the low degree of inflammation in obesity contributes to systemic metabolic dysfunction. Recent experimental studies proposed some effects of alteration in gut microbiota on inflammatory factors. This study aimed to assess the anti-inflammatory effects of a synbiotic supplement on inflammation markers in overweight and obese children and adolescents.
Methodsthis randomized triple-masked controlled trial was conducted among 70 participants aged 6 to 18 years, with a body mass index (BMI) equal or higher than the 85th percentile. They were randomly assigned into two groups of equal number to receive synbiotic or placebo for eight weeks.
Resultsfifty-six of 70 participants (80%) completed the study. Compared with the placebo group, the synbiotic group had significant decrease in mean values of tumor necrosis-α and interleukin-6, with significant increase in adiponectin; these changes were no longer significant after adjustment for BMI. There was no significant change in the mean values of high-sensitive C-reactive protein.
Conclusionthe present findings suggest the positive influence of synbiotic supplementation on inflammation factors, which are dependent to its effect on weight reduction in overweight and obese children.
o baixo grau de inflamação na obesidade contribui para disfunção metabólica sistêmica. Estudos experimentais recentes propuseram alguns efeitos de alteração na microbiota intestinal sobre fatores inflamatórios. O objetivo deste estudo foi avaliar os efeitos anti-inflamatórios de um suplemento simbiótico sobre marcadores de inflamação em crianças e adolescentes com sobrepeso e obesos.
Métodoseste ensaio clínico controlado randomizado triplo-cego foi conduzido entre 70 participantes com idade entre seis e 18 anos, com índice de massa corporal (IMC) igual ou acima do 85° percentil. Eles foram aleatoriamente divididos em dois grupos de igual número de participantes para receber simbiótico ou placebo por oito semanas.
Resultadosno todo, 56 de 70 participantes (80%) concluíram o estudo. Em comparação ao grupo placebo, o grupo simbiótico teve redução significativa nos valores médios de necrose tumoral-α e interleucina-6, com aumento significativo na adiponectina; essas alterações não eram mais expressivas após o ajuste do IMC. Não houve alteração importante nos valores médios da proteína C-reativa altamente sensível.
Conclusãonossas conclusões sugerem a influência positiva da suplementação simbiótica sobre fatores inflamatórios, dependente de seu efeito sobre a redução de peso em crianças com sobrepeso e obesas.
The emerging global epidemic of obesity is a serious health problem at individual and public health levels. This is of special concern for the pediatric age group. Obesity is associated with chronic low-grade inflammation, which contributes to the systemic metabolic dysfunction linked to obesity-linked disorders, such as metabolic syndrome.1
Increased expression and production of cytokines and acute phase reactants such as C-reactive protein (CRP), interleukins (ILs), tumor necrosis factor α (TNF- α), or lipopolysaccharides (LPS) result in the low degree of inflammation among obese individuals.2,3
Some studies proposed that gut microbiota may participate in the whole-body metabolism by affecting energy balance, glucose metabolism, and low-grade inflammation associated with obesity and related metabolic disorders.4
It is documented that gut microbiota are different among obese and eutrophic individuals.5,6 Gut microbiota-derived LPS are known as a factor involved in the onset and progression of inflammation and metabolic disorders.7 LPS are a component of Gram-negative bacteria cell walls, which are among the most potent and well-studied inducers of inflammation. 4
Moreover, any change in the gut microbiota may lead to change in the production of endotoxin and thus change in the LPS levels.7,8
Although the intestinal epithelium acts as a continuous barrier to avoid LPS translocation, some events can damage this barrier. For instance, a study demonstrated that the modulation of gut bacteria following a high-fat diet strongly increased the intestinal permeability by reducing the expression of genes coding.9
Therefore, it can be assumed that regulating gut microbiota may be an appropriate strategy to control obesity and its related disorders.
Therefore, it was hypothesized that supplementation with synbiotics, which modulates gut microbiota and their production, may be effective in changing markers of inflammation in individuals with excess weight. This study aimed to investigate the effect of synbiotic supplementation on inflammatory factors in overweight and obese children and adolescents.
MethodThe detailed methods of this study have been previously published;10 the findings on markers of inflammation, which have not been reported before, are presented here. The study was conducted from September to November of 2011 at the Isfahan University of Medical Sciences (IUMS), Isfahan, Iran. It was a randomized triple-blinded controlled trial, i.e. the researchers, participants and statistician were masked to the groups under study.
