Intussusception surveillance was initiated after the nationwide introduction of live attenuated monovalent rotavirus vaccine (RV1). The objective is to assess the epidemiology of intussusception and compare the number of cases before and after the introduction of rotavirus vaccine.
MethodsCases of intussusception occurring between March 2006 and January 2008 were identified through a prospective enhanced passive surveillance system established in sentinel state hospitals. Retrospective review of medical records was used to identify cases, which occurred in sentinel hospitals between January 2001 and February 2006.
ResultsFrom 2001 to 2008, 331 intussusception cases were identified, 59.5% were male, with peak incidence among those 18–24 weeks of age. Overall <10% of cases were among infants 6–14 weeks of age (when the first dose of RV1 is administered). The most frequently observed signs or symptoms of intussusception included vomiting (89.4%), bloody stool (75.5%), and abdominal distention (71.8%). A majority (92.1%) of the case-patients required surgery for treatment; 31.8% of those who underwent surgery required bowel resection, and 13 (3.9%) died. Among the 21 hospitals that reported cases throughout the entire surveillance period (2001–2008), the number of intussusception events during 2007 (n=26) and 2008 (n=19) was not greater than the average annual number (n=31, range 24–42) during baseline years 2001–2005.
ConclusionsAlthough this analysis did not identify an increase in intussusception cases during the two years after RV1 introduction, these results support the need for special epidemiologic methods to assess the potential link between rotavirus vaccine and this very rare adverse event.
A vigilância da intussuscepção foi iniciada após a introdução da vacina monovalente viva atenuada contra rotavírus (RV1) em todo o país. O objetivo é avaliar a epidemiologia da intussuscepção e comparar a quantidade de casos antes e depois da introdução da vacina contra rotavírus.
MétodosOs casos de intussuscepção entre março de 2006 e janeiro de 2008 foram identificados por meio de um sistema de vigilância passivo prospectivo aprimorado estabelecido em hospitais-sentinela estaduais. A análise retrospectiva de prontuários médicos foi utilizada para identificar os casos que ocorreram em hospitais-sentinela entre janeiro de 2001 e fevereiro de 2006.
ResultadosDe 2001-2008, identificamos 331 casos de intussuscepção, 59,5% dos quais ocorreram em pacientes do sexo masculino, com pico de incidência entre aqueles com 18-24 semanas de idade. Em geral, < 10% dos casos ocorreram entre neonatos com 6-14 semanas de idade (quando a 1ª dose de RV1 é administrada). Os sinais ou sintomas de intussuscepção observados com mais frequência incluíam vômito (89,4%), fezes com sangue (75,5%) e distensão abdominal (71,8%). A maioria (92,1%) dos pacientes precisou de cirurgia para o tratamento; 31,8% dos que se submeteram à cirurgia precisaram de ressecção intestinal, e 13 (3,9%) vieram a óbito. Entre os 21 hospitais que relataram casos durante todo o período de vigilância (2001-2008), a quantidade de casos de intussuscepção em 2007 (n=26) e 2008 (n=19) não foi maior que a quantidade média anual (31, faixa de 24-42) durante os anos-base de 2001-2005.
ConclusõesEmbora esta análise não tenha identificado um aumento nos casos de intussuscepção nos dois anos após a introdução da RV1, esses resultados justificam a necessidade de métodos epidemiológicos especiais para avaliar a possível associação entre a vacina contra rotavírus e esse evento adverso muito raro.
Rotavirus is a leading cause of severe diarrhea, accounting for ∼453,000 deaths annually among children <5 years of age worldwide.1 The World Health Organization (WHO) has recommended global introduction of one of the two licensed rotavirus vaccines [RotaTeq or RV5 (Merck®, PA, USA) and Rotarix or RV1 (Rotarix®, GlaxoSmithKline Biologicals, Rixensart, Belgium)] in national immunization programs for preventing severe rotavirus disease.2 In March 2006, the Brazilian Ministry of Health introduced RV1, a live attenuated monovalent vaccine derived from human G1P[8] strain, simultaneously in all 27 states, through its National Immunization Program (Programa Nacional de Imunização [PNI]).
