To analyze the fecal microbiota composition of children living in an urban slum in Brazil, with or without small intestinal bacterial overgrowth, and to investigate the occurrence of stunting and anemia.
MethodsA total of 100 children were studied, aged 5–11 years, from the municipality of Osasco, São Paulo. Small intestinal bacterial overgrowth was screened through hydrogen and methane breath test with lactulose. Weight and height were measured, and the height-for-age and body mass-for-age anthropometric indexes were calculated. The occurrence of anemia was investigated by capillary hemoglobin. Analysis of bacterial phylum, genus, and species was performed by real-time polymerase chain reaction in fecal samples.
ResultsSmall intestinal bacterial overgrowth was identified in 61.0% of the children. A lower mean of height-for-age Z-score ([−0.48±0.90] vs. [−0.11±0.97]; p=0.027), as well as capillary hemoglobin ([12.61±1.03g/dL] vs. [13.44±1.19g/dL]; p<0.001) was demonstrated in children with SIBO when compared with children without small intestinal bacterial overgrowth. Children with small intestinal bacterial overgrowth presented a higher frequency of Salmonella spp., when compared to those without small intestinal bacterial overgrowth (37.7% vs. 10.3%; p=0.002). Higher counts of total Eubacteria (p=0.014) and Firmicutes (p=0.038) were observed in children without small intestinal bacterial overgrowth; however, a higher count of Salmonella (p=0.002) was found in children with small intestinal bacterial overgrowth.
ConclusionChildren who lived in a slum and were diagnosed with small intestinal bacterial overgrowth showed lower H/A Z-scores and hemoglobin levels. Furthermore, differences were observed in the fecal microbiota of children with small intestinal bacterial overgrowth, when compared to those without it; specifically, a higher frequency and count of Salmonella, and lower counts of Firmicutes and total Eubacteria.
Analisar a composição da microbiota fecal de crianças moradoras de uma favela urbana no Brasil, com e sem sobrecrescimento bacteriano no intestino delgado, e investigar a ocorrência de déficit de crescimento e anemia.
MétodosForam estudadas 100 crianças, com idade entre 5 e 11 anos, na cidade de Osasco, São Paulo. Sobrecrescimento bacteriano no intestino delgado foi pesquisado por teste respiratório do hidrogênio e metano no ar expirado com lactulose. Foram mensurados peso, estatura e calculados os índices antropométricos estatura para idade e índice de massa corporal para idade. Foi investigada a ocorrência de anemia, pela avaliação da hemoglobina capilar. A análise dos filos, gêneros e espécies bacterianas em amostras de fezes foi realizada por polymerase chain reaction em tempo real.
ResultadosSobrecrescimento bacteriano no intestino delgado foi diagnosticado em 61,0% das crianças avaliadas. Foi verificada menor média do escore Z do índice estatura para idade (-0,48±0,90 vs.-0,11±0,97 DP) e de hemoglobina capilar (12,61±1,03 vs. 13,44±1,19g/dL) no grupo de crianças com sobrecrescimento bacteriano no intestino delgado, quando comparadas àquelas sem sobrecrescimento bacteriano no intestino delgado (p<0,05). Nas crianças com sobrecrescimento bacteriano no intestino delgado foi observada maior frequência de Salmonella spp., quando comparadas àquelas sem sobrecrescimento bacteriano no intestino delgado (37,7% vs. 10,3%; p=0,002). Maior contagem de Eubactérias totais (p=0,014) e Firmicutes (p=0,038) foi observada nas crianças sem sobrecrescimento bacteriano no intestino delgado, enquanto que as crianças com sobrecrescimento bacteriano no intestino delgado apresentaram maior contagem de Salmonella (p=0,002).
ConclusãoNas crianças com diagnóstico de sobrecrescimento bacteriano no intestino delgado verificaram-se menores valores de estatura para idade e de hemoglobina. Foram constatadas diferenças na microbiota fecal das crianças com sobrecrescimento bacteriano no intestino delgado, especificamente, maior frequência e contagem de Salmonella spp. e menores contagens de Firmicutes e Eubactérias totais.