The trial protocol was in accordance with the Declaration of Helsinki, and was approved by the Research and Ethics Committee of IUMS. The trial was registered under trial registry code IRCT201103081434N4 at the national registry for clinical trials, which is a member of the World Health Organization.
After providing detailed information, an informed consent was signed by parents, and oral assent from participants was obtained.
Participants70 apparently healthy children and adolescents, aged 6 to 18 years, with a body mass index (BMI) equal to or higher than the age- and gender-specific 85th revised percentiles of the Centers for Disease Control and Prevention,11 which are in close agreement with the percentiles of Iranian children and adolescents,12 were selected by random sampling from children who were referred to the Pediatric Obesity and Metabolic Syndrome Clinic of the Child Growth and Development Research Center of the IUMS. Participants were randomized to either synbiotic (n = 35) or placebo (n = 35) groups through random table numbers. Children with syndromal obesity, endocrine disorders, any physical disability, history of chronic medication use, use of mineral and/or vitamin supplements, history of any chronic diseases and/or chronic medication use, or those under special diets were not included in the study. The trial duration was eight weeks, and both groups received similar counseling for lifestyle modification regarding dietary and physical activity habits.
Physical examinationThe participants’ age and birth date were recorded. All anthropometric measurements were made by the same trained person and under the supervision of the same pediatrician. Physical examination was conducted under standard protocols through calibrated instruments at the beginning and end of the trial.
Body weight was measured with a digital floor scale (Seca - Hamburg, Germany) with 100g accuracy, without shoes and with minimum clothing. Height was measured, with 1mm accuracy, with a non-stretch tape.
Waist and hip circumferences were measured with a non-elastic tape. Waist circumference was measured at a point midway between the lower border of the rib cage and the iliac crest at the end of normal expiration. Hip circumference was measured at the maximum girth of the buttocks, and waist-to-hip ratio was calculated.
Biochemical measurementsParticipants were instructed to fast for 12hours before blood sampling. With one of the parents accompanying his/her child, blood samples were taken from the antecubital vein between 08:00 and 09:30 am. After collecting blood samples, the participants were served a healthy snack provided by the project team. CRP, TNF-α, IL-6, and adiponectin were measured enzymatically with standard auto-analyzer kits (Pars Azmoun, Iran).
Synbiotic administrationSynbiotic capsules (Protexin - London, England) were used, each containing 2.0 × 108 colony-forming units (CFU)/day. They contained a combination of viable frozen-dried)Lactobacillus casei, Lactobacillus rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, Lactobacillus acidophilus, Bifidobacterium longum, Lactobacillus bulgaricus) of human origin with prebiotics (frocto oligosaccharides), vitamin E, vitamin A, and vitamin C. The children and adolescents assigned to the synbiotic group were instructed to take one capsule a day before a main meal for eight weeks.
The placebo was prepared in the Pharmaceutics Department of the Faculty of Pharmacy of the IUMS. It contained maltodextrine and consisted of capsules with shape, taste, and smell identical to the synbiotic capsules.
In addition to regular visits of participants, medication adherence was tracked by stool sample collection and the count of bacteria in stool, as well as by weekly phone call to participants.
Stool sample collectionStool samples were collected from both synbiotic and placebo groups at baseline and on the days 15 and 60 of the trial. Samples were kept refrigerated in sealed plastic feces containers for less than six hours until they were transferred to the laboratory, where they were examined at the earliest possible time.
MediaMRS agar (Merck - Germany, pH = 5.7) and MRS agar (Merck - Germany, pH = 5.7) combined with 1% muprocin (Sigma - United States) and 0.5% systein hydrochloride (Sigma - United States) were used for enumeration of Lactobacillus and Bifidobacterium colonies, respectively.
Bacterial countsEach fecal sample (0.5g) was placed in the sterilized tube combined with 5mL of sterile normal saline, mixed thoroughly, and centrifuged for 5minutes at 100rpm. 1mL of the upper phase was serially tenfold-diluted to a 10−7 dilution.
100μL of the proper dilution was surface-cultured on both types of plates. Plates were incubated anaerobically with 5% CO2, using a CO2-injected incubator. MRS plates were kept at 37° to 38°C for 48hours, and the plates containing MRS-muprocin-hydrochloride were kept at the same temperature for 72hours.
Colony counting was performed by an expert and expressed as a log of the CFU per gram of fresh feces.