A key issue for rotavirus vaccine immunization programs is the need for safety monitoring with regard to intussusception, a form of intestinal obstruction occurring at a background rate of approximately 50 per 100,000 infants.3 An earlier rotavirus vaccine (Rotashield, Wyeth Vaccines, PA, USA) based on a different (rhesus) strain than the current WHO recommended vaccines was found to be associated with an increased risk of intussusception, with the vaccine causing roughly ten excess cases per 100,000 vaccinated infants.4 Large clinical trials have not detected a risk of intussusception associated with either currently used vaccines5,6; however, post-licensure surveillance in Mexico and Australia has observed a small risk of intussusception after the initial dose.7,8 In Mexico, an association was found between RV1 and intussusception, with the vaccine causing one to four excess cases of intussusception per 100,000 vaccinated infants.8,9 In Australia, a possible temporal clustering of intussusception episodes was noted during the seven days after the initial dose of both RV1 and RV5, though there was no increase in overall risk at 12 months of age.7 In Brazil, no increased risk was identified after the first dose, but a potential small risk was identified after the second dose of RV1 (excess of 1.5 cases per 100,000 vaccinated infants).9 No definite increased risk of intussusception has been identified after use of RV5 in the United States, but an excess risk less than one in 65,000 vaccinated infants has not been excluded.10 Marked declines in severe and fatal diarrhea were demonstrated in early adopter countries after the introduction of rotavirus vaccine.11 Because the documented benefits of vaccination have far outweighed the low-level risk of intussusception observed in some settings, regulatory agencies have continued to recommend rotavirus vaccination for all children worldwide.2 Ongoing post-licensure monitoring of the safety and effectiveness of rotavirus vaccines is crucial for assessing the risk/benefit profile of rotavirus vaccines.
This study's objective was to monitor and characterizes the intussusception events in a subset of participating hospitals and compares the number of intussusceptions cases before and after the introduction of rotavirus vaccine.
Materials and methodsIntussusception case definitionIntussusception case-patients were included if registered as State of São Paulo residents, aged less than 1 year, and diagnosed with definite intussusception based on Level I of diagnostic certainty as defined by the Brighton Collaboration Intussusception Working Group criteria.12 Diagnosis of intussusception is classified as Level 1 if confirmed by air or liquid contrast enema, ultrasound (with confirmed reduction on subsequent ultrasound or enema), or at surgery or autopsy.
Setting and data sourcesSão Paulo is a highly industrialized Brazilian state. It is also the most populous: over 41 million people live there (41,579,695 in 2011). The newborn cohort has been decreasing in recent years. In 2001, 686,533 children were born in São Paulo, while 605,558 were born in 2008.13 Two-dose rotavirus vaccine coverage among children <1 year of age reached 85.1% in 2007 and 86.3% in 2008.14 In São Paulo state, there are 9549 clinical pediatric beds and 1048 pediatric surgery beds distributed across 893 public and private hospitals.
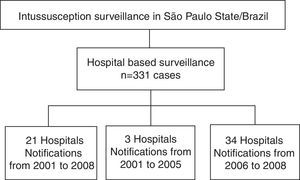
Data on intussusception were obtained from 58 hospitals in São Paulo state from 2001 through 2008. These sentinel hospitals were selected from those with pediatric surgery services that served as the primary referral hospital for intestinal obstruction in each metropolitan area or groups of municipalities (Fig. 1). Not all of these hospitals conducted surveillance during the entire 2001–2008 study period. Thus, for comparison of the pre-vaccine and post-vaccine periods, data was only used from 21 of the 58 hospitals that conducted intussusception surveillance for the entire 2001–2008 period. These hospitals contain 10.2% of the clinical pediatrics beds and 12.7% of the pediatric surgery beds of São Paulo state, but accounted for approximately 30% of the discharge diagnoses of intestinal obstruction identified among children younger than 1 year of age admitted in public hospitals in São Paulo during 2001–2005.
