.8% (238/8,238; 1:33) and 2.5% (66/2,629; 1:39; p=0.305), respectively. The secular trend showed a statistically significant increase in DM pregnancies between 2001-2005 (2.4%, 95% CI: 2.1-2.8) and 2006 -2010 (3.2%, 95% CI: 2.8-3.6) periods (p=0.019).
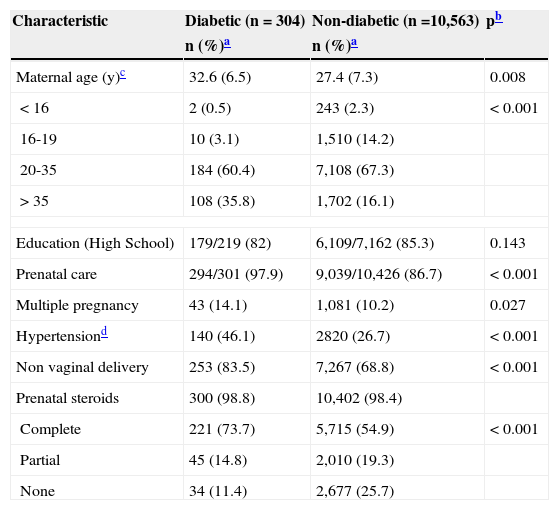
Table 1 lists the demographic, pregnancy, and delivery characteristics among DM compared with NDM women. Briefly, mothers in the DM group were older and were more likely to have attended more prenatal care; have had multiple pregnancies, hypertensive disorders, cesarean deliveries; and to have received a complete course of prenatal steroids. No interaction was found between hypertensive disorders and SGA (p for interaction=0.701).
Comparison of maternal demographic, pregnancy and delivery characteristics, diabetic versus nondiabetic mothers.
| Characteristic | Diabetic (n=304) | Non-diabetic (n =10,563) | pb |
|---|---|---|---|
| n (%)a | n (%)a | ||
| Maternal age (y)c | 32.6 (6.5) | 27.4 (7.3) | 0.008 |
| <16 | 2 (0.5) | 243 (2.3) | < 0.001 |
| 16-19 | 10 (3.1) | 1,510 (14.2) | |
| 20-35 | 184 (60.4) | 7,108 (67.3) | |
| >35 | 108 (35.8) | 1,702 (16.1) | |
| Education (High School) | 179/219 (82) | 6,109/7,162 (85.3) | 0.143 |
| Prenatal care | 294/301 (97.9) | 9,039/10,426 (86.7) | < 0.001 |
| Multiple pregnancy | 43 (14.1) | 1,081 (10.2) | 0.027 |
| Hypertensiond | 140 (46.1) | 2820 (26.7) | < 0.001 |
| Non vaginal delivery | 253 (83.5) | 7,267 (68.8) | < 0.001 |
| Prenatal steroids | 300 (98.8) | 10,402 (98.4) | |
| Complete | 221 (73.7) | 5,715 (54.9) | < 0.001 |
| Partial | 45 (14.8) | 2,010 (19.3) | |
| None | 34 (11.4) | 2,677 (25.7) | |
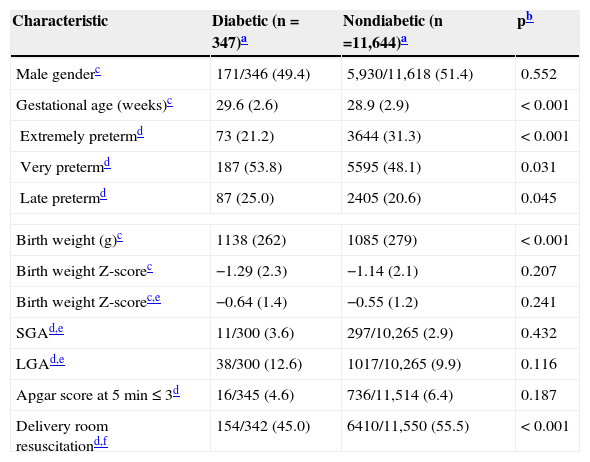
Table 2 present infants characteristics, and due to multiple births, the numbers are higher than those of the mothers. The DM group had a slightly higher GA, lower proportion of extremely preterm infants, and lower need of delivery room resuscitation, but higher mean birth weight than the NDM group. The mean standardized birth weight score (Z-score) was nearly identical for both groups.