Over the last few years, several studies have been carried out aiming to broaden the knowledge about the human intestinal microbiota composition. The stool contains a large biomass of bacterial cells, representing a combination of mucosal bacteria and those transiently present in the intestinal lumen.1 However, little is known about the bacterial communities that adhere to and colonize the small intestine, because of the technical difficulties to collect samples for analysis of the intestinal contents in this gastrointestinal tract region.2
An increase in the amount of bacteria in the small intestine, especially of species common to the colon, characterizes small intestinal bacterial overgrowth (SIBO).3 This clinical condition is often associated with environmental enteropathy, recently renamed environmental enteric dysfunction,4 in individuals exposed to unhealthy environments.5 Thus, morphological and functional alterations of the small intestine can be observed, derived from a local inflammatory process4,5 through the action of pathogenic bacteria, especially Gram-negative,3 triggering a picture of chronic malabsorption of nutrients and consequent growth deficit in children,4–6 even when they are asymptomatic.4,7
Respiratory tests are a non-invasive alternative for SIBO investigation.8 In healthy individuals, hydrogen and methane production occurs predominantly by anaerobic bacterial fermentation in the large intestine. In the presence of SIBO, the production of these gases can also be observed in the small intestine, through the action of contaminating bacteria.8 In this context, a study carried out by the present research group7 in children exposed to poor living conditions found that those diagnosed with SIBO had a higher fermentation potential not only in the small intestine, but also in the colon, suggesting a situation of dysbiosis throughout the entire gastrointestinal tract in the presence of this clinical condition.
Considering that the intestinal microbiota composition can be influenced by the environment and the living conditions to which the individual is exposed9 and the negative consequences of the environmental enteric dysfunction in childhood,4,5 the present study aimed to analyze the fecal microbiota composition of children with and without SIBO living in an urban slum in Brazil, as well as to investigate the occurrence of growth deficit and anemia in these children.
MethodsDesignThis was a cross-sectional study carried out in the city of Osasco, metropolitan region of São Paulo, Brazil. The study population consisted of children of low socioeconomic status, living in an urban slum, constituting a convenience sample.
Inclusion criteria were age between 5 and 11 years, absence of diarrhea (liquid stools), and non-use of antibiotics for at least one month. Failure to perform a respiratory test and/or non-delivery of stool sample constituted sample losses. Children with clinical evidence of severe chronic diseases (e.g., heart disease) were not included in the study.
With the help of a community leader, participants were invited to the study. A total of 122 children, accompanied by their parents, volunteered to participate; however, 22 did not meet the criteria for study enrollment.
This project was approved by the Research Ethics Committee of the Universidade Federal de São Paulo. A signed informed consent was obtained from each participant's parents/guardians at the time of study enrollment.
Housing conditions, anthropometrics and hemoglobin level measurementInformation was obtained on the housing conditions from the parents/guardians. To measure weight and height, a digital scale (Filizola SA Pesagem e Automação, São Paulo, Brazil) was used, with a 150kg capacity and sensitivity of 100g, and a vertical anthropometer (Seca GmbhCo. Kg., Hamburg, Germany) with 190cm measuring capacity and sensitivity of 0.1cm. The height-for-age (H/A) and body mass index-for-age (BMI/A) Z-scores were obtained.10
Capillary hemoglobin levels, obtained from a blood sample collected by digital pulp puncture, were determined in a portable photometer (Hemocue®, Ängelholm, Sweden), considering anemia as the presence of hemoglobin levels below 11.5g/dL.11
Breath test with lactuloseThe breath test was performed after 8h of fasting and oral hygiene with antiseptic solution. After the collection of a baseline expired air sample, 10g of lactulose7,12 (Daiichi Sankyo, São Paulo, Brazil) were administered orally in a 10% aqueous solution. New samples of expired air were collected at 15, 30, 45, 60, 90, 120, and 180min after lactulose ingestion. Samples were collected in a single forced expiration, in hermetically sealed bags. Hydrogen (H2) and methane (CH4) concentrations were measured by gas chromatography (MicroLyzer SC, Quintron Instrument Co. Inc., Wisconsin, USA).