Two approaches were used to ascertain intussusception cases. To establish a pre-vaccine baseline, intussusception cases hospitalized between January 2001 and February 2006 were identified at each sentinel hospital through retrospective review of discharge diagnoses coded as intestinal obstruction (K56), according to the International Classification of Diseases, 10th edition (ICD-10), and diagnoses of intussusception in radiology or surgical log-books. The search was conducted during 2006 and data were abstracted from medical records using standardized forms. Cases occurring between March 2006 and December 2008 were identified through a prospective surveillance system that was established in sentinel private and public state hospitals. Medical and surgical staff at sentinel hospitals participated in an initial training in identifying intussusception cases using the validated Brighton Collaboration Level I case definition. Hospital staff, particularly pediatric surgeons, were asked to report cases electronically using a standard data collection form. Periodic visits and calls were made to the hospital to encourage reporting and to address concerns. Periodic review of surgical and radiology logs was conducted to ensure the thoroughness of reporting.
Statistical analysisThe demographic and clinical characteristics of case-patients from all hospitals were summarized using descriptive statistics. To examine potential seasonal variation and age distribution of intussusception cases, the number of intussusception hospitalizations in São Paulo was plotted by year and week of age. For this analysis, cases from all 58 surveillance hospitals were used.
It was also of interest to monitor changes in number of intussusception cases before and after the introduction of vaccine. Thus, the number of intussusception cases per year was plotted for those 21 hospitals that conducted surveillance during the entire 2001–2008 period. Because rotavirus vaccine coverage among infants was high after 2006,14 this study compared the number of intussusception events during 2007 and 2008 with the average annual number of cases during the baseline years 2001–2005, considering 2006 as a transition year when rotavirus vaccine was being introduced. The number of intussusception cases from these 21 hospitals was also plotted by calendar year, according to four age groups: <6 weeks, 6–14 weeks, 15–24 weeks, and 25–52 weeks. All statistical analyses were performed using Epi-Info 3.5.1 (Epi Info™, GA, USA) and Microsoft Excel 2007 (Microsoft®, WA, USA).
EthicsThe study was approved by the Ethics Committee of Irmandade da Santa Casa de Misericórdia de São Paulo, registration No. 38053.
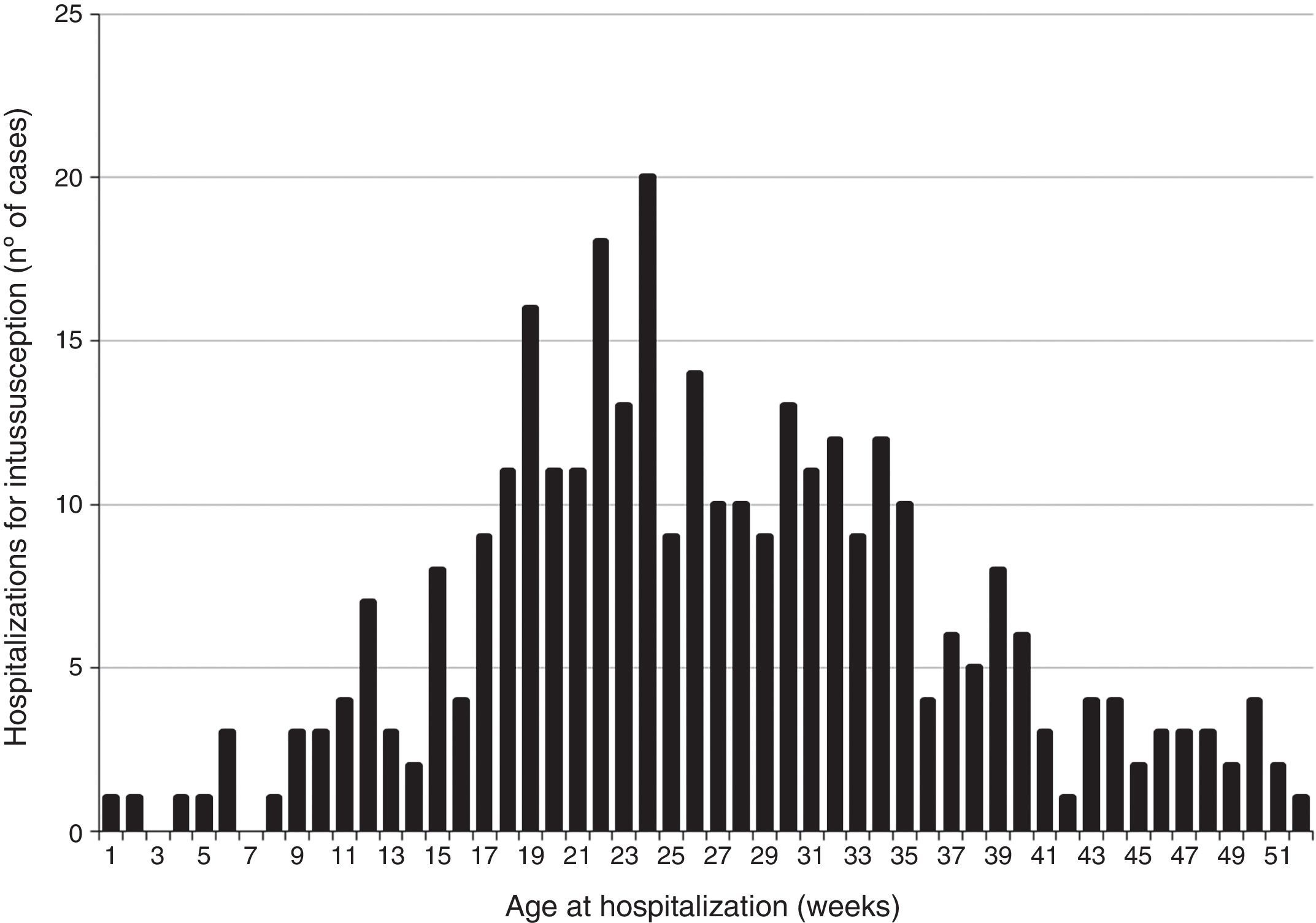
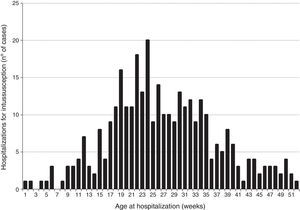
ResultsClinical and demographic characteristics of casesBetween 2001 and 2008, a total of 331 intussusception cases were identified in São Paulo state (Fig. 1). Intussusception cases were more likely to be male (59.5%) and had a median age of 26 weeks (Table 1), with peak incidence among those 18–24 weeks of age (Fig. 2). Only 9.1% of the intussusception events occurred among children <15 weeks of age, when the initial dose of rotavirus vaccines is typically administered in Brazil, whereas 36.6% occurred among those 15–24 weeks of age, when the second dose of rotavirus vaccine is typically administered, and 54.4% among those 25–52 weeks of age. Intussusception events occurred year-round, without evidence of a seasonal peak (data not shown).
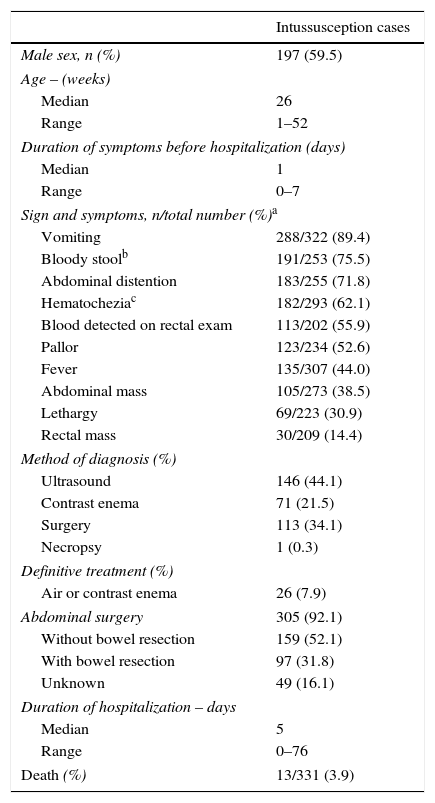
Demographic and clinical characteristics of hospitalized intussusception cases. State of São Paulo, Brazil, 2001–2008 (n=331).
