The aim of this review was to summarize the most common extrapulmonary manifestations in pediatric patients with COVID-19, as well as to discuss clinical, epidemiological, and pathophysiological aspects of these clinical presentations in children.
Source of dataAn extensive search of literature was performed in order to identify pediatric cases with extrapulmonary manifestations between January 1, 2020 and June 21, 2020. Generic keywords, such as “Novel coronavirus” or “Novel coronavirus 2019” or “2019 nCoV” or “COVID-19” or “SARS-CoV-2” were searched on PubMed database, associated either with age filters or generic pediatric terms.
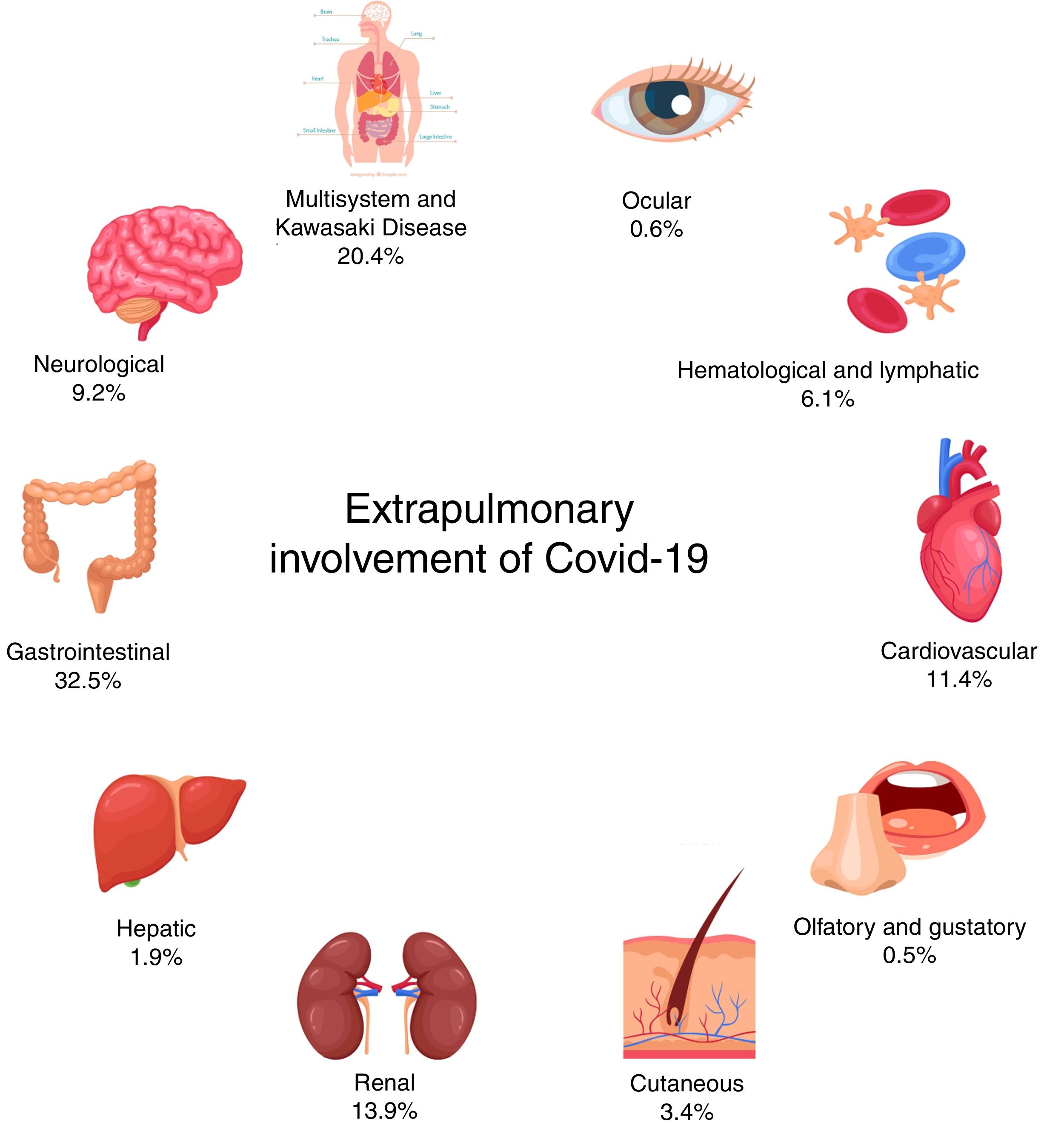
Summary of findingsA total of 28 articles, including 199 patients, were considered suitable to review and data extraction. The main findings were summarized in tables. The main non-pulmonary manifestations in pediatric patients, in decreasing order of frequency, were gastrointestinal, renal, cardiovascular, neurological, hematological and lymphatic, cutaneous, hepatic, ocular, olfactory, and gustatory. Multisystem impairment and Kawasaki-like disease were also described.
ConclusionsDifferences in immune response of children and variations of tissue expression of angiotensin converting enzyme 2, the virus receptor, are likely to influence clinical, epidemiological, and pathophysiological patterns of the disease.
In December 2019, a case series of pneumonia with unknown origin was reported in Wuhan, the capital of Hubei province, China. Later, it became known that a new type of enveloped RNA betacoronavirus, officially named severe acute respiratory syndrome coronavirus 2] (SARS-CoV-2), had emerged.1 In January 30, 2020, the (World Health Organization (WHO) declared a public health emergency of international concern,2 and in March 11, 2020, recognized as a global pandemic.3
During infection, the SARS-CoV-2 enters into host cells by binding the spike protein (protein S) expressed in the viral envelope with the membrane-bound angiotensin-converting enzyme 2 (ACE2), the virus receptor present in lung cells. This binding leads to the endocytosis of the SARS-CoV-2 and ACE2 complex, resulting in the viral entry into the cell.4
ACE2 is a crucial enzyme of the renin-angiotensin-aldosterone system (RAAS), which has major role in the control of blood pressure, cardiovascular, renal, immune, and neural systems homeostasis.5 Then, due to this intersection the interaction of SARS-CoV-2 with ACE2, some scientists hypothesize that the coronavirus disease 2019 (COVID-19) produces RAAS imbalance, which may explain some clinical findings.6 At the beginning of the pandemic, COVID-19 was primarily considered a pulmonary disease with extrapulmonary manifestations. Currently, data support that SARS-CoV-2 infection is a systemic disease with pulmonary involvement. Additionally, studies have shown that ACE2 is also expressed in other organs, with high expression in the ileum and kidney, followed by adipocytes, heart, brain stem, small intestine enterocytes, stomach, liver, and vasculature, leading to more possibilities of extrapulmonary manifestations.7–10
Despite all the scientific community’s and health workers’ effort, by July 21, 2020, the SARS-CoV-2 infection had affected over 14 million people and killed 607,781 patients, mostly elderly individuals with associated comorbidities, including hypertension and diabetes.11 It appears that children are less frequently infected and less severely affected by COVID-19, unlike other respiratory diseases.12 However, due to the outrageous number of infected children, some fatal cases are being reported, associated with extrapulmonary complications, pointing to a need of medical and scientific attention.13–40
In this study, the authors aimed to perform an extensive search of extrapulmonary manifestation in pediatric cases of COVID-19.
MethodologyOutcomeThe expected outcome of this study was to qualitatively summarize the signs and symptoms of the SARS-CoV-2 infection in children currently reported in the literature, along with clinical and epidemiological features presented.
Search strategyTwo authors independently performed a search at PubMed platform, searching for articles published between January 1, 2020 to June 21, 2020. First, a search was made utilizing the generic keywords “Novel coronavirus” or “Novel coronavirus 2019” or “2019 nCoV” or “COVID-19” or “SARS-CoV-2.”. Then, PubMed age filters, ranging from 0 to 18 years, were applied. The authors also restricted the search, using the “Article Type” filter to only show “Case Reports”, “Clinical Study”, “Letter”, and “Observational Study.” To minimize any ineffectiveness of the age filters, a second search was made by disabling the filters and adding the terms “children”, “infant”, “neonate” and “adolescent” to the generic terms specified in the first search.
Data extractionOne author extracted data from the selected studies and included the findings in Table 1. Then, a second author reviewed all selected studies and the table to avoid any errors in data extraction.
- A
Study identification: First author, publication date, country, and type of study;
- B
Participants: Age, gender, and sample size;
- C
Variables: Source of SARS-CoV-2 exposure, signs and symptoms, co-morbidities and co-infections, complications during the disease, main treatment, and outcome.