SIBO was characterized by an increase in the concentrations of H2≥20ppm and/or of CH4≥10ppm in the expired air, in relation to the concentrations in fasting samples, up to 60min after the test.7,12
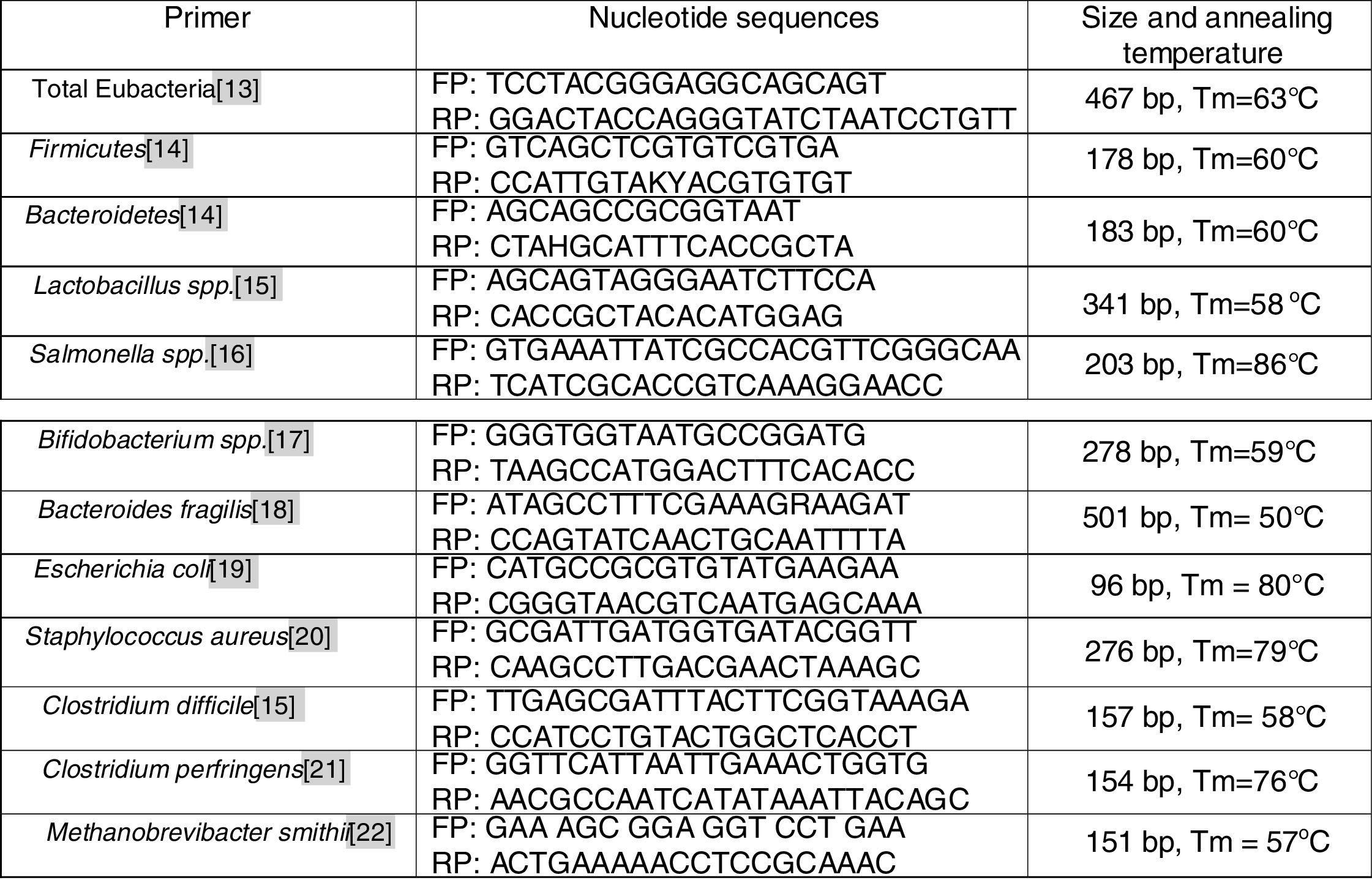
Real-time polymerase chain reaction (PCR)Stool collection was performed by the children's parents/guardians, after receiving instructions. The samples were stored in a universal stool collector and then stored in a domestic freezer for up to 24h (between evacuation and delivery). In the laboratory, a fecal aliquot of approximately 1g was transferred to a sterile cryotube containing ASL buffer from the QiaAmp mini Stool kit (Qiagen, Hilden, Germany) and kept at −20°C until DNA extraction; the bacterial genomic DNA was extracted from the samples according to the protocol suggested by the manufacturer. The purified DNA was diluted in a buffer solution to a final volume of 200μL. DNA quantification was performed on a Nanodroop 1000 spectrophotometer (ThermoScientific – Waltham, USA). All DNA samples were diluted to the concentration of 20ng/μL and stored at −20°C. The primers (Fig. 1) were used for identification and quantification of total Eubacteria,13Firmicutes and Bacteroidetes14 phyla, Lactobacillus spp.,15Salmonella spp.,16Bifidobacterium spp.17 genera, and the following species: Bacteroides fragilis,18Escherichia coli,19Staphylococcus aureus,20Clostridium difficile,15Clostridium perfringens,21 and Methanobrevibacter smithii.22 DNA from all fecal samples was submitted to the real-time PCR assay.