| Intussusception cases | |
|---|---|
| Male sex, n (%) | 197 (59.5) |
| Age – (weeks) | |
| Median | 26 |
| Range | 1–52 |
| Duration of symptoms before hospitalization (days) | |
| Median | 1 |
| Range | 0–7 |
| Sign and symptoms, n/total number (%)a | |
| Vomiting | 288/322 (89.4) |
| Bloody stoolb | 191/253 (75.5) |
| Abdominal distention | 183/255 (71.8) |
| Hematocheziac | 182/293 (62.1) |
| Blood detected on rectal exam | 113/202 (55.9) |
| Pallor | 123/234 (52.6) |
| Fever | 135/307 (44.0) |
| Abdominal mass | 105/273 (38.5) |
| Lethargy | 69/223 (30.9) |
| Rectal mass | 30/209 (14.4) |
| Method of diagnosis (%) | |
| Ultrasound | 146 (44.1) |
| Contrast enema | 71 (21.5) |
| Surgery | 113 (34.1) |
| Necropsy | 1 (0.3) |
| Definitive treatment (%) | |
| Air or contrast enema | 26 (7.9) |
| Abdominal surgery | 305 (92.1) |
| Without bowel resection | 159 (52.1) |
| With bowel resection | 97 (31.8) |
| Unknown | 49 (16.1) |
| Duration of hospitalization – days | |
| Median | 5 |
| Range | 0–76 |
| Death (%) | 13/331 (3.9) |
The most frequently observed signs or symptoms (Table 1) included vomiting (89.4%), bloody stool (75.5%), and abdominal distention (71.8%). Mean duration of symptoms before presenting for medical care was roughly one day. Diagnosis of intussusception was made by ultrasonography, contrast enema, and surgery in 44.1%, 21.5%, and 34.1% of the case-patients, respectively. A majority (92.1%) of the case-patients were surgically treated for intussusception, with at least 31.8% of those who underwent surgery requiring bowel resection. Among the 331 case-patients hospitalized for intussusception during the study period, 13 (3.9%) died. Among those surviving, the mean duration of hospitalization was approximately five days (range: 0–76 days).
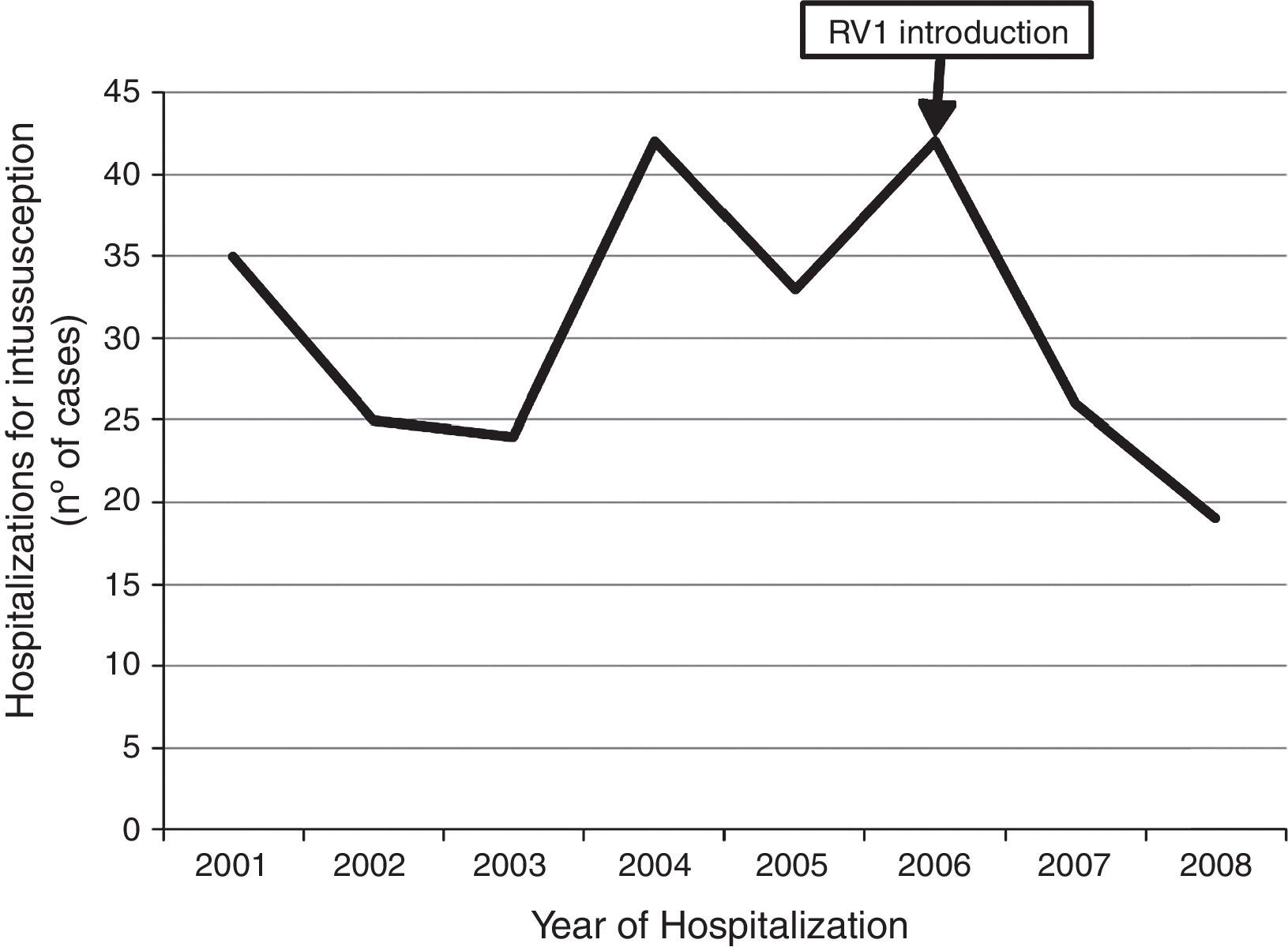
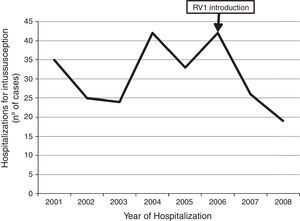
Temporal distribution of casesAmong the 331 case-patients, 246 (74.3%) were from 21 hospitals that identified and reported intussusception during the entire surveillance period, 2001–2008. The overall demographic and clinical characteristics of cases from these 21 hospitals were similar to those of cases from hospitals that only reported cases for part of the surveillance period (data not shown). When restricting the analysis to cases at the 21 hospitals with stable reporting during the entire 2001–2008 surveillance period, no increase was observed in the number of reported intussusception events during the surveillance period after vaccine introduction. The observed number of intussusception events during 2007 (n=26) and 2008 (n=19) was somewhat lower than the average annual number of intussusception cases (n=32 cases per year, range 24–42 cases per year) during 2001–2005 (Fig. 3).