Comparison of infants characteristics, diabetic versus nondiabetic mothers.
| Characteristic | Diabetic (n=347)a | Nondiabetic (n =11,644)a | pb |
|---|---|---|---|
| Male genderc | 171/346 (49.4) | 5,930/11,618 (51.4) | 0.552 |
| Gestational age (weeks)c | 29.6 (2.6) | 28.9 (2.9) | < 0.001 |
| Extremely pretermd | 73 (21.2) | 3644 (31.3) | < 0.001 |
| Very pretermd | 187 (53.8) | 5595 (48.1) | 0.031 |
| Late pretermd | 87 (25.0) | 2405 (20.6) | 0.045 |
| Birth weight (g)c | 1138 (262) | 1085 (279) | < 0.001 |
| Birth weight Z-scorec | −1.29 (2.3) | −1.14 (2.1) | 0.207 |
| Birth weight Z-scorec,e | −0.64 (1.4) | −0.55 (1.2) | 0.241 |
| SGAd,e | 11/300 (3.6) | 297/10,265 (2.9) | 0.432 |
| LGAd,e | 38/300 (12.6) | 1017/10,265 (9.9) | 0.116 |
| Apgar score at 5 min≤3d | 16/345 (4.6) | 736/11,514 (6.4) | 0.187 |
| Delivery room resuscitationd,f | 154/342 (45.0) | 6410/11,550 (55.5) | < 0.001 |
SGA, small for gestational age (less than two Z scores apart from its expected median); LGA, large for gestational age (more than two Z scores apart from its expected median).
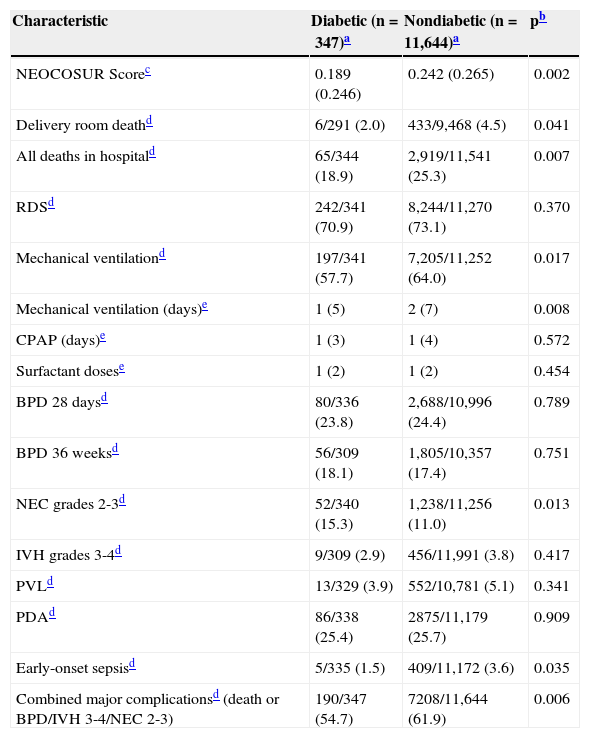
Table 3 compares the mortality rates and major neonatal complications between the two groups. The NEOCOSUR Score, delivery room death, in-hospital mortality, mechanical ventilation, early-onset sepsis, and combined major complications rates were significantly lower in the diabetic group. Noteworthy, the rate of NEC was the only morbidity significantly higher in the DM group.