Dietary recordsNutrient intakes were estimated using three-day dietary record (two weekdays and one weekend day) at the beginning and at the end of the study. Participants were asked to write down the type and amount of food eaten, using scales or household measures to gauge portion sizes where possible. Three-day averages of energy and macronutrient intakes were analyzed using the Nutritionist 4 software (First Databank Inc., Hearst Corp. - San Bruno, CA, United States). Data entry was performed by a trained dietitian. If a participant ate a food not included in the database, another food with very similar nutrient composition was selected. Nutrient information was also obtained through food labels or recipes from participants. Participants were encouraged to keep their dietary intakes and exercise patterns monitored by the Physical Activity Questionnaire for Older Children (PAQ-C) unchanged during the experiment.
Statistical analysisthe Statistical Package for Social Sciences (SPSS) version 16.0 for Windows (SPSS - Chicago, IL, United States) was used for statistical analysis. Descriptive data were expressed as mean±standard deviation (SD). After assessment of the normal distribution by the Kolmogorov-Smirnov test, intra-group changes were compared by the paired Student's t-test, and Student's t-test for independent variables was used for inter-group comparison. Data of overweight and obese participants were also compared. Paired Student's t-test was also used for comparing before-and-after intervention within each group. Ptime, Pgroup and Ptime×group were measured for each variable through analysis of the covariance (ANCOVA). Total fecal bacterial counts of the two groups studied were compared at baseline, and on the day 15 and 60 of the trial. p-values lower than 0.05 were considered as statistically significant.
ResultsOverall, 56 out of 70 participants (80%) completed the study; the rest did not have complete adherence. The participants who completed the trial demonstrated good compliance with the supplement consumption, and no adverse effects or symptoms were reported.
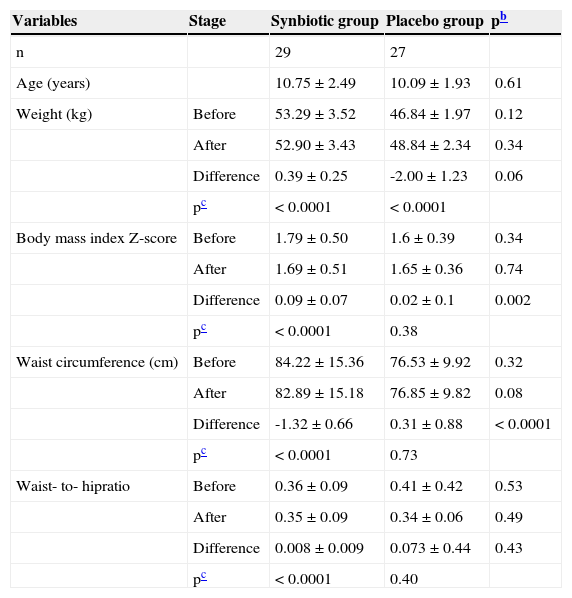
Physical characteristicsThe demographics and physical characteristics at the beginning of the study did not differ significantly between the two groups. Table 1 presents the comparisons of anthropometric measures between the synbiotic and placebo groups, as well as intra-group differences before and after the trial. The BMI Z-score decreased significantly in the synbiotic group (1.79±0.09 vs. 1.69±0.09, respectively, p < 0.0001), whereas the corresponding figure was not significant in the placebo group (1.67±0.07 vs. 1.65±0.07, respectively, p = 0.38). Likewise, the change in the mean waist circumference was significant in the synbiotic group (84.22±2.85 vs. 82.89±2.82cm, respectively, p < 0.0001), but not significant in the placebo group (76.53±1.91 vs. 76.85±1.92cm, respectively, p = 0.73)
Anthropometric measuresa of participants before and after the trial.