All reactions were carried out in duplicate, in a final volume of 10μL containing 5μL of Rotor-gene SYBR Green PCR Master Mix (Qiagen – Hilden, Germany), 0.2μL (10pmol/μL) of the forward and reverse primers of each bacteria, 0.5μL of the DNA sample (20ng/μL), and 4.1μL of DEPC (diethylpyrocarbonate) water (Qiagen – Hilden, Germany). Thermocycling was performed on the Rotor-gene Q equipment (Qiagen – Hilden, Germany) under the following conditions: 5min at 95°C, followed by 40 cycles of 95°C for 10s and 60°C for 15s. The product dissociation cycle for the melting curve was 95°C for 1m and one phase for the melting curve that ranged from 70°C to 95°C, with a gradual increase in the temperature of 1°C/s.
An internal reaction control was carried out for all samples, using primers designed to detect total Eubacteria,23 working as a standard for the relative quantification of total bacterial DNA. As negative control, a reaction containing all the reagents was used, except for the DNA sample.
The standard curve for all analyses was performed by the amplification of a TopoTA plasmid (Invitrogen®, USA), which contained the gene fragment for each bacterium, previously amplified by conventional PCR, and its specificity was confirmed by sequencing and alignment in the BLAST system (Canablast®, Canada).
Statistical analysisThe Mann–Whitney or Student's t-test was used to analyze the results when comparing two independent groups for continuous numerical variables, while the chi-squared test was used for categorical variables. The calculations were performed using the Sigma Stat program (Systat software, Inc, version 3.1, USA) setting the level for rejection of the null hypothesis at 5%.