Infant mortality and morbidities, diabetic versus nondiabetic mothers.
| Characteristic | Diabetic (n=347)a | Nondiabetic (n=11,644)a | pb |
|---|---|---|---|
| NEOCOSUR Scorec | 0.189 (0.246) | 0.242 (0.265) | 0.002 |
| Delivery room deathd | 6/291 (2.0) | 433/9,468 (4.5) | 0.041 |
| All deaths in hospitald | 65/344 (18.9) | 2,919/11,541 (25.3) | 0.007 |
| RDSd | 242/341 (70.9) | 8,244/11,270 (73.1) | 0.370 |
| Mechanical ventilationd | 197/341 (57.7) | 7,205/11,252 (64.0) | 0.017 |
| Mechanical ventilation (days)e | 1 (5) | 2 (7) | 0.008 |
| CPAP (days)e | 1 (3) | 1 (4) | 0.572 |
| Surfactant dosese | 1 (2) | 1 (2) | 0.454 |
| BPD 28 daysd | 80/336 (23.8) | 2,688/10,996 (24.4) | 0.789 |
| BPD 36 weeksd | 56/309 (18.1) | 1,805/10,357 (17.4) | 0.751 |
| NEC grades 2-3d | 52/340 (15.3) | 1,238/11,256 (11.0) | 0.013 |
| IVH grades 3-4d | 9/309 (2.9) | 456/11,991 (3.8) | 0.417 |
| PVLd | 13/329 (3.9) | 552/10,781 (5.1) | 0.341 |
| PDAd | 86/338 (25.4) | 2875/11,179 (25.7) | 0.909 |
| Early-onset sepsisd | 5/335 (1.5) | 409/11,172 (3.6) | 0.035 |
| Combined major complicationsd (death or BPD/IVH 3-4/NEC 2-3) | 190/347 (54.7) | 7208/11,644 (61.9) | 0.006 |
RDS, respiratory distress syndrome; CPAP, continuous positive airway pressure; BPD, bronchopulmonary dysplasia; NEC, necrotizing enterocolitis; IVH, intraventricular hemorrhage; PVL, periventricular leukomalacia; PDA, patent ductus arteriosus.
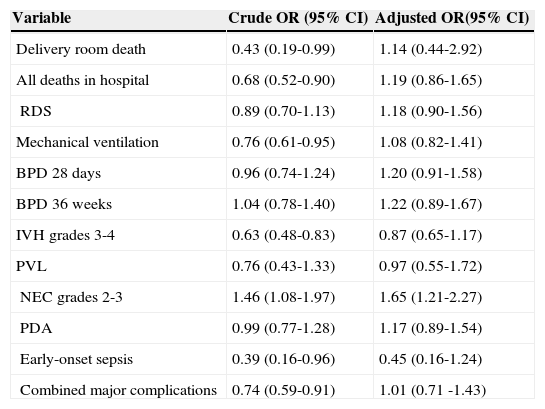
After adjustment in the logistic regression analyses, NEC grades 2-3 was the only condition independently associated with DM group (Table 4).
Crude and adjusted odds Ratios and 95% CI for mortality and morbidities among preterm VLBW infants of diabetic mothers.
| Variable | Crude OR (95% CI) | Adjusted OR(95% CI) |
|---|---|---|
| Delivery room death | 0.43 (0.19-0.99) | 1.14 (0.44-2.92) |
| All deaths in hospital | 0.68 (0.52-0.90) | 1.19 (0.86-1.65) |
| RDS | 0.89 (0.70-1.13) | 1.18 (0.90-1.56) |
| Mechanical ventilation | 0.76 (0.61-0.95) | 1.08 (0.82-1.41) |
| BPD 28 days | 0.96 (0.74-1.24) | 1.20 (0.91-1.58) |
| BPD 36 weeks | 1.04 (0.78-1.40) | 1.22 (0.89-1.67) |
| IVH grades 3-4 | 0.63 (0.48-0.83) | 0.87 (0.65-1.17) |
| PVL | 0.76 (0.43-1.33) | 0.97 (0.55-1.72) |
| NEC grades 2-3 | 1.46 (1.08-1.97) | 1.65 (1.21-2.27) |
| PDA | 0.99 (0.77-1.28) | 1.17 (0.89-1.54) |
| Early-onset sepsis | 0.39 (0.16-0.96) | 0.45 (0.16-1.24) |
| Combined major complications | 0.74 (0.59-0.91) | 1.01 (0.71 -1.43) |
VLBW, very low birth weight; OR, odds ratio; CI, confidence interval.