| Variables | Stage | Synbiotic group | Placebo group | pb |
|---|---|---|---|---|
| n | 29 | 27 | ||
| Age (years) | 10.75±2.49 | 10.09±1.93 | 0.61 | |
| Weight (kg) | Before | 53.29±3.52 | 46.84±1.97 | 0.12 |
| After | 52.90±3.43 | 48.84±2.34 | 0.34 | |
| Difference | 0.39±0.25 | -2.00±1.23 | 0.06 | |
| pc | < 0.0001 | < 0.0001 | ||
| Body mass index Z-score | Before | 1.79±0.50 | 1.6±0.39 | 0.34 |
| After | 1.69±0.51 | 1.65±0.36 | 0.74 | |
| Difference | 0.09±0.07 | 0.02±0.1 | 0.002 | |
| pc | < 0.0001 | 0.38 | ||
| Waist circumference (cm) | Before | 84.22±15.36 | 76.53±9.92 | 0.32 |
| After | 82.89±15.18 | 76.85±9.82 | 0.08 | |
| Difference | -1.32±0.66 | 0.31±0.88 | < 0.0001 | |
| pc | < 0.0001 | 0.73 | ||
| Waist- to- hipratio | Before | 0.36±0.09 | 0.41±0.42 | 0.53 |
| After | 0.35±0.09 | 0.34±0.06 | 0.49 | |
| Difference | 0.008±0.009 | 0.073±0.44 | 0.43 | |
| pc | < 0.0001 | 0.40 |
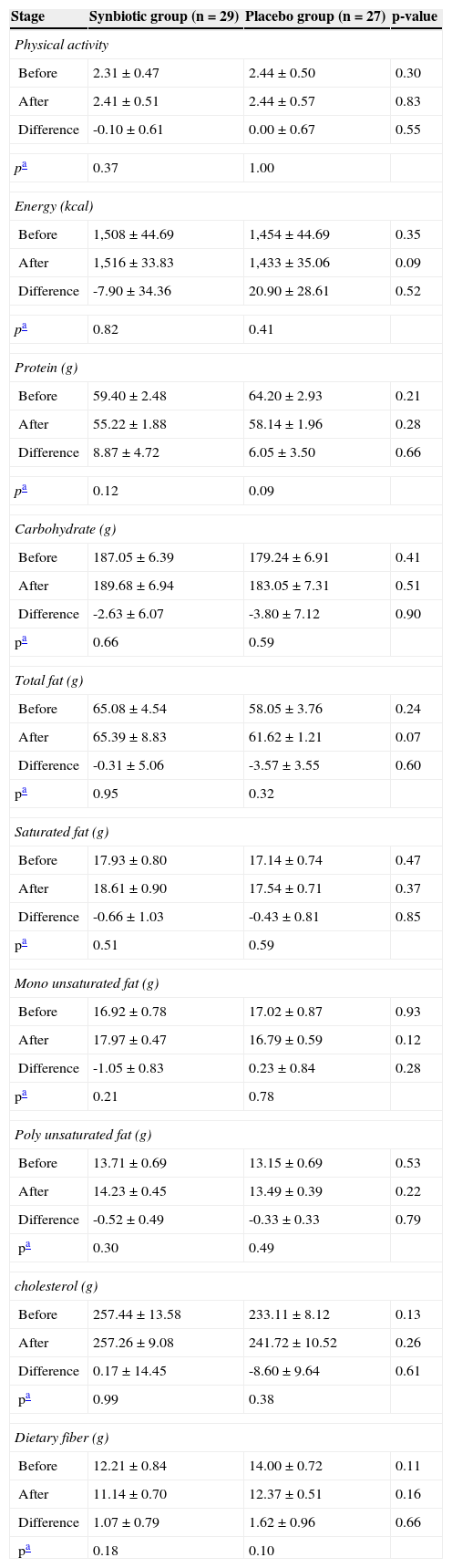
The energy and nutrient intake, as well as the levels of physical activity, were not significantly different between the two groups, and did not change significantly during the study (Table 2).
Dietary intake and physical activity level of participants throughout the trial.