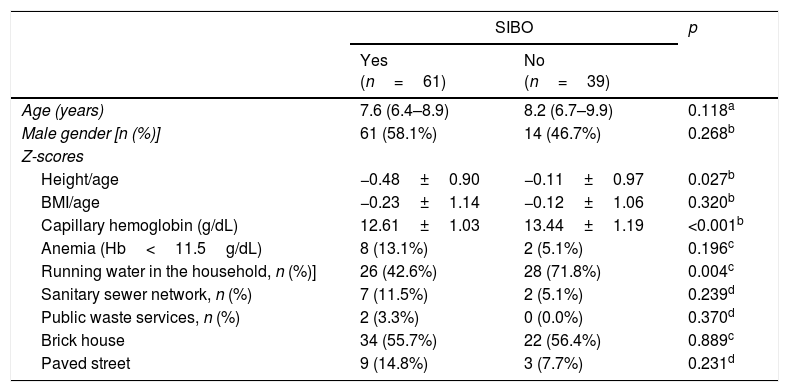
ResultsSIBO was diagnosed in 61/100 (61.0%) children. Table 1 presents the demographic and anthropometric data, frequency of anemia, and mean hemoglobin values. The group of children with SIBO presented lower values of H/A and hemoglobin Z-scores (p<0.05) when compared with those without SIBO. An association was also found between SIBO and absence of running water supply at the household.
Anthropometric data and living conditions of children living in an urban slum, with or without small intestine bacterial overgrowth (SIBO).
| SIBO | p | ||
|---|---|---|---|
| Yes (n=61) | No (n=39) | ||
| Age (years) | 7.6 (6.4–8.9) | 8.2 (6.7–9.9) | 0.118a |
| Male gender [n (%)] | 61 (58.1%) | 14 (46.7%) | 0.268b |
| Z-scores | |||
| Height/age | −0.48±0.90 | −0.11±0.97 | 0.027b |
| BMI/age | −0.23±1.14 | −0.12±1.06 | 0.320b |
| Capillary hemoglobin (g/dL) | 12.61±1.03 | 13.44±1.19 | <0.001b |
| Anemia (Hb<11.5g/dL) | 8 (13.1%) | 2 (5.1%) | 0.196c |
| Running water in the household, n (%)] | 26 (42.6%) | 28 (71.8%) | 0.004c |
| Sanitary sewer network, n (%) | 7 (11.5%) | 2 (5.1%) | 0.239d |
| Public waste services, n (%) | 2 (3.3%) | 0 (0.0%) | 0.370d |
| Brick house | 34 (55.7%) | 22 (56.4%) | 0.889c |
| Paved street | 9 (14.8%) | 3 (7.7%) | 0.231d |
BMI, body mass index; Hb, hemoglobin.
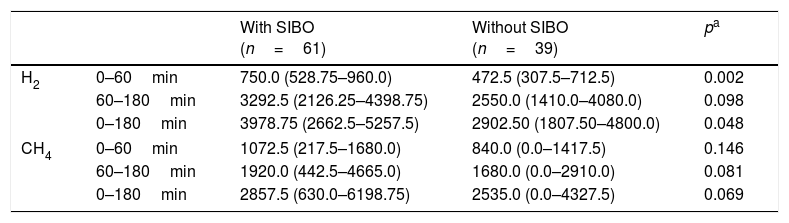
Table 2 shows the hydrogen and methane production, obtained from the breath test with lactulose, and expressed as individual areas under the curve. It was observed that children with SIBO showed a higher hydrogen production during the first hour of the test (p=0.002), presumably in the small intestine. This difference was not verified with methane concentrations. Between 60 and 180min, a period during which gas production occurs predominantly in the large intestine, children with SIBO showed higher hydrogen and methane concentrations; however, the differences did not reach statistical significance (p=0.081 and 0.098, respectively).
Area under the curve of the concentration in PPM/min, of hydrogen (H2) and methane (CH4) obtained from the breath test with lactulose of children living in an urban slum, with or without small intestine bacterial overgrowth (SIBO) during the first 60min, between 60 and 180min and the entire test period.