aAdjusted for maternal age, multiple pregnancy, maternal hypertensive disorders, prenatal steroid treatment, mode of delivery, need for delivery room resuscitation, gender, gestational age, and birth weight Z-score.
Public centers (n=9,090) showed an increased risk of NEC grades 2-3 compared with private centers (n=2,901) for VLBWI born to DM mothers (adjusted OR: 1.67; 95% CI: 1.22-2.28; p < 0.001); the remaining perinatal outcomes were not significantly different between centers. Also, NEC grades 2-3 was the only disease that showed an increased risk in the 2006-2010 period (n=6,684) compared with the 2001-2005 period (n=5,307) for VLBWI born to DM mothers (p=0.001), whereas the other outcomes did not differ among periods.
DiscussionTo the best of the authors’ knowledge, this is the first study in Latin America to investigate the association between maternal DM and perinatal outcomes in VLBWI in the past decade. The sample size was large, and the study period allowed for the analysis of temporal trends.
The current study unexpectedly demonstrated that the risk of mortality or early morbidity was not increased in a large number of small preterm infants born to DM mothers, all born with a birth weight of<1,500g, when compared with infants born to NDM mothers in a regional birth cohort over a ten-year period. Moreover, multivariable analysis did not identify maternal DM as a risk factor for mortality or early morbidity, except NEC, in this population of preterm VLBWI.
In recent years, the care of obstetric DM patients has changed, and it is likely that this could have resulted in the trend for improvements in detection, glycemic control, and pregnancy outcomes among obstetric DM patients.
In a recent review of the prevalence of gestational DM in European countries, the reported figures were between 2.0% and 6.0% in over half of the studies.2 In a previous study of DM in VLBW pregnancies, the prevalence was higher (5.4%) than the present study's figure of 2.8%.6 However, estimating the prevalence of gestational DM is made difficult by a lack of a universally accepted diagnostic criteria.19
Poor glycemic control is associated with an increased risk of pre-eclampsia,20 and may explain the fact that the rate of maternal hypertensive disorders were more prevalent in the DM group. Also, it was reported that obesity, in addition to DM, was associated with a greater risk of preeclampsia than either factor alone, thereby implicating other potential mechanisms such as inflammation in the development of preeclampsia in this high-risk group.21
Women with DM were more likely to have cesarean-section and vaginal operative delivery than normoglycemic women. In addition, the trend for increased pre-pregnancy BMI observed in Latin America,22 coupled with higher multiple births and hypertension, might have partly explained the avoidance of vaginal delivery as the best choice for DM patients in the present study.2
In international literature, patients suffering from gestational or pre-existing DM are generally exposed to a higher risk of preterm delivery.23 A possible explanation is that DM patients who did not achieve the desired level of glycemic control reflect a subgroup of patients with poorer compliance and higher risk of preterm VLBW delivery.
Length of gestation was somewhat greater, whereas a lower proportion of extremely preterm infants were observed in the DM group. This may be due to modern management and adequate glycemic control in pregnancy that led to prolonged gestation and lower risk of VLBW neonatal mortality and morbidity in pregnancies complicated by DM.
There were no significant differences in anthropometric characteristics between the two groups at birth (except birth weight), in agreement with a population-base study in Israel.7
Apgar scores were similar in both groups in accordance with previous studies,7–9 although resuscitation provided was significantly lower in the DM group.
The significantly lower NEOCOSUR Score observed in the DM group is in accordance with total mortality during hospital stay that was significantly increased in the NDM group.