| Stage | Synbiotic group (n = 29) | Placebo group (n = 27) | p-value |
|---|---|---|---|
| Physical activity | |||
| Before | 2.31±0.47 | 2.44±0.50 | 0.30 |
| After | 2.41±0.51 | 2.44±0.57 | 0.83 |
| Difference | -0.10±0.61 | 0.00±0.67 | 0.55 |
| pa | 0.37 | 1.00 | |
| Energy (kcal) | |||
| Before | 1,508±44.69 | 1,454±44.69 | 0.35 |
| After | 1,516±33.83 | 1,433±35.06 | 0.09 |
| Difference | -7.90±34.36 | 20.90±28.61 | 0.52 |
| pa | 0.82 | 0.41 | |
| Protein (g) | |||
| Before | 59.40±2.48 | 64.20±2.93 | 0.21 |
| After | 55.22±1.88 | 58.14±1.96 | 0.28 |
| Difference | 8.87±4.72 | 6.05±3.50 | 0.66 |
| pa | 0.12 | 0.09 | |
| Carbohydrate (g) | |||
| Before | 187.05±6.39 | 179.24±6.91 | 0.41 |
| After | 189.68±6.94 | 183.05±7.31 | 0.51 |
| Difference | -2.63±6.07 | -3.80±7.12 | 0.90 |
| pa | 0.66 | 0.59 | |
| Total fat (g) | |||
| Before | 65.08±4.54 | 58.05±3.76 | 0.24 |
| After | 65.39±8.83 | 61.62±1.21 | 0.07 |
| Difference | -0.31±5.06 | -3.57±3.55 | 0.60 |
| pa | 0.95 | 0.32 | |
| Saturated fat (g) | |||
| Before | 17.93±0.80 | 17.14±0.74 | 0.47 |
| After | 18.61±0.90 | 17.54±0.71 | 0.37 |
| Difference | -0.66±1.03 | -0.43±0.81 | 0.85 |
| pa | 0.51 | 0.59 | |
| Mono unsaturated fat (g) | |||
| Before | 16.92±0.78 | 17.02±0.87 | 0.93 |
| After | 17.97±0.47 | 16.79±0.59 | 0.12 |
| Difference | -1.05±0.83 | 0.23±0.84 | 0.28 |
| pa | 0.21 | 0.78 | |
| Poly unsaturated fat (g) | |||
| Before | 13.71±0.69 | 13.15±0.69 | 0.53 |
| After | 14.23±0.45 | 13.49±0.39 | 0.22 |
| Difference | -0.52±0.49 | -0.33±0.33 | 0.79 |
| pa | 0.30 | 0.49 | |
| cholesterol (g) | |||
| Before | 257.44±13.58 | 233.11±8.12 | 0.13 |
| After | 257.26±9.08 | 241.72±10.52 | 0.26 |
| Difference | 0.17±14.45 | -8.60±9.64 | 0.61 |
| pa | 0.99 | 0.38 | |
| Dietary fiber (g) | |||
| Before | 12.21±0.84 | 14.00±0.72 | 0.11 |
| After | 11.14±0.70 | 12.37±0.51 | 0.16 |
| Difference | 1.07±0.79 | 1.62±0.96 | 0.66 |
| pa | 0.18 | 0.10 | |
Data are presented as means±standard error (SE) for nutrient.
Data are presented as means ±standard deviation for physical activity.
pa, p-value resulted from paired Student's t-test for difference within groups throughout the study.
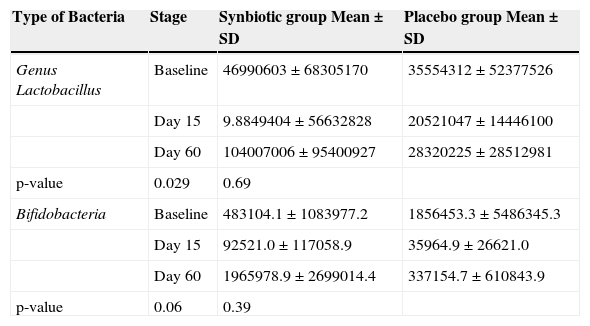
As presented in Table 3, during the experimental period, the fecal total counts increased significantly in the synbiotic group compared to the placebo group.9
Bacterial count in stool at three times during the trial.
| Type of Bacteria | Stage | Synbiotic group Mean±SD | Placebo group Mean±SD |
|---|---|---|---|
| Genus Lactobacillus | Baseline | 46990603±68305170 | 35554312±52377526 |
| Day 15 | 9.8849404±56632828 | 20521047±14446100 | |
| Day 60 | 104007006±95400927 | 28320225±28512981 | |
| p-value | 0.029 | 0.69 | |
| Bifidobacteria | Baseline | 483104.1±1083977.2 | 1856453.3±5486345.3 |
| Day 15 | 92521.0±117058.9 | 35964.9±26621.0 | |
| Day 60 | 1965978.9±2699014.4 | 337154.7±610843.9 | |
| p-value | 0.06 | 0.39 |
SD, standard deviation.