| With SIBO (n=61) | Without SIBO (n=39) | pa | ||
|---|---|---|---|---|
| H2 | 0–60min | 750.0 (528.75–960.0) | 472.5 (307.5–712.5) | 0.002 |
| 60–180min | 3292.5 (2126.25–4398.75) | 2550.0 (1410.0–4080.0) | 0.098 | |
| 0–180min | 3978.75 (2662.5–5257.5) | 2902.50 (1807.50–4800.0) | 0.048 | |
| CH4 | 0–60min | 1072.5 (217.5–1680.0) | 840.0 (0.0–1417.5) | 0.146 |
| 60–180min | 1920.0 (442.5–4665.0) | 1680.0 (0.0–2910.0) | 0.081 | |
| 0–180min | 2857.5 (630.0–6198.75) | 2535.0 (0.0–4327.5) | 0.069 | |
The following were identified in all children (100.0%): Bacteroides fragilis, Escherichia coli, Lactobacillus spp., Bifidobacterium spp., and Methanobrevibacter smithii. As for the other genera and species analyzed, variable frequencies were observed in children with and without SIBO, respectively: Salmonella spp. (37.7% vs. 10.3%; p=0.002), Staphylococcus aureus (52.5% vs. 41.0%; p=0.267), Clostridium difficile (44.3% vs. 41.0%; p=0.751), and Clostridium perfringens (91.8% vs. 92.3%; p=0.928).
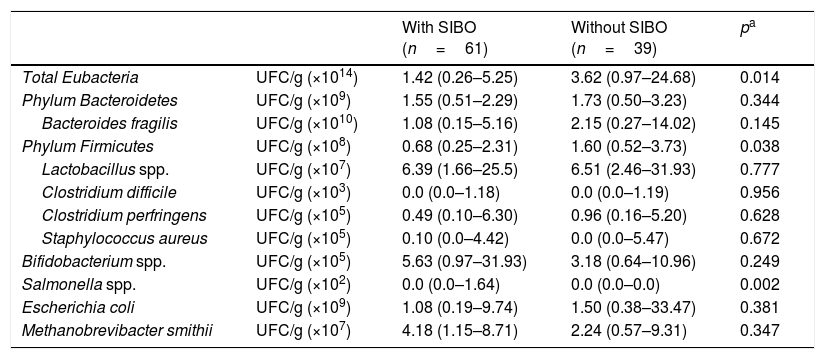
A higher total count of Eubacteria (p=0.014) and the Firmicutes phylum (p=0.038) was verified in the group of children without SIBO; however, a higher Salmonella count (p=0.002) was observed in children with SIBO. The quantification of bacterial phyla, genera, and species, according to the presence or absence of SIBO, is presented in Table 3.
Bacterial phylum, genus, and species (colony forming units: CFU/g of feces) that represent the fecal microbiota of children living in urban slums, with or without small intestine bacterial overgrowth (SIBO).
| With SIBO (n=61) | Without SIBO (n=39) | pa | ||
|---|---|---|---|---|
| Total Eubacteria | UFC/g (×1014) | 1.42 (0.26–5.25) | 3.62 (0.97–24.68) | 0.014 |
| Phylum Bacteroidetes | UFC/g (×109) | 1.55 (0.51–2.29) | 1.73 (0.50–3.23) | 0.344 |
| Bacteroides fragilis | UFC/g (×1010) | 1.08 (0.15–5.16) | 2.15 (0.27–14.02) | 0.145 |
| Phylum Firmicutes | UFC/g (×108) | 0.68 (0.25–2.31) | 1.60 (0.52–3.73) | 0.038 |
| Lactobacillus spp. | UFC/g (×107) | 6.39 (1.66–25.5) | 6.51 (2.46–31.93) | 0.777 |
| Clostridium difficile | UFC/g (×103) | 0.0 (0.0–1.18) | 0.0 (0.0–1.19) | 0.956 |
| Clostridium perfringens | UFC/g (×105) | 0.49 (0.10–6.30) | 0.96 (0.16–5.20) | 0.628 |
| Staphylococcus aureus | UFC/g (×105) | 0.10 (0.0–4.42) | 0.0 (0.0–5.47) | 0.672 |
| Bifidobacterium spp. | UFC/g (×105) | 5.63 (0.97–31.93) | 3.18 (0.64–10.96) | 0.249 |
| Salmonella spp. | UFC/g (×102) | 0.0 (0.0–1.64) | 0.0 (0.0–0.0) | 0.002 |
| Escherichia coli | UFC/g (×109) | 1.08 (0.19–9.74) | 1.50 (0.38–33.47) | 0.381 |
| Methanobrevibacter smithii | UFC/g (×107) | 4.18 (1.15–8.71) | 2.24 (0.57–9.31) | 0.347 |
In the present study, differences were observed in the fecal microbiota composition of children with SIBO living in an urban slum; more precisely, higher frequency and counts of Salmonella spp. and lower counts of Firmicutes and total Eubacteria were observed in children with SIBO when compared to those without it.