Study characteristics and epidemiological and clinical data analysis of pediatric patients infected with Sars-Cov-2.
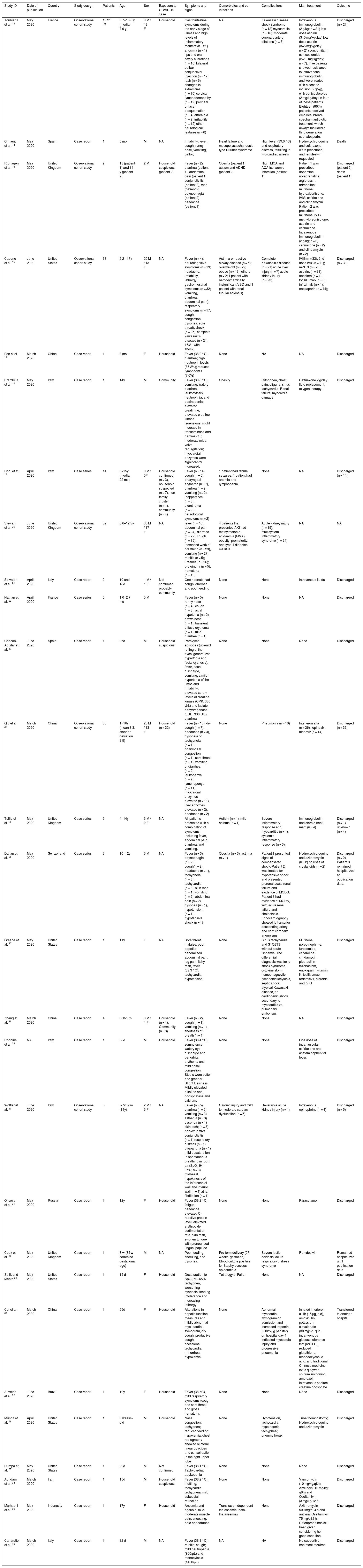
| Study ID | Date of publication | Country | Study design | Patients | Age | Sex | Exposure to COVID-19 case | Symptoms and signs | Comorbidies and co-infections | Complications | Main treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Toubiana et al. 13 | May 2020 | France | Observational cohort study | 19/21 (a) | 3.7−16.6 y (median 7.9 y) | 9 M / 12 F | Household | Gastrointestinal symptoms during the early stage of illness and high levels of inflammatory markers (n = 21) anosmia (n = 1) lips and oral cavity alterations (n = 16) bilateral bulbar conjunctival injection (n = 17) rash (n = 6) changes to extremities (n = 10) cervical lymphadenopathy (n = 12) perineal or face desquamation (n = 4) arthralgia (n = 2) irritability (n = 12) other neurological features (n = 6) | NA | Kawasaki disease shock syndrome (n = 12) myocarditis (n = 16), moderate coronary artery dilations (n = 5) | Intravenous immunoglobulin (2 g/kg; n = 21) low dose aspirin (3−5 mg/kg/day) low dose aspirin (3−5 mg/kg/day; n = 21) concomitant corticosteroids (2−10 mg/kg/day; n = 7). Five patients showed resistance to intravenous immunoglobulin and were treated with a second infusion (2 g/kg), with corticosteroids (2 mg/kg/day) in four of these patients. Eighteen (86%) patients received empirical broad-spectrum antibiotic treatment, which always included a third generation cephalosporin. | Discharged (n = 21) |
| Climent et al. 14 | May 2020 | Spain | Case report | 1 | 5 mo | M | NA | Irritability, fever, cough, runny nose, vomiting, pallor, | Heart failure and mucopolysaccharidosis type I-Hurler syndrome | High fever (39.6 °C) and respiratory distress, resulting in two cardiac arrests | Hydroxychloroquine and ceftriaxone were prescribed, and remdesivir requested | Death |
| Riphagen et al. 15 | May 2020 | United Kingdom | Observational cohort study | 2 | 13 (patient 1) and 14 y (patient 2) | 2 M | Household suspicious (patient 2) | Fever (n = 2), diarrhea (patient 1), abdominal pain (patient 1), conjunctivitis (patient 2), rash (patient 2), odynophagia (patient 2) headache (patient 1) | Obesity (patient 1), autism and ADHD (patient 2) | Right MCA and ACA ischaemic infarction (patient 1) | Patient 1 was prescribed dopamine, noradrenaline, argipressin, adrenaline milrinone, hydroxicortisone, IVIG, ceftriaxone and clindamycin. Patient 2 was prescribed milrinone, IVIG, methylprednisolone, aspirin and ceftriaxone. Intravenous immunoglobulin (2 g/kg; n = 2) ceftriaxone (n = 2) and clindamycin (n = 2) | Discharged (patient 2), death (patient 1) |
| Capone et al. 16 | June 2020 | United States | Observational cohort study | 33 | 2.2 - 17y | 20 M / 13 F | NA | Fever (n = 4); neurocognitive symptoms (n = 19; headache, irritability, lethargy); gastrointestinal symptoms (n = 32; vomiting, diarrhea, abdominal pain); respiratory symptoms (n = 17; cough, congestion, dyspnea, sore throat); shock (n = 25); complete kawasaki's disease (n = 21, 16/21 with shock) | Asthma or reactive airway disease (n = 5); overweight (n = 2); obese (n = 13); others (n = 2; 1 patient with hemodynamically insignificant VSD and 1 patient with renal tubular acidosis) | Complete Kawasaki's disease (n = 21) acute liver injury (n = 7) acute kidney injury (n = 23) | IVIG (n = 33); 2nd dose IVIG n = 11); mPDN (n = 23); aspirin, (n = 29); anakinra (n = 4); tocilizumab (n = 3); infliximab (n = 1); enoxaparin (n = 14); | Discharged (n = 33) |
| Fan et al. 17 | March 2020 | China | Case report | 1 | 3 mo | F | Household | Fever (38.2 °C); diarrhea; high neutrophil levels (86.2%); reduced lymphocites (7.6%) | None | NA | NA | Discharged |
| Brambilla et al. 18 | May 2020 | Italy | Case report | 1 | 14y | M | Community | Fever (39.8 °C), vomiting, watery diarrhea, leukocytosis, neutrophilia, and eosinopenia, elevated creatinine, elevated creatine kinase isoenzyme, slight increase in transaminase and gamma-GT; moderate mitral valve regurgitation; myocardial enzymes were significantly increased. | Obesity | Orthopnea, chest pain, oliguria, sinus tachycardia; Renal failure; myocardial damage | Ceftriaxone 2 g/day; fluid replacement; oxygen therapy; | Discharged |
| Dodi et at 19 | April 2020 | Italy | Case series | 14 | 0−15y (median 22 mo) | 9 M / 5F | Household confirmed (n = 3), household suspected (n = 7), non family cluster (n = 1), community (n = 4) | Fever (n = 14), cough (n = 5), pharyngeal erythema (n = 7), diarrhea (n = 2), vomiting (n = 2), inappetence (n = 3), exanthema (n = 2), neurological symptoms (n = 2) | 1 patient had febrile seizures. 1 patient had anemia and lymphopenia. | None | NA | Discharged (n = 14) |
| Stewart et al. 20 | June 2020 | United Kingdom | Observational cohort study | 52 | 5.6–12.9y | 35 M / 17 F | NA | fever (n = 46), abdominal pain (n = 24), diarrhea (n = 22), cough (n = 15), increased work of breathing (n = 23), vomiting (n = 27), rhinitis (n = 5); uraemia (n = 26); proteinuria (n = 5), hematuria (n = 12) | 4 patients that presented AKI had methylmalonic acidaemia (MMA), obesity, prematurity, and type 1 diabetes mellitus. | Acute kidney injury (n = 15); multisystem inflammatory syndrome (n = 24) | NA | NA |
| Salvatori et al. 21 | April 2020 | Italy | Case report | 2 | 10 and 18d | 1 M / 1 F | Not confirmed, probably community | One neonate had cough, diarrhea and poor feeding | None | None | Intravenous fluids | Discharged |
| Nathan et al. 22 | April 2020 | France | Case series | 5 | 1.6−2.7 mo | 5 M | Fever (n = 5), runny nose (n = 4), cough (n = 3), axial hypotonia (n = 2), drowsiness (n = 1), transient diffuse erythema (n = 1), mild diarrhea (n = 1) | None | None | NA | Discharged | |
| Chacón-Aguilar et al. 23 | June 2020 | Spain | Case report | 1 | 26d | M | Household suspicious | Paroxymal episodes (upward rolling of the eyes, generalized hypertonia and facial cyanosis), fever, nasal discharge, vomiting, a mild hypertonia of the limbs and irritability, elevated serum levels of creatine kinase (CPK, 380 U/L) and lactate dehydrogenase (LDH, 390 U/L), diarrhea | None | None | None | Discharged |
| Qiu et al. 24 | March 2020 | China | Observational cohort study | 36 | 1−16y (mean 8.3; standart deviation 3.5) | 23 M / 13 F | Household (n = 32) | Fever (n = 13), dry cough (n = 7), headache (n = 3), dyspneia or tachypneia (n = 1), pharyngeal congestion (n = 1), sore throat (n = 1), vomiting or diarrhea (n = 2), leukopenya (n = 7), lymphopenya (n = 11), myocardial enzymes elevated (n = 11), liver enzymes elevated (n = 2), headache (n = 2) | None | Pneumonia (n = 19) | Interferon alfa (n = 36), lopinavir–ritonavir (n = 14) | Discharged (n = 36) |