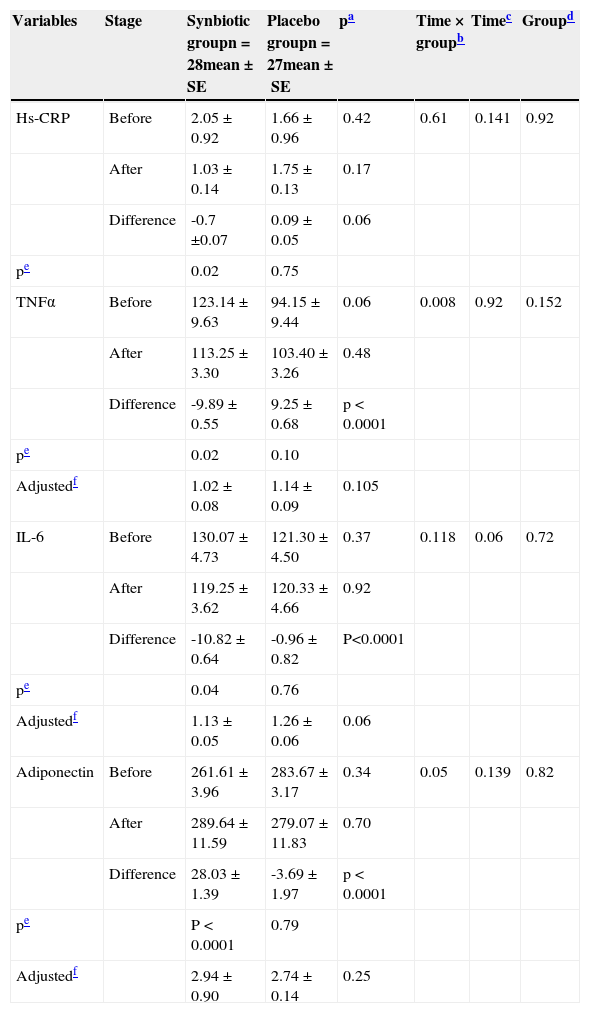
The mean (SE) of inflammation factors before and after receiving synbiotic supplement and placebo are presented in Table 4. Compared with the placebo group, the supplement group presented a significant decrease in TNF-α, IL-6 levels, and a significant increase in adiponectin; these changes were no longer significant after adjustment for BMI. There was no significant change in high-sensitivity C-reactive protein (hs-CRP) level.
Effects of synbiotic and placebo consumption on inflammation markers.
| Variables | Stage | Synbiotic groupn = 28mean±SE | Placebo groupn = 27mean±SE | pa | Time × groupb | Timec | Groupd |
|---|---|---|---|---|---|---|---|
| Hs-CRP | Before | 2.05±0.92 | 1.66±0.96 | 0.42 | 0.61 | 0.141 | 0.92 |
| After | 1.03±0.14 | 1.75±0.13 | 0.17 | ||||
| Difference | -0.7 ±0.07 | 0.09±0.05 | 0.06 | ||||
| pe | 0.02 | 0.75 | |||||
| TNFα | Before | 123.14±9.63 | 94.15±9.44 | 0.06 | 0.008 | 0.92 | 0.152 |
| After | 113.25±3.30 | 103.40±3.26 | 0.48 | ||||
| Difference | -9.89±0.55 | 9.25±0.68 | p < 0.0001 | ||||
| pe | 0.02 | 0.10 | |||||
| Adjustedf | 1.02±0.08 | 1.14±0.09 | 0.105 | ||||
| IL-6 | Before | 130.07±4.73 | 121.30±4.50 | 0.37 | 0.118 | 0.06 | 0.72 |
| After | 119.25±3.62 | 120.33±4.66 | 0.92 | ||||
| Difference | -10.82±0.64 | -0.96±0.82 | P<0.0001 | ||||
| pe | 0.04 | 0.76 | |||||
| Adjustedf | 1.13±0.05 | 1.26±0.06 | 0.06 | ||||
| Adiponectin | Before | 261.61±3.96 | 283.67±3.17 | 0.34 | 0.05 | 0.139 | 0.82 |
| After | 289.64±11.59 | 279.07±11.83 | 0.70 | ||||
| Difference | 28.03±1.39 | -3.69±1.97 | p < 0.0001 | ||||
| pe | P < 0.0001 | 0.79 | |||||
| Adjustedf | 2.94±0.90 | 2.74±0.14 | 0.25 |
Hs-CRP, high sensitivity C-reactive protein; IL-6, Interleukin 6; TNFα, tumor necrosis factor α.