In a previous study carried out by the present research team, a finding that motivated the study of the fecal microbiota of children exposed to poverty and diagnosed with SIBO was a differentiated pattern of fermentation in the colon, characterized by the higher production of hydrogen in the breath test.9 This result led to the assumption that individuals with SIBO possibly have a situation of dysbiosis in the different intestinal segments, and not only in the small intestine. However, this pattern of higher production of hydrogen and even methane in the colon of children with SIBO, although suggestive, was not confirmed by the present results.
The study of the intestinal bacterial composition is made possible by the analysis of fecal samples.1 In turn, endoscopic procedures, associated with analyses of the intestinal content (jejunal aspirate), would be necessary for the characterization of the small intestine microbiota, considered the gold standard in the diagnosis of SIBO.3,8,24 However, the invasive characteristics and the high cost8 of this method make it unfeasible for the evaluation of asymptomatic or non-specific individuals, in addition to the fact that its use may not be ethically appropriate for research purposes.24
In healthy individuals, the bacterial colonization in the proximal small intestine (102CFU/g of intestinal contents) is small when compared with that in the colon (1010–1012CFU/g feces). The lower bacterial density in both the stomach and small intestine is due to the action of gastric juice and digestive enzymes, in addition to peristaltic movements, as part of the migrating motor complex (MMC) observed in these segments.24 Conversely, the characterization of SIBO is usually associated with qualitative and quantitative changes in bacterial genera and species in the small intestine.3
The bacteria involved in the occurrence of SIBO are mainly Gram-negative, which have lipopolysaccharide (LPS) in their cell membranes. LPS is associated with the onset of a local inflammatory process, causing mucosal lesions and increased intestinal permeability,3,6 with a consequent malabsorption syndrome4–6 and high nutrient fermentation in the colon.3 An inhibitory action of MMC is also attributed to the bacterial LPS, which would cause a stasis of the luminal content in the interdigestive period, favoring the excessive growth, in the small intestine, of bacteria common to the colon.24
Intestinal enteric dysfunction is associated with infection by potentially pathogenic microorganisms, which permeates a condition of intestinal dysbiosis.5Salmonella and Escherichia coli25 are species with high pathogenic potential strains, very often with diarrhea as a gastrointestinal symptom. In the present study, a high frequency of Salmonella spp. and a higher count in fecal samples was observed in children with SIBO when compared with those without it, a result that indicates a higher number of asymptomatic carriers than expected.
A lower quantification of Firmicutes was observed in the SIBO group. According to some authors, a greater variability in intestinal bacterial composition may reflect a greater resistance to pathogen invasion.26 Intestinal bacterial diversity appears to confer resilience and, consequently, greater stability of the bacterial ecosystem.27 However, the higher quantification of a bacterial phylum is not necessarily associated with a greater number or diversity of colonizing bacterial genera and species. Similarly, the higher quantification of total Eubacteria in the group of children with SIBO can be reflect a higher bacterial concentration.
The genetic variability of the microorganisms that make up the microbiota of individuals with and without SIBO could be identified with the use of new generation sequencing technologies, which may be the subject of future studies. The technique used in the present study constitutes a limiting factor, since it only allows the assessment of some pre-selected bacterial groups.