| Tullie et al. 25 | May 2020 | United Kingdom | Case series | 5 | 4−14y | 3 M / 2 F | NA | All patients presented with a combination of symptoms including fever, abdominal pain, diarrhea, and vomiting. | Autism (n = 1), mild asthma (n = 1) | Severe inflammatory response and myocarditis (n = 1), systemic inflammatory response (n = 3), | Immunoglobulin and steroid treat- ment (n = 4) | Discharged (n = 1), unknown (n = 4) |
| Dallan et al. 26 | May 2020 | Switzerland | Case series | 3 | 10−12y | 3 M | NA | Fever (n = 3), odynophagia (n = 2), cough(n = 2), headache (n = 1), tachypneia (n = 3), tachycardia (n = 3), skin rash (n = 1), vomiting (n = 2), abdominal pain (n = 2), dyspnea (n = 1), hypotension (n = 1), hypotensive shock (n = 1) | Obesity (n = 3), asthma (n = 1) | Patient 1 presented signs of compensated shock. Patient 2 was treated for hypotensive shock and presented prerenal acute renal failure and evidence of MODS. Patient 3 had evidence of MODS, with acute renal failure and cholestasis.. Echocardiography showed left anterior descending artery and right coronary aneurysms | Hydroxychloroquine and azithromycin (n = 2) boluses of crystalloids (n = 2) | Discharged (n = 2). Patient 3 remained hospitalized at publication date. |
| Greene et al. 27 | May 2020 | United States | Case report | 1 | 11y | F | NA | Sore throat, malaise, poor appetite, generalized abdominal pain, leg pain, itchy rash, fever (39.3 °C), tachycardia, hypotension | None | Sinus tachycardia and S1Q3T3 without acute ischemia. The differential diagnosis was toxic shock syndrome, cytokine storm, hemophagocytic lymphohistiocytosis, septic shock, atypical Kawasaki disease, or cardiogenic shock secondary to myocarditis vs. pulmonary embolism. | Milrinone, norepinephrine, furosemide, ceftaroline, clindamycin, piperacillin-tazobactam, enoxaparin, vitamin K, tocilizumab, redemsivir, steroids and IVIG | Discharged |
| Zhang et al. 28 | March 2020 | China | Case report | 4 | 30h-17h | 3 M / 1 F | Household (n = 1); Community (n = 3) | Fever (n = 2), cough (n = 1), vomiting (n = 1), shortness of breath (n = 1) | None | None | NA | Discharged |
| Robbins et al. 29 | NA | Italy | Case report | 1 | 58d | M | Household | Fever (38.4 °C), somnolence, watery eye discharge and periorbital erythema and mild nasal congestion. Stools were softer and greener. Slight fussiness Mildly elevated alkaline and phosphatase and calcium. | None | None | One dose of intramuscular ceftriaxone and acetaminophen for fever. | Discharged |
| Wolfler et al. 30 | June 2020 | Italy | Observational cohort study | 5 | ∼7y (2 m -14y) | 2 M / 3 F | NA | Fever (n = 5) diarrhea (n = 5) vomiting (n = 3) asthenia (n = 3) dyspnea (n = 1) skin rash; (n = 3) non-exudative conjunctivitis (n = 1) respiratory distress (n = 1) oligoanuria (n = 1) mild desaturation in spontaneous breathing in room air (SpO2 94–96%; n = 3) midbasal hypokinesis of the inferoseptal wall and inferior wall (n = 4) atrial fibrillation (n = 1) | Cardiac injury and mild to moderate cardiac dysfunction (n = 5) | Reversible acute kidney injury (n = 1) | Intravenous epinephrine (n = 4) | Discharged (n = 5) |
| Olisova et al. 31 | May 2020 | Russia | Case report | 1 | 12y | F | Household | Fever (38.2 °C), fatigue, headache, elevated C-reactive protein level, elevated erythrocyte sedimentation rate, skin rash, swollen tongue with pronounced lingual papillae | None | None | Paracetamol | Discharged |
| Cook et al. 32 | May 2020 | United Kingdom | Case report | 1 | 8 w (35 w corrected gestational age) | M | NA | Poor feeding, sneezing, and dyspnea. | Pre-term delivery (27 weeks' gestation). Blood culture positive for Staphylococcus epidermidis | Severe lactic acidosis, acute respiratory distress syndrome | Remdesivir | Remained hospitalized until publication date |
| Salik and Mehta 33 | May 2020 | United States | Case report | 1 | 15 d | F | Household | Desaturation to SpO2 60–65%, tachypnea, worsening cyanosis, feeding intolerance and increasing lethargy. | Tetralogy of Fallot | None | NA | Discharged |
| Cui et al. 34 | March 2020 | China | Case report | 1 | 55d | F | Household | Alterations in hepatic function measures and mildly abnormal myo- cardial zymogram, dry cough, productive cough, occasional tachycardia, rhinorrhea, hypoxemia | None | Abnormal myocardial zymogram on admission and increased troponin I (0.025 μg per liter) on hospital day 4 indicated myocardia injury and progressive pneumonia | Inhaled interferon α-1b (15 μg, bid), amoxicillin potassium clavulanate (30 mg/kg, q8h, intra- venous glucose tolerance test [IVGTT]), reduced glutathione, ursodeoxycholic acid, and traditional Chinese medicine lotus qingwen, sputum suctioning, ambroxol, intravenous sodium creatine phosphate | Transferred to another hospital |
| Almeida et al. 35 | June 2020 | Brazil | Case report | 1 | 10y | F | Household | Fever (38 °C), mild respiratory symptoms (cough and sore throat) and gross hematuria. | None | None | None | Discharged |
| Munoz et al. 36 | April 2020 | United States | Case report | 1 | 3 weeks-old | M | Household | Nasal congestion; tachypnea; reduced feeding; hypoxemia; chest radiography showed bilateral linear opacities and consolidation in the right upper lobe | None | Hypotension, tachycardia, hypothermia, tachypnea; pneumothorax | Tube thoracostomy; Hydroxychloroquine and azithromycin | Discharged |
| Dumpa et al. 37 | May 2020 | United States | Case report | 1 | 22d | M | Not confirmed | Fever (38.1 °C); Tachycardia; Leukopenia | None | None | None | Discharged |
| Aghdam et al. 38 | March 2020 | Iran | Case report | 1 | 15d | M | Household suspicious | Fever (38.2 °C), mottling, tachycardia, tachypneia, mild subcostal retraction | None | None | Vancomycin (10 mg/kg/q8h), Amikacin (10 mg/kg/ q8h) and Oseltamivir (3 mg/kg/12 h) | Discharged |
| Marhaeni et al. 39 | May 2020 | Indonesia | Case report | 1 | 17y | F | Household | Anosmia and ageusia, mild-moderate muscle pain, sneezing, pale appearance | Transfusion-dependent thalassemia (beta-thalassemia) | None | Azithromycin 500 mg/q24 h and antiviral Oseltamivir 75 mg/q12 h. Deferiprone has still been given, considering her good condition. | Discharged |
| Canarutto et al. 40 | March 2020 | Italy | Case report | 1 | 32 d | M | NA | Fever (38.3 °C); rhinitis; cough; mild neutropenia (900/μL) and monocytosis (1400/μL) | NA | NA | No supportive treatment required | Discharged |
Gamma-GT, gamma-glutamyl transpeptidase; SpO2, oxygen saturation; IVIG, intravenous immune globulin; mPDN,methylpredisonolone; VSD, ventricular septal defect; MODS, multiple organ dysfunction syndrome; IVGTT, intravenous glucose tolerance test; MCA, middle cerebral artery; ACA, anterior cerebral artery; ADHD, attention deficit hyperactivity disorder; (a): This study was included due to the relevant data regarding kawasaki Disease and pediatric COVID-19. Only 19 of 21 patients were Sars-Cov-2 positive.