DiscussionEfforts to build capacity for intussusception monitoring by the Division of Immunization of the State of São Paulo identified several important lessons for future rotavirus vaccine safety monitoring efforts in Brazil. First, the age distribution of intussusception in São Paulo is similar to that in other regions of the world, with <10% of cases occurring among those <15 weeks of age.15–17 At this age, the first dose of rotavirus vaccine – the dose with the highest potential risk of intussusception – is typically administered. This finding indicates that it would be necessary to expand surveillance to a large number of hospitals in order to identify enough cases to assess risk of intussusception after the initial dose of rotavirus vaccination. Identifying a four- to five-fold increase in risk of intussusception relative to background might not be possible when assessing trends of intussusception among all infants under one year of age who typically have background rates of ∼40–50 per 100,000.18 Second, surgery is the most common treatment of intussusception in Brazil, compared with more developed settings where non-surgical treatment with air/contrast enema is used more often.19–21 This finding has implications for resource-poor settings, in that networking with surgeons and hospitals with surgical centers could enhance surveillance for intussusception after the introduction of vaccine. Third, while cautious interpretation is warranted, this study did not observe a substantial increase in the number of intussusception cases during the post-vaccine introduction period, when some 85% of the infants had received vaccination in Brazil.
Brazil was one of the early adopters of rotavirus vaccine, and has documented large reductions in hospitalizations and deaths related to diarrhea among children under five years age since the introduction of rotavirus vaccines.14 While clinical trials for rotavirus vaccines have not identified a vaccine-associated increased risk of intussusception, large post-licensure evaluations in Mexico and Australia have found a low risk after the first dose of both rotavirus vaccines, amounting to roughly one to four excess cases of intussusception for every 100,000 vaccinated children.7–9 While a cautious interpretation is warranted, since the present surveillance was not intended to quantify risk of intussusception associated with RV1, it is reassuring that there was no large increase in intussusception cases at a population level following vaccine introduction in the state of São Paulo, Brazil.