All values are expressed as mean±standard error (SE).
p-values present the comparison of baseline (computed by Student's t-test for independent samples) and end-point values between two groups (computed by analysis of the covariance [ANCOVA]).
To the best of the authors’ knowledge, this trial was the first of its kind in the pediatric age group to investigate the effect of synbiotic supplementation on inflammatory factors in overweight and obese children and adolescents. It was observed that the intake of synbiotic had favorable results in weight reduction of obese children and adolescents, as well as in significant changes of serum TNFα, IL-6, and adiponectin, without change in the hs-CRP level. However, the changes in inflammation markers were dependent on weight reduction.
During the study period, the energy, macronutrient, and also antioxidant intake, and the level of physical activities did significantly change within each group, besides an increase in concentrations of protective bacteria, as shown by the microbiological data, and their metabolic activities suggest that the obtained results may be due to the synbiotic supplementation.
Cani et al. recently demonstrated that mice fed a high-fat diet were characterized by an increase in gut permeability and metabolic endotoxaemia or LPS,10 which consists in leakage into the body from the Gram-negative part of the intestinal microbiota, and its factors are involved in the onset and progression of inflammation and metabolic diseases.4 Thus, probiotics may be effective in improving the gut-barrier and suppressing Gram-negative bacteria in the gastrointestinal channel.13,14 It was demonstrated that probiotics strains such as Lactobacillus rhamnosus GG and Lactobacillus casei DN-114–001 protect the epithelial barrier function against Escherichia coli-induced redistribution of the tight-junction proteins,15,16 and that some probiotic strains, such as L. plantarum 299v, can mitigate bacterial translocation.17,18
High-fat diet contributes to the disruption of the tight-junction proteins (zonula occludens-1 Z°−1 and occludin) involved in the gut barrier function,10,19 and modulation of gut microbiota of mice with prebiotic would lead to lower levels of several plasma cytokines, such as TNF-α and IL-6, due to promotion of tight-junction proteins (ZO-1 and occludin) disruption. These data confirm that obese mice exhibit an altered gut barrier, characterized by a disruption of tight-junction proteins. Cani et al. concluded that the modulation of the gut microbiota using prebiotics in obese mice could act favorably on the intestinal barrier, thereby reducing endotoxaemia and systemic and liver inflammation, with beneficial consequences on associated metabolic disorders.19,20
Furthermore, it has been demonstrated in healthy humans that L. plantarum WCSF1 increased the relocation of occludin and ZO-1 into the tight junction area between duodenal epithelial cells.21
The presents findings are consistent with those on the connection between gut microbiota, inflammation, and homeostasis, and their role in the pathogenesis of obesity and related disorders,19,22 as well as with the findings on the relationship of diet and gut microbiota with homeostasis, as investigated in experimental models of diet-induced obesity.23,24 It appears that, because of the association between obesity and inflammation,25 it can be proposed that the favorable effects of probiotics in controlling inflammation may play a role in obesity prevention and control.
Among the probable mechanisms, it has been demonstrated that the modulation of the gut microbiota increases villus height and crypt depth, and leads to a thicker mucosal layer in the jejunum and in the colon.26 These effects are due to fermentation products of bacteria, mainly short-chain fatty acids (SCFAs), such as butyrate, which acts as an energetic substrate for the colonocytes and have a trophic effect on mucosa.27,28 In the present study, this mechanism was highlighted, because an increase in the concentrations of protective bacteria due to the synbiotic supplementation was documented.
Plasma adipocytokine levels rise with an increase in adipose tissue and adipocyte volume, except for plasma adiponectin, which is lower in obesity.29,30 In the present study, the increase in plasma adiponectin was due to weight reduction and suppression of fat tissue.
In conclusion, the present findings suggest the positive influence of synbiotic supplementation on inflammation factors, whose changes are dependent on weight reduction in overweight and obese children.
FundingThis trial was conducted as a thesis funded by the Isfahan University of Medical Sciences, Isfahan, Iran
Conflicts of InterestThe authors declare no conflicts of interest.
This study was funded by Vice Chancellery for Research and Technology, Isfahan University of Medical Sciences, Isfahan, Iran. Guarantor: Prof. Roya Kelishadi
Please cite this article as: Kelishadi R, Farajian S, Safavi M, Mirlohi M, Hashemipour M. A randomized triple-masked controlled trial on the effects of synbiotics on inflammation markers in overweight children. J Pediatr (Rio J). 2014;90:161–8.