To elaborate Table 2, one author extracted data from Table 1 and summarized into the following listed below. Then, a second author double-checked all data included to avoid any errors in extraction.
- A
Signs and symptoms were classified by organs and systems affected;
- B
Number of times the symptom or sign was described;
- C
Percentage of symptom/sign concerning each organ/system;
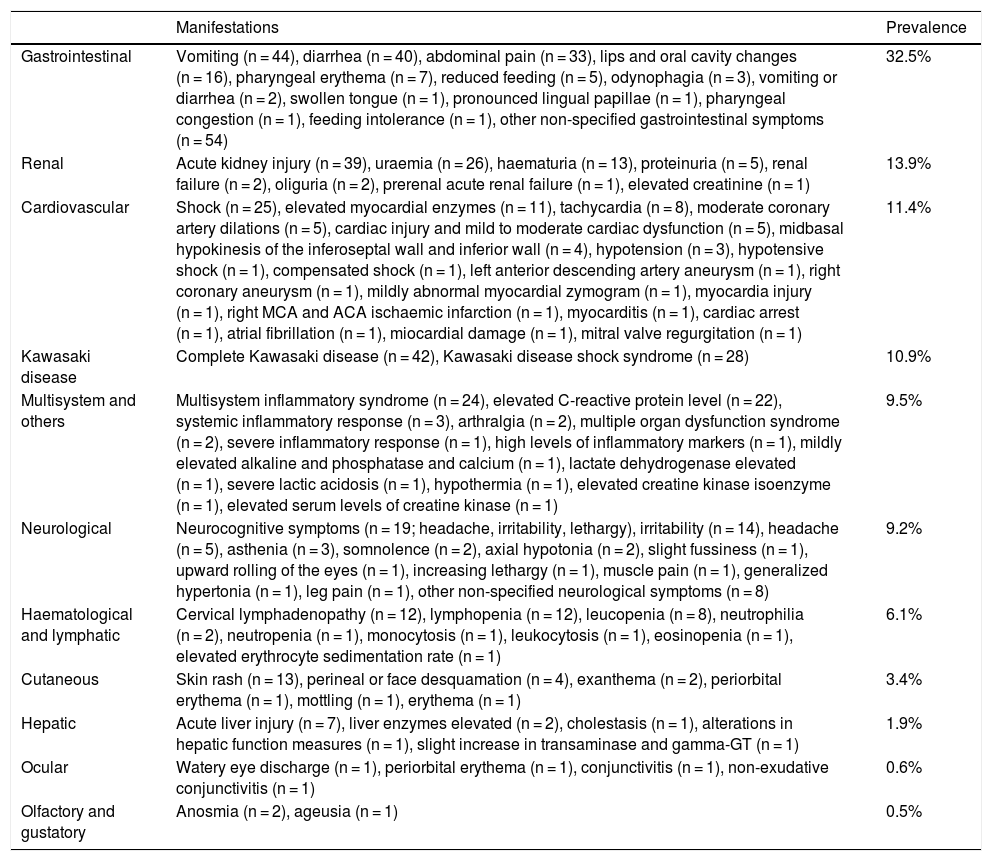
Summary and prevalence rates of extrapulmonary manifestations of COVID-19 in children.
| Manifestations | Prevalence | |
|---|---|---|
| Gastrointestinal | Vomiting (n = 44), diarrhea (n = 40), abdominal pain (n = 33), lips and oral cavity changes (n = 16), pharyngeal erythema (n = 7), reduced feeding (n = 5), odynophagia (n = 3), vomiting or diarrhea (n = 2), swollen tongue (n = 1), pronounced lingual papillae (n = 1), pharyngeal congestion (n = 1), feeding intolerance (n = 1), other non-specified gastrointestinal symptoms (n = 54) | 32.5% |
| Renal | Acute kidney injury (n = 39), uraemia (n = 26), haematuria (n = 13), proteinuria (n = 5), renal failure (n = 2), oliguria (n = 2), prerenal acute renal failure (n = 1), elevated creatinine (n = 1) | 13.9% |
| Cardiovascular | Shock (n = 25), elevated myocardial enzymes (n = 11), tachycardia (n = 8), moderate coronary artery dilations (n = 5), cardiac injury and mild to moderate cardiac dysfunction (n = 5), midbasal hypokinesis of the inferoseptal wall and inferior wall (n = 4), hypotension (n = 3), hypotensive shock (n = 1), compensated shock (n = 1), left anterior descending artery aneurysm (n = 1), right coronary aneurysm (n = 1), mildly abnormal myocardial zymogram (n = 1), myocardia injury (n = 1), right MCA and ACA ischaemic infarction (n = 1), myocarditis (n = 1), cardiac arrest (n = 1), atrial fibrillation (n = 1), miocardial damage (n = 1), mitral valve regurgitation (n = 1) | 11.4% |
| Kawasaki disease | Complete Kawasaki disease (n = 42), Kawasaki disease shock syndrome (n = 28) | 10.9% |
| Multisystem and others | Multisystem inflammatory syndrome (n = 24), elevated C-reactive protein level (n = 22), systemic inflammatory response (n = 3), arthralgia (n = 2), multiple organ dysfunction syndrome (n = 2), severe inflammatory response (n = 1), high levels of inflammatory markers (n = 1), mildly elevated alkaline and phosphatase and calcium (n = 1), lactate dehydrogenase elevated (n = 1), severe lactic acidosis (n = 1), hypothermia (n = 1), elevated creatine kinase isoenzyme (n = 1), elevated serum levels of creatine kinase (n = 1) | 9.5% |
| Neurological | Neurocognitive symptoms (n = 19; headache, irritability, lethargy), irritability (n = 14), headache (n = 5), asthenia (n = 3), somnolence (n = 2), axial hypotonia (n = 2), slight fussiness (n = 1), upward rolling of the eyes (n = 1), increasing lethargy (n = 1), muscle pain (n = 1), generalized hypertonia (n = 1), leg pain (n = 1), other non-specified neurological symptoms (n = 8) | 9.2% |
| Haematological and lymphatic | Cervical lymphadenopathy (n = 12), lymphopenia (n = 12), leucopenia (n = 8), neutrophilia (n = 2), neutropenia (n = 1), monocytosis (n = 1), leukocytosis (n = 1), eosinopenia (n = 1), elevated erythrocyte sedimentation rate (n = 1) | 6.1% |
| Cutaneous | Skin rash (n = 13), perineal or face desquamation (n = 4), exanthema (n = 2), periorbital erythema (n = 1), mottling (n = 1), erythema (n = 1) | 3.4% |
| Hepatic | Acute liver injury (n = 7), liver enzymes elevated (n = 2), cholestasis (n = 1), alterations in hepatic function measures (n = 1), slight increase in transaminase and gamma-GT (n = 1) | 1.9% |
| Ocular | Watery eye discharge (n = 1), periorbital erythema (n = 1), conjunctivitis (n = 1), non-exudative conjunctivitis (n = 1) | 0.6% |
| Olfactory and gustatory | Anosmia (n = 2), ageusia (n = 1) | 0.5% |
One author selected potential articles based on the study title and abstract. Whenever a study appeared to meet the inclusion criteria, the authors obtained full text. Once the search was completed and full articles were obtained, two authors independently made the definitive inclusion of the article by reviewing the text of the selected studies. Any differences between authors’ selections were solved by discussion between them. If these authors were not able to achieve a consensus, a third author was consulted. One study13 did not fit the inclusion criteria described below, since two out of 21 participants did not test positive for SARS-CoV-2. However, the authors in consensus decided to include the article due to its relevance and minor impact on qualitative study reliability.
Inclusion criteriaThe following checklist was used to determine whether a study was suitable for the present review.
- 1
Population: Children and adolescents (0–18 years) with laboratory confirmed infection of SARS-CoV-2
- 2
Study design: retrospective studies (cross-sectional studies, case-control studies, case series, and case reports)
- 3
Variables: Study information, epidemiological characteristics, clinical features, laboratory findings, extrapulmonary signs and symptoms, main treatment, and outcome.