Trend analysis cannot exclude a risk of similar magnitude to that seen in Mexico and Australia after the initial dose, particularly because background rates of intussusception are very low (∼10–20 per 100,000 infant years during 6–12 weeks of age when the first dose is administered in Brazil).3,9 Assessing trends in narrow age windows might be useful, but sample size is typically insufficient for confidently excluding risk, even with national datasets from countries with large birth cohorts, such as the United States.3
Analytic epidemiologic studies, such as those employing the case-series or the case–control method, would be necessary for assessing a magnitude of risk of one to two excess adverse events per 100,000 vaccinated children. Surveillance platforms similar to that established by the State of São Paulo are the backbone for such epidemiologic studies, provided that intussusception case-finding is active and independent of the vaccination status. Indeed, several of these hospitals from São Paulo enhanced and continued surveillance as part of a nationwide study in Brazil that assessed risk of intussusception after RV1 using self-controlled case-series and case–control design. In that nationwide study, no risk of intussusception was found after the first dose of rotavirus vaccine in Brazil, but a low-level risk was detected after the second dose.9 Initiating this type of active hospital based surveillance for specific outcomes such as intussusception would be valuable for safety monitoring in other settings without any existing national safety monitoring platforms, particularly for building capacity for safety monitoring and for obtaining established platforms that can be utilized for specialized studies for assessing risk as new safety concerns arise.
In the present cohort, 90% of the intussusception case-patients required surgery. This is comparable to treatment standards in resource poor settings, while in industrialized countries only 10–50% of patients require surgical treatment.15,19–21 The reasons for the high rates of surgical treatment among cases remain a subject for further investigation. Delays in presentation and treatment may also explain the higher in-hospital mortality of 4–5% in some regions of Brazil9 and ∼12–13% reported from Africa,15 compared with 1% in Mexico9 and <1% in industrialized countries.19–21 Improving diagnosis and early intervention with non-invasive reduction techniques have the potential to reduce morbidity and mortality in Brazil. Further studies identifying risk-factors for severe outcomes related to intussusception in Brazil are warranted.
This evaluation has several limitations. First, this study considered sentinel services. The reported cases do not represent all intussusception cases of São Paulo state during the studied period. The selected services have 30% of pediatrics beds of the State of São Paulo's public health system, which covers ∼60% the population. Without a precise population denominator, it is not possible to calculate incidences. Results in absolute numbers can be influenced by population changes, such as decrease in birth rate over time. Second, using different surveillance methods before and after the introduction of vaccine limits the ability to confidently compare the rate of events between the two periods, particularly because of differences in methods of ascertaining cases. These results alone cannot be used to refute a casual association between rotavirus vaccination and intussusception, and cannot be directly compared with data from post-licensure monitoring in Mexico and Australia.7,8 Third, vaccination history of cases was not available. Moreover, the completeness and accuracy of data describing the clinical presentation and management of intussusceptions reflects recording completeness in medical records. Fourth, intussusception is a rare event, especially among infants aged <3 months; relatively small changes in absolute numbers in narrower age ranges, such as infants aged 6–14 weeks, could result an increase or decrease in trend by chance alone. Last, hospital based surveillance may not be representative of all intussusception events, particularly in resource poor settings where access to pediatric diagnostic facilities and treatment is limited.
Since 2006, rotavirus vaccines have been introduced in over 30 countries worldwide.22 While the benefits of rotavirus vaccination in terms of reducing severe and fatal diarrhea have far outweighed the low risk of intussusception, the ongoing monitoring of intussusception after the introduction of vaccine is important for ensuring vaccine safety and maintaining public trust in the rotavirus vaccine program. The present experience, establishing intussusception surveillance for monitoring post licensure rotavirus vaccine safety in São Paulo, should provide valuable information for other similar countries that are introducing rotavirus vaccine and do not have existing safety monitoring systems.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Fernandes EG, Leshem E, Patel M, Flannery B, Pellini AC, Veras MA, et al. Hospital-based surveillance of intussusception among infants. J Pediatr (Rio J). 2016;92:181–7.