The prevalence of respiratory morbidity was similar in the two study populations, while severity, illustrated by the need for ventilatory support and median number of days on mechanical ventilation, were statistically lower in the DM group. Clinical studies on the effects of maternal diabetes on fetal pulmonary maturation have produced conflicting data,9,24 possibly due to the differences in diabetic control, prenatal steroids, sex distribution, GA, mode of delivery, birth asphyxia, definition of RDS, and the severity of disease in different study populations. The present findings further support this observation; i.e., after controlling for GA, sex, and mode of delivery, there was no increase in the likelihood of RDS.
This was also true for other major complications of prematurity, such as rates of BPD at 28 days’ or 36 weeks’ postmenstrual age, PDA, IVH, or PVL. Conversely, early-onset sepsis and combined major complications were more frequent in the NDM group.
In bivariate analyses, the odds ratio of delivery room death, all deaths in hospital, mechanical ventilation, IVH grades 3-4, early-onset sepsis and combined major complications were significantly lower in the VLBWI of DM group than in the control group. The authors speculate that these differences may have been in part a result of a combination of factors such as (1) a slightly higher GA and birth weight in the DM group and (2) a significantly higher rate of completed courses of prenatal steroids in the DM group, which may be linked to better prenatal care. In the present study, approximately 80% of infants in both groups were exposed to prenatal steroids. Moreover, a Cochrane analysis revealed that prenatal steroid therapy decreases the risks of BPD and IVH.25 A third factor was differences in mode of delivery: there was a higher rate of cesarean deliveries in the group with DM. Although the topic is highly controversial, some retrospective studies indicate that there might be a reduced rate of complications related to prematurity when the infant is delivered by cesarean section.26 Other influential factors include differences in the rate of maternal hypertensive disorders, which were, as expected, more prevalent in the group with DM; it appears that preeclampsia might reduce the risk for developing RDS.27
In the multivariable analyses, only NEC persisted independently associated with a higher risk in VLBWI born to DM mothers. Its pathogenesis is multifactorial and involves an overreactive response of the immune system to an insult (i.e. infectious or response to translocation of normal enteric bacteria).28 Because cesarean section-born individuals do not make contact at birth with maternal vaginal and intestinal bacteria, this could lead to long-term changes in the gut microbiota that could contribute to NEC. Furthermore, NEC was the only morbidity more frequently observed in public centers and showed a significant trend in the study period. In a population study NEC was not associated with DM, although rates halved that observed in the present study.7
This was a retrospective cohort study including 22 different maternity units in the Latin-American region, and consequently some inconsistency in the screening methods and diagnosis of DM are to be expected. This may have led to the underreporting of DM cases in some units, and consequently the inclusion of some women with occult diabetes in the NDM group.
Another limitation of the present study is that the type of DM and the degree of glycemic control was not prospectively recorded in the database. Although pre=gestational and gestational DM involve distinct metabolic alterations, the infant population of this study represents the group of VLBWI born after in utero exposure to diabetic milieu in the mother. In addition previous studies showed no statistically significant differences in sociodemographic conditions or perinatal outcomes between the two groups.7,29 Besides the smaller rate of pregestational DM (0.2 to 0.3%) in other studies,1 it is likely that the present observations represent the general trends in outcome of these infants.
The present data suggest that with modern management and adequate prenatal care there is no significant increase in mortality rates or early morbidity rates in VLBWI born to mothers with DM, except NEC. It appears that, with reasonable diabetic control, prematurity rather than the diabetic state determines the neonatal outcome.
Conflicts of interestThe authors declare no conflicts of interest.