Reviews, nationwide aggregated data, and studies reporting the same patients' data were removed to avoid overlapping and duplicate publications. Publications that either reported asymptomatic cases or only respiratory symptoms associated with fever were also excluded.
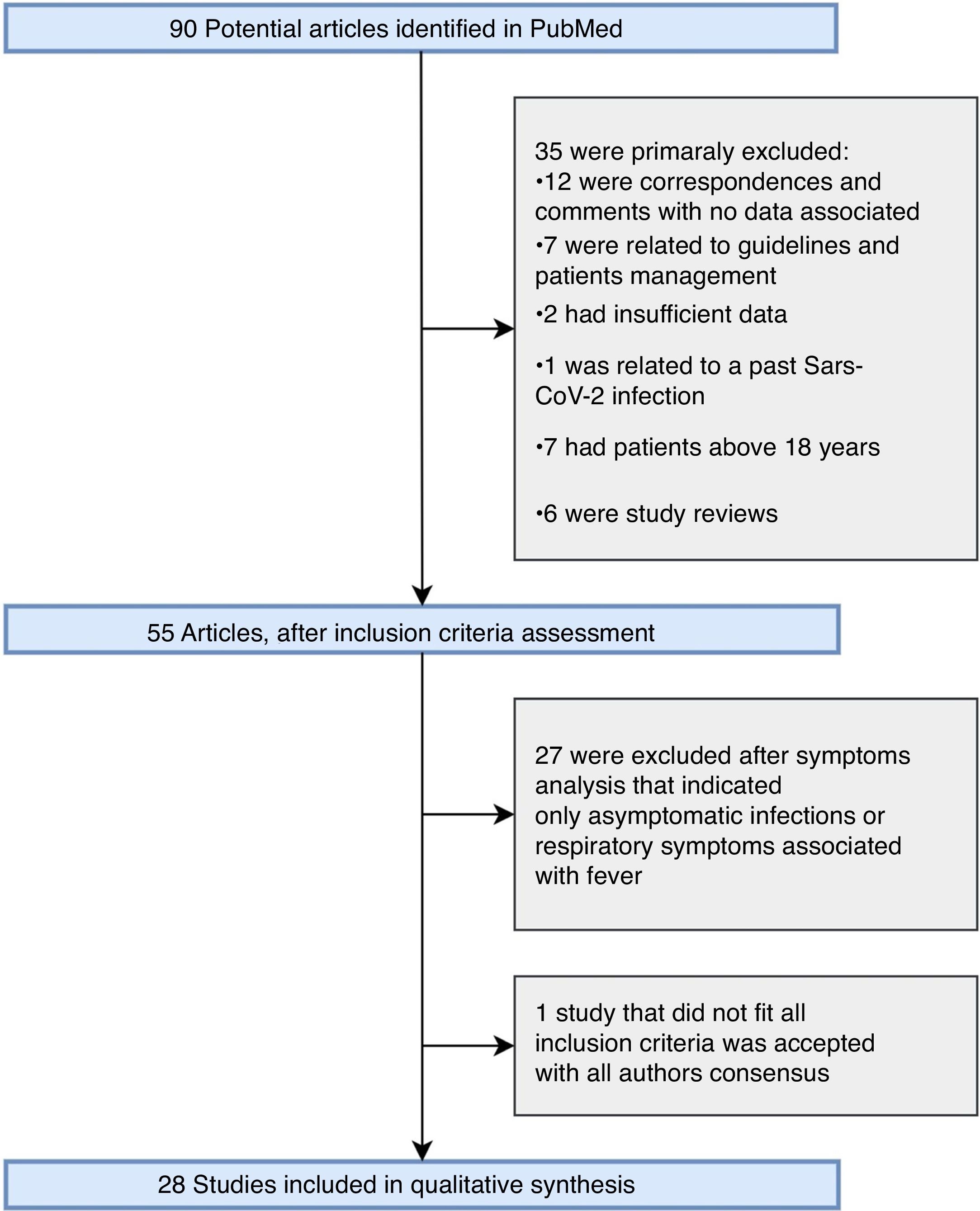
ResultsThe strategy retrieved 90 potential articles (Fig. 1). After full-text assessment, 35 articles that did not meet the inclusion criteria were excluded. Subsequently, 27 articles that either only reported asymptomatic cases or only respiratory symptoms associated with fever were excluded. Articles from Brazil (one study), China (four studies), France (three studies), Indonesia (one study), Iran (one study), Italy (five cases), Russia (one study), Spain (two studies), Switzerland (one study), United Kingdom (four studies), and United States (five studies) were assessed. The study included 28 studies, described in Table 1, including 18 case reports, four case series, and sux observational studies. A total of 199 pediatric patients with COVID-19 were presented in these studies, including 121 male patients and 70 females. One study did not report the sex of eight patients.13 Two patients demised.14,15
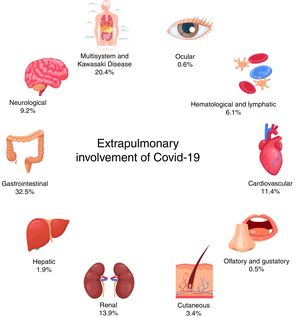
The main extrapulmonary manifestations found were gastrointestinal (GI), with high prevalence of diarrhea, abdominal pain, and vomiting.15–30 Other GI symptoms found were related to inflammatory process, such as pharyngeal erythema,19 swollen tongue, and pronounced lingual papillae.31 Reduced feeding,21,32 odynophagia,15,26 and feeding intolerance33 were also reported and are important signs for pediatric cases. Acute liver injury16 and hepatic enzymes alterations were also observed (Fig. 2).18,24,29,34
Renal, cardiovascular and neurological manifestations were also observed. Renal manifestations were reported with hematuria,20,35 proteinuria,20 uremia,20 and increased creatinine18 as the main signs. Acute kidney injury was identified in 38 patients,16,20 representing a frequent complication. Cardiac manifestations were often described as alterations in myocardial enzymes,18,24,34 tachycardia,26,27,34,36–38 and shock,16 with eight and 25 presentations, respectively. Vascular manifestations included aneurysms26 and ischaemic infarctions15 were identified and associated with obesity. Cardiac damage and dysfunction was observed in five patients.30 Neurological manifestations were frequently described as neurocognitive symptoms, including headache, irritability, lethargy, and somnolence.13–16,23,24,26,29,31,33 Axial hypotonia,22 generalized hypertonia,23 and muscular pain39 indicated the presence of neuromuscular manifestations.
Inflammatory markers were often elevated and described as a multisystemic manifestation, sometimes leading to multiorgan dysfunction complications.13,23,31 Hematologic and lymphatic manifestations were also reported, with variations in leukocytes,18,24,37 lymphocytes,17,19,24 neutrophil count,17,18,40 and erythrocyte sedimentation rate.31 Twelve cases of lymphadenopathy were described.13
Kawasaki-like disease (KD) and cutaneous manifestations were described and often associated (Fig. 3).13,27 Dermatologic presentations were defined as skin rashes,13,15,26,27,31 perineal or facial desquamation,13 erythemas22 and exanthems.19 Complete Kawasaki disease and KD shock syndrome was confirmed in 21 and 28 patients, respectively.13,16,27 Bilateral conjunctival injections were described in 17 patients and were all associated with KD. Other ocular manifestations included watery eye discharge,23 conjunctivitis,15 and periorbital erythema.29 Other isolated presentations were also reported, such as anosmia,13,39 ageusia,39 arthralgia.13 Full cases description and prevalence rates are described in Tables 1 and 2, respectively.