The present study did not find other differences in the fecal microbiota composition in children with and without SIBO. Based on this observation, the power of the test was analyzed; the results of Escherichia coli counts were considered for the calculation, since the interpretation of its results shows biological plausibility in the presence of SIBO. Considering the statistical test (Mann–Whitney) and effect size (d=0.45), calculated from the means and standard deviations of the Escherichia coli count (CFU/g of feces) of both groups, it was observed that the power (1−β) for this analysis was 56.6%. To obtain a power of 80%, maintaining the effect size and α=5%, a total of 164 individuals would be required (Software G*Power, version 3.1.9.2). Therefore, this also constitutes a study limitation, which may justify the non-attainment of statistical evidence in some analyses.
The occurrence of SIBO in children exposed to unhealthy housing conditions and to vehicles of contamination is the main indicator of environmental enteropathy.7 In the present study, the prevalence of SIBO (61.0%) was high, being significantly higher than that observed in other studies.7,12 In this context, the lower access to running water in the households of children with SIBO is noteworthy. A previous study,7 carried out in this same community, showed that 41.2% of the households had a clandestine water supply; in the group of children with SIBO, the presence of fecal coliforms was verified in 80.8% of analyzed water samples.
In environmental enteropathy, the presence of small intestinal villous atrophy, cryptic hyperplasia, and lymphoplasmacytic infiltrate in the lamina propria can be observed.5 Macronutrient and micronutrients malabsorption is characterized, and these digestive-absorptive dysfunctions may be associated with the occurrence of short stature in children from developing countries.24
Different authors, when studying the behavior of environmental enteropathy biomarkers in children exposed to poor living conditions in the Northeast of Brazil6 and in Bangladesh,28 found a reduction in the intestinal barrier action and absorptive function due to the increase in intestinal permeability verified by serum levels of zonulin6 and lactulose and mannitol absorption test,6,28 respectively. An association with the systemic inflammatory response induced by microbial products, such as LPS,6 was also verified; the biomarkers were shown to be factors associated with growth deficits in children. However, these data need to be analyzed with caution, due to the complexity of the mechanisms involved in the intestinal and systemic inflammatory response.28
The greater susceptibility of children living in slums to nutritional disorders has been already well demonstrated in the literature.7,9 However, the present findings also indicate a higher frequency of low nutritional stature in SIBO patients, when compared to those without SIBO, when both groups are exposed to the same risk factors.
A study carried out in Bangladesh with 90 2-year-old children living in poverty found that the main factor associated with SIBO was the reduction in the H/A Z-score, in comparison to the birth parameters, regardless of whether children had or not recent or frequent diarrheal disease.29
Another result that, similar to short stature, reinforces the occurrence of a malabsorption syndrome, was the lower mean levels of hemoglobin found in the group of children with SIBO. Another study30 carried out with children living in a slum found an association between the occurrence of anemia and an intestinal function abnormality, characterized by lower absorption of d-xylose.
It is important to emphasize the originality of the present findings, which may help to understand the environmental enteric dysfunction and its consequences, more precisely the association with SIBO and changes in the intestinal microbiota. It should be emphasized the finding of lower H/A and hemoglobin levels in children with SIBO, when compared to those without it, even though they lived in the same urban slum. This result may suggest that exposure to microorganisms with high pathogenic potential, characterized here by the higher frequency and counts of Salmonella in children with SIBO, could represent an important factor associated with the development of short stature and anemia.
Much remains to be elucidated about bacterial communities and their interactions with the human organism. However, based on the hypothesis that individuals susceptible to bacterial contamination by potentially pathogenic species may present serious damage in their health and nutrition, it is necessary to emphasize the importance of effective public policies for the improvement of housing conditions and basic sanitation of the vulnerable population, thus contributing to the eradication of environmental enteropathy.
FundingFundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Mello CS, Rodrigues MS, Filho HB, Melli LC, Tahan S, Pignatari AC, et al. Fecal microbiota analysis of children with small intestinal bacterial overgrowth among residents of an urban slum in Brazil. J Pediatr (Rio J). 2018;94:483–90.