We thank all the Neocosur centers that participated in this study. The present study included the following collaborators from the Neocosur Network:
Argentina: Guillermo Colantonio, Gabriel Musante,Luis Prudent, Liliana Rochinotti, Ines Galindez, Mariana Sorgetti, Lorena Soler (Clinica y Maternidad Suizo Argentina, Buenos Aires); Isabel Kurlat, Oscar Di Siervi, Adriana Escarate (Hospital de Clínicas José de San Martin, Buenos Aires); Gonzalo Mariani, Jose María Ceriani, Silvia Fernandez, Carlos Fustiñana (Hospital Italiano, Buenos Aires); Jorge Tavosnaska, Liliana Roldan, Hector Sexer, Elizabeth Lombardo (Hospital Juan Fernandez, Buenos Aires); Gabriela Torres, Daniel Agost, Augusto Fischetti, Monica Rinaldi (Hospital Lagomaggiore, Mendoza); Carlos Grandi, Claudio Solana, Javier Meritano, Miguel Larguia (Maternidad Sarda, Buenos Aires), Marcelo Decaro, Lionel Cracco, Gustavo Bassi, Noemi Jacobi, Andrea Brum, Nestor Vain (Sanatorio de la Trinidad, Buenos Aires); Adriana Aguilar, Miriam Guerrero, Edgardo Szyld, Alcira Escandar (Hospital Dr. Diego Paroissien, Buenos Aires); Horacio Roge, Mario Marsano, Elisa Fehlmann, Jorge Rios (Hospital Español de Mendoza, Mendoza).
Brasil: Vanda Simões, Marynea do Vale Nunes, Marilia Martins (Hospital Universitário Materno Infantil, Universidade Federal do Maranhão).
Chile: Jorge Fabres, Alberto Estay, Alvaro Gonzalez, Sandra Vignes, Mariela Quezada, Jose L. Tapia, Soledad Urzua (Hospital Clinico Universidad Catolica de Chile, Santiago); Rodrigo Ramírez, Maria Eugenia Hübner, Jaime Burgos, Jorge Catalan (Hospital Clinico Universidad de Chile, Santiago); Lilia Campos, Aldo Bancalari, Lilian Cifuentes, Jorge Leon, Eduardo Broitman, Roxana Aguilar (Hospital Guillermo Grant, Concepción); Jane Standen, Marisol Escobar, Alejandra Nuñez (Hospital Gustavo Fricke, Viña del Mar); Agustina González, Ana Luisa Candia, Lorena Tapia, Giovanna Loguercio, Claudia Avila (Hospital San Jose, Santiago); Claudia Toro, Patricia Mena, Angelica Alegria, Adolfo Llanos (Hospital Dr. Sotero del Rio, Santiago); Veronica Peña, Marianne Bachler, Patricia Duarte (Hospital San Borja Arriaran, Santiago); Ivonne D‘Apremont,Guillermo Marshall, Sandra Vignes, Mariela Quezada, Luis Villarroel, Angelica Dominguez (Unidad Base de Datos, Pontifícia Universidad Catolica, Santiago).
Paraguay: Jose Lacarruba, Elizabeth Cespedes, Ramon Mir, Elvira Mendieta, Larissa Genes, Carlos Caballero (Departamento de Pediatria, Hospital de Clinicas de Asuncion, Asuncion).
Peru: Jaime Zegarra, Veronica Webb, Fabiola Rivera,Marilu Rospigliosi, Silvia Febres, Enrique Bambaren (Hospital Cayetano Heredia, Lima); Rosa Unjan, Walter Cabrera, Raul Llanos, Anne Castañeda, Oscar Chumbes, Roberto Rivera (Hospital Guillermo Almenara, Lima).
Uruguay: Ruben Panizza, Sandra Gugliucci, Silvia Fernandez, Eduardo Mayans, Alicia Prieto, Cristina Hernandez (Facultad de Medicina, Servicio de Recien Nacidos, Montevideo).
Please cite this article as: Grandi C, Tapia JL, Cardoso VC. Impact of maternal diabetes mellitus on mortality and morbidity of very low birth weight infants: a multicenter Latin America study. J Pediatr (Rio J). 2015;91:234–41.
Study conducted at Department of Pediatrics, Faculty of Medicine, Catholic University, Santiago, Chile.