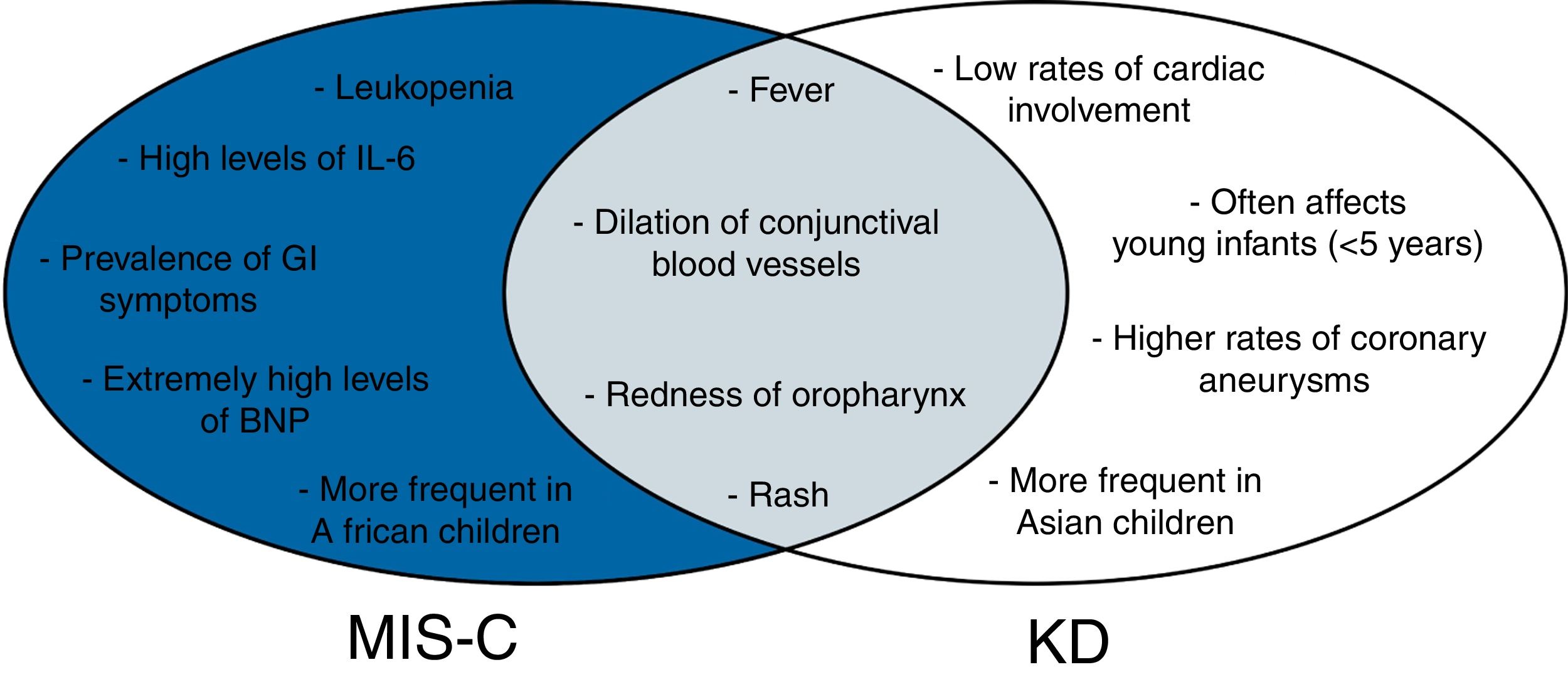
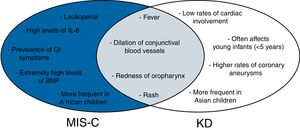
Cross-sectional diagram of MIS-C and KD symptoms.
Blue section of the diagram indicates symptoms related only to MIS-C. Gray section refers to symptoms common to both MIS-C and KD. White section indicates symptoms related only to KD. MIS-C, multisystem inflammatory syndrome in children; KD, Kawasaki disease; GI, gastrointestinal.
In the respiratory route infection, protein S, expressed in the viral envelope, interacts with the membrane-bound ACE2 that is expressed in type I and type II alveolar pneumocytes. This link stimulates a clathrin-dependent endocytosis, creating a low pH endosome containing the protein S and ACE2 complex inside the host cell.4 This infection mechanism explains the primary lung manifestations, due to the presence of viral particles, infiltration of inflammatory cells and cytopathic effects on lung cells.41
However, ACE2 is not only expressed on type I and type II pneumocytes. Hamming et al.42 had investigated the tissue distribution of ACE2 in healthy human organs. An interesting result found in that study was the high and strong presence of ACE2 in epithelial cells of the lung, the small intestine, and the kidney. ACE2 was also expressed in vascular endothelial cells, in smooth muscle cells, in the basal epidermal layer of in the skin, and in the oral and nasal mucosa. These results suggest other viral transmission routes related to ACE2 expression. Furthermore, the ubiquitous expression of ACE2 in human body explains the great variability of extrapulmonary manifestations on COVID-19.
Additionally, as a result of the endocytosis of the SARS-CoV-2 along with ACE2, the membrane-bound enzyme is reduced in several organs and system.43 This downregulation affects the entire RAAS, which is responsible for the blood pressure control and several organ homeostasis·5 Therefore, COVID-19 is conceived as a systemic disease, being RAAS molecules critical for pathophysiological mechanisms.6 Hence, SARS-CoV-2 infection may be considered a systemic condition with pulmonary involvement rather than a respiratory disease with extrapulmonary manifestations.
Children’s susceptibility to COVID-19As previously mentioned, children are less infected and less affected by SARS-CoV-2, unlike other respiratory diseases. The milder presentation of COVID-19 can be related to the immature immune system memory of children, as they are yet building their pool of B and T-cells. Therefore, they are more prepared to deal with completely novel antigens, such as SARS-CoV-2, than elderly people, as they present lower amounts of lymphocytes. There are two populations of memory B-cells: CD27Bright and CD27Dull. CD27Bright is known for producing specific antibodies against the antigen, while CD27Dull synthesizes antibodies with wide range of activity. In children, CD27Dull prevails over CD27Bright, explaining their higher ability to face new diseases. In elderly individuals, the inverse happens.12
The mechanisms of this innate immune system were suggested in the study by Paki and Iwasaki;44 Interferon (IFN) response is an adaptive form of the immune system and its timing can vary with viral load and/or genetic differences. An early IFN response can lead to rapid viral clearance before its replication, resulting in milder diseases. Delayed IFN response can enable viral persistence, increase levels of inflammatory markers such as IL-6, and cause severe cases. IFN induction can be delayed and minimized in older hosts, explaining the increasing vulnerability with aging.
GI manifestations of pediatric COVID-19As shown in Table 2, GI manifestations are the most common extrapulmonary signs and symptoms of SARS-CoV-2 infection in pediatric patients. Digestive symptoms are presented in 8-10% of pediatric cases of COVID-19, including diarrhea, abdominal pain and discomfort and vomiting.45 A systematic review observed that the prevalence of GI symptoms was 9.6% in children and 9.7% in adults, a difference that is not statistically significant. A review performed by Tian et al.46 found, that when compared with adults, children present similar percentages of diarrhea, but a considerable higher rate of vomiting. The implications of these findings will be discussed later on this article.
GI injury mechanismAs previously mentioned, the ACE2 receptor, presented in type I and II pneumocytes, is a crucial component for viral endocytosis during SARS-CoV-2 infection. In addition, epithelial cells of the small intestine is another tissue that highly express ACE2 in cell membrane,42 creating another potential region for SARS-CoV-2 infection and enteric manifestations of COVID-19. The pathophysiology of these GI symptoms is not well elucidated, but some potential mechanisms are described in a study of Ye et al.47 The most logical mechanism relates the high expression of ACE2 in the epithelial cells of small intestine that may cause a direct viral infection into the bowel. However, this hypothesis remains speculative, as one study found that SARS-CoV-2 was not always detected in stool samples of COVID-19 patients with GI manifestations.
Although scientific data did not describe renin angiotensin system (RAS) primarily affecting the intestine, this system regulates the homeostasis of intestinal amino acids, the expression of antimicrobial peptides, the and ecology of the gut microbiome.48 Some studies speculate that the downregulation of ACE2 expression in the epithelial cells of the intestine may affect the absorption of tryptophan – a peptide that belongs to a pathway that regulates the antimicrobial peptides expression and, therefore, the composition of the intestinal flora.47 Therefore, any dysregulation of this pathway could lead to GI symptoms, such as diarrhea.
Other mechanisms were also discussed in the study by Ye et al.,47 including side effects of antibiotics and the establishment of a gut-lung axis that would allow a immune reaction of a lung infection to be also present in the bowel, causing diarrhea and intestinal immune damage.
Pathophysiological considerationsAs previously mentioned in this article, no significant difference between children and adults regarding the prevalence of GI symptoms was found, suggesting that both groups are equally susceptible to these manifestations. However, in 54% of adults, GI symptoms were reported to be associated with more severe/critical illness,49 while Tian et al. found that, out of 57 children, only one was critically ill with GI presentations. Thus, despite the fact that these groups quantitatively present the same rate of GI symptoms, children appear to exhibit GI symptoms even with less severe illness.
A recent study by Vuille-dit-Bille et al.50 demonstrated a positive correlation of ACE2 expression in the small intestine and age, with increased ACE2 mRNA expression with aging. Therefore, children might express less ACE2 receptors compared with older patients, being more vulnerable to ACE2-downregulation of the tryptophan pathway, leading to more susceptibility of diarrhea. Hence, the present authors speculate that, although children and adults have similar rates of GI symptoms, children GI symptoms are usually associated as a primary response of SARS-CoV-2 infection, due to this minor expression of ACE2, and represent milder symptoms. Additionally, one study showed that inflammatory cytokines IL-6, IL-10, and TNF-alpha were intensively elevated in adult patients with diarrhea,51 and related to cytokine storm and multi-organ damage. Thus, the present authors also suspect that most severe GI presentations in adults may be caused by a secondary response of systemic inflammation triggered by SARS-CoV-2.
Hepatic manifestationsLiver injury is another extrapulmonary manifestation of COVID-19, being described as any liver damage presented during the disease progression. This manifestation often occurs in more severe cases rather than mild presentations.52 Two important biochemical markers of liver injury are increased serum levels of ALT and AST enzymes. Children are susceptible to liver injury, as shown by a meta-analysis of 551 laboratory-confirmed pediatric COVID-19 patients reporting that 9% (35/290) presented increased ALT and 18% (58/280), high levels of AST.53
The mechanism of SARS-CoV-2 liver damage is not clearly elucidated; it can be either resulted from direct SARS-CoV-2 infection of hepatic cells or drug induced liver injury. Some possible mechanisms described by Sun et al.52 are:
- A
Immune damage from inflammatory response
- B
Direct cytopathic effect, as liver presents ACE2 receptors
- C
Anoxia due to respiratory failure
- D
Drug-induced liver damage
- E
Reactivation of pre-existing liver disease
To date, there is no scientific data to elucidate whether children are equally susceptible to liver damage as adults.
Renal manifestations of SARS-CoV-2 infectionA meta-analysis including pediatric patients with SARS-CoV-2 infection conducted by Zhang et al.53 demonstrated that 5% (22/139) had increased urea and 4% (48/184) had increased creatinine. A cohort study by Stewart et al.54 showed a frequency of increased serum creatinine in 46% (n = 24), and 29% (n = 15) of hospitalised patients that meet the diagnosis criteria for acute kidney injury. Out of these 15 inpatients with AKI, 93% (n = 14) were admitted to the pediatric intensive care unit (PICU) and 73% (n = 11) were associated with with multisystem inflammatory syndrome in children (MIS-C) temporarily associated with SARS-CoV-2. These data suggest that AKI may not have a high prevalence in pediatric patients with COVID-19. However, when present, AKI may be the main cause of critical illness in pediatric patients, requiring PICU admission.
Mechanisms of renal injury in children with Covid-19As previously mentioned in this article, ACE2 receptors play a critical role in COVID-19 pathophysiology, due to the high affinity of the SARS-CoV-2 protein S to membrane-bound ACE2.4 Histologic studies have shown that ACE2 expression is about 100 times greater in the kidneys compared to the lungs,55 the main pathway for viral infection. Therefore, it is reasonable to predict that the kidney may be vulnerable to SARS-CoV-2 infection, resulting in an injury caused by the direct cytopathic effect of the viral infection.56 A renal post-mortem histopathological analysis of 26 adult patients with COVID-19 showed a prominent proximal acute tubular injury (ATI) associated with the loss of brush border. Interestingly, it is known that the ACE2 is highly expressed in the brush border of tubular cells and some coronavirus-like particles were found in the renal proximal tubular epithelium,42 supporting the cytopathic mechanisms of kidney injury.
As well as this cytopathic mechanism, a systemic condition is another potential mechanism for kidney injury. Cytokine release syndrome (CRS) is being described in pediatric cases of COVID-1957 and might be a cofactor of renal manifestations. Hirano and Murakami described the potential role of ACE-AngII-AT1R axis dysregulation of the RAS during the later phase of infection leading to downregulation of ACE2 that causes CRS, characterized by high expression of IL-6 and TNF.41,59 Intrarenal inflammation, increased vascular permeability, volume depletion, and cardiomyopathy, caused by these cytokines, might also lead to kidney failure.56 Other minor potential mechanisms of kidney injury are being identified, including systemic effects of septic shock and the deposition of immune-complexes in the kidney.56
Pathophysiological considerationsThere is few available data that elucidates pathophysiological differences between adults and children. Vinturache et al.58 observed greater expression of the AT1R and the AT2R in kidneys and vasculature in the course of fetal life than at any other time during the lifespan in response to cardiovascular and renal demands. Ang II can bind to both receptors. As previously mentioned, the binding of Ang II to AT1R is associated to pro-inflammatory activity and cytokine release.41,59 In turn, the binding of Ang II to AT2R triggers vasodilatory effects, anti-inflammatory actions, and natriuresis.60 Hence, AT1R expression leads to inflammatory effects, while AT2R mediates protective effects. This study has shown that the decline rate of expression of AT2R is greater than that of AT1R, causing a predominance of AT1R over AT2R with aging. Therefore, children might present less severe cases of kidney injury associated with COVID-19 due to this greater expression of AT2R than adults. However, further studies on ACE2 expression and other RAS molecules concentrations in children are needed.
Cardiovascular manifestations of pediatric COVID-19As shown in Tables 1 and 2, some pediatric patients presented cardiovascular manifestations, such as tachycardia, increased myocardial enzymes, cardiac injury, and myocardial damage. The literature also reports acute myocardial injury and arrhythmias.61 In adults, elevated troponin was present in myocarditis, and myoglobin and muscle enzymes were elevated due to COVID-19-associated muscular injury. These findings were observed only in children with MIS-C.62,63 Some mechanisms of cardiovascular complications are:61
- A
Direct myocardial injury, since cardiac tissue highly expressed ACE2 receptors, making direct cytopathic effect of SARS-Cov-2 infection possible;
- B
Systemic inflammation with high levels of circulating cytokines, which can lead to multi-organ damage, including the heart;
- C
Altered myocardial demand/supply ratio in systemic infection, which can increase cardiometabolic demand and respiratory failure resulting in hypoxia and acute myocardial damage;
- D
Electrolyte imbalance, since SARS-CoV-2 directly affects RAS, which is responsible for electrolyte homeostasis and can cause arrhythmias due to hypokalemia;
- E
Coronary thrombosis;
- F
Adverse effects of various therapies.
No data on the pathophysiological and clinical differences of pediatric patients was retrieved in the literature.
Neurological manifestationsAs shown, neurological signs and symptoms are being observed in pediatric SARS-CoV-2 infection. The main clinical manifestations found in the present study are neurocognitive symptoms, which include headache, irritability, and lethargy. Myalgia and other isolated symptoms were found and described in Table 2, suggesting neuromuscular involvement.
Studies have shown the presence of ACE2 in the nervous system, making these neurological manifestations possible, also due to direct cytopathic effect. Reports indicate the detection of SARS-CoV-2 RNA in cerebrospinal fluid (CSF), confirming the viral presence and activity in central nervous system. Other mechanisms may also cause neural damage, such as hypoxic injury and immune response.64
Neurologic manifestations in younger children are not always documented, due to difficulties arising from the undeveloped communication that makes the diagnosis harder and subjective. However, physicians and pediatricians should consider COVID-19 as a differential diagnosis of neurologic manifestations in children.
Hematologic manifestationsLaboratory abnormalitiesAccording to the systematic review by Zhang et al., 15% (47/243) of pediatric patients had leukocytosis, 14% (48/314) had leukopenia, 35% had lymphocytosis (67/157), and 13% (61/323) had lymphopenia.53 Compared to adults, children present significant minor rate of lymphopenia and a higher rate of lymphocytosis. A meta-analysis of adult patients showed a pooled frequency of 57.4% of increased lymphocytes and 8.2% of decreased lymphocytes. Differences in leukocytes rate were observed, as the frequency of leukocytosis and leukopenia in adults were 9.8% and 20.1%, respectively.65
Gupta et al. suggested that the mechanisms for lymphopenia are direct viral entry, leading to cytopathic effects on lymphocytes, apoptosis-mediated lymphocytes depletion, and inhibition of lymphocytes proliferation due to lactic acid effects. An increase in leukocyte count may be induced by hyperinflammatory response to SARS-CoV-2 infection or other bacterial infections.66 The available data are not sufficient to establish pathophysiological mechanisms that explain differences of these laboratorial findings between adults and children.
CoagulopathiesCoagulation disorders are a usual complication in adult patients,67 but no pediatric reports were retrieved. D-dimer levels were elevated in 29.3% adult patients65 and in 12% of children.53 The mechanisms behind COVID-19-associated coagulopathy are not fully elucidated, but it is suspected that cytokine storm activates a thrombo-inflammatory pathway that can lead to abnormalities in coagulation.68
Kawasaki disease manifestation in pediatric patients and Covid-19Kawasaki disease is a self-limited acute vasculitis presented in 0.01% of children. The first target of this disease is medium-sized muscular arteries, but several vessels can be affected, such as the coronary arteries. Unlike other vasculitis, KD progression is similar to infectious diseases, with rash, mucosal inflammation, and limb alterations.69
Although the etiology of Kawasaki disease remains unknown, pathologic, epidemiologic, ultrastructural and immunologic evidence may suggest that an infectious agent leads to a sequence of events causing the disease. In addition, antibiotic treatment failure supports the hypothesis that the etiological agent of KD is not a bacterial pathogen.70 However, several studies over many decades until 2008 were unable to connect a microorganism to this disease, showing that no known infectious agent can be considered the cause of KD.71
Due to HCoV-NH, the connection between SARS and KD was already studied,72 suggesting that KD and coronaviruses infection are associated. Between January 1, 2015 and February 17, 2020, 19 patients were diagnosed with KD in the General Pediatric Unit of Hospital Papa Giovanni XXIII (Bergamo, Italy). Meanwhile, between February 18 and April 20, 2020, ten patients were diagnosed with the disease in this same hospital. Interestingly, eight out these ten patients presented a positive IgM and/or IgG test for SARS-CoV-2.73 This recent case series reports that six patients had cardiac involvement, five exhibited Kawasaki disease shock syndrome and five presented macrophage activation syndrome, while in the first cohort of 19 patients only two patients had cardiac involvement. These findings suggest that KD in COVID-19 patients result in more severe complications.
MIS-C and Kawasaki disease overlapping symptomsIn April 2020, an alarming warning was made by the National Health Service in England about cases of SARS-CoV-2 positive in older school-aged children and adolescents presenting with fever, hypotension, severe abdominal pain, and cardiac dysfunction. High levels of IL-6 and other inflammatory markers suggested cytokine storm, and children generally required inotropic support to increase cardiac output. Later, the Centers for Disease Control and Prevention (CDC) defined this condition as MIS-C. Some clinical features of MIS-C overlap KD symptoms, including fever, dilation of conjunctival blood vessels, rash, and redness of the oropharynx.74,75 While patients can simultaneously meet both MIS-C and KD diagnosis criteria (OCS2), clinical and epidemiological features distinguish both diseases.
According to two recent cohorts studies, one from 26 states in the United States and the other from New York State,62,63 respectively 92% and 80% of pediatric patients with MIS-C present GI symptoms. Moreover, the latter study63 reported only 27% rate of respiratory symptoms of SARS-CoV-2-associated MIS-C, suggesting that MIS-C is predominantly reported with GI rather than respiratory symptoms. Furthermore, MIS-C laboratory findings include leukopenia and extremely elevated levels of ventricular natriuretic peptide, while these alterations are not found in KD’s patients.74 Coronary aneurysms are a typical finding of KD, while reports of this presentation are rare in MIS-C.69,74 Previousl epidemiological reports suggest that the mean age of children with KD is 2 years, with few reports beyond late childhood, while MIS-C can affect older children and adolescents.69 Data implies that Asian children are more prone to have KD, and African children are more susceptible to MIS-C.62,63,74
The distinction between KD or MIS-C can be hard to make, leading to misdiagnose. In the present review, several cases of MIS-C and other severe undefined inflammatory presentations associated with other symptoms, compatible with KD signs, were retrieved.25–27 Further studies regarding MIS-C definition, pathogenesis, epidemiology, and other relevant factors are necessary. Meanwhile, pediatricians should evaluate clinical, epidemiological, and laboratorial data to consider both KD and MIS-C as diagnosis, especially in severe cases.
Cutaneous manifestationsCutaneous manifestations are related not only to SARS-CoV-2 infection, but also to several other infectious diseases, such as toxic shock syndrome, meningococcemia, rickettsial diseases, measles, and scarlet fever.76 In the present study, 19 manifestations of dermatologic symptoms were observed, including skin rash, mottling, exanthema, erythema, periorbital erythema, and peritoneal or facial desquamation. These skin changes are rarely observed, but they can still go unnoticed due to lack of attention, which raises concern about COVID-19 transmission by misdiagnosed asymptomatic children.77
The physiopathological mechanism of these lesions remains poorly understood. A review by Kaia et al. indicates two major groups, related to two distinct possible mechanisms. One is related to the immune response to viral nucleotides, similar to viral exanthemas. The other one relates cutaneous eruptions to systemic consequences of COVID-19, such as vasculitis and thrombotic vasculopathy.78 The present authors speculate that children can be less susceptible to the first mechanism, due to differences in the innate immune system.
Olfactory and gustatory manifestationsAnosmia and ageusia are common symptoms of COVID-19 in adults,79 which can be attributable to an easier self-diagnosis. However, in children, the description of the absence of smell and taste may be difficult and hinders diagnosis. Additionally, some cases of anosmia and ageusia are described in the present review, but in a very small sample when compared with adults.
A recent study by Brann et al.80 showed that ACE2 and a protease involved in the viral endocytosis called TMPRSS2 are expressed in the olfactory mucosa, but not (or weakly) in the olfactory receptors neurons. Additionally, anosmia symptoms usually disappear within 1–2 weeks,81 which exclude neural damage as a cause of the lack of smell and suggest a non-neural cause of anosmia.
The mechanism of SARS-CoV-2 ageusia remains speculative. Retrospective studies from MERS and SARS-CoV suggest that coronaviruses occupy binding sites of sialic acid on taste buds and this can lead to degradation of gustatory particles. Other possibility of lack of taste may be attributable to the simultaneous manifestation of anosmia, as these two sensory systems are associated.81
Pathophysiological considerationsThese potential mechanisms described in literature are insufficient to fully explain any pathophysiological differences for these manifestations between children and adults. However, it is known that children have lower expression of ACE2 in the oral and nasal mucosa.82 The present authors suspect that this lower expression of ACE2 might be extended to the non-nervous olfactory tissue and this could explain the lower prevalence of anosmia in kids. Supporting this idea, a recent study of animal models has shown that ACE2 and TMPRSS2 expression in animals increase with aging. However, this remains highly speculative, and histological studies are necessary to elucidate this hypothesis.
Ocular manifestationsOcular involvement is rarely reported in both adult and pediatric cases of COVID-19;64 only three symptoms were retrieved in the literature researched: watery eye discharge, periorbital erythema and conjunctivitis. In addition, both ACE2 and TMPRSS2 are expressed in human ocular surface, explaining ocular susceptibility to direct cytopathic effect of SARS-CoV-2 infection. RAS involvement should also be considered, as the eye presents its own RAS molecules in the aqueous humor dynamics,83 but the implications of this finding in SARS-CoV-2 infection remains unknown. Therefore, pediatricians should consider SARS-CoV-2 infection as a differential diagnosis during COVID-19 pandemic and be aware of eye-to-eye transmission.84
ConclusionApart from fever and respiratory signs and symptoms, SARS-CoV-2 infection often causes extrapulmonary manifestations, including KD, GI, renal, cardiovascular, neurological, haematological, cutaneous, hepatic, ocular, olfactory, and gustatory. The main potential pathophysiological mechanisms regarding these signs are direct cytopathic effects of tissues with ACE2 expression and immune-mediated inflammatory responses, as well some medication induced side effects. The present findings highlight the importance for pediatricians and other physicians to consider extrapulmonary manifestations as a differential diagnosis of SARS-CoV-2 infection in pediatric patients, especially during the COVID-19 pandemic. Therefore, laboratory tests, including serum levels of ALT, AST, creatinine, inflammatory markers, and myocardial enzymes in children infected with SARS-CoV-2, should be consider in order to identify any non-pulmonary manifestations of the disease and to prevent poor outcomes.
Conflicts of interestThe authors declare no conflicts of interest.
This study was partially supported by Brazilian National Council of Research Development [CNPq - Grant # 302153/2019-5], Coordination of High Education Level Personnel [CAPES] and Foundation of Research of Minas Gerais [FAPEMIG]. Elements from Fig. 2 were designed by macrovector/Freepik.