Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease (COVID-19) is a rare and challenging diagnosis requiring early treatment. The diagnostic criteria involve clinical, laboratory, and complementary tests. This review aims to draw pediatrician attention to this diagnosis, suggesting early treatment strategies, and proposing a pediatric emergency care flowchart.
SourcesThe PubMed/MEDLINE/WHO COVID-19 databases were reviewed for original and review articles, systematic reviews, meta-analyses, case series, and recommendations from medical societies and health organizations published through July 3, 2020. The reference lists of the selected articles were manually searched to identify any additional articles.
Summary of the findingsCOVID-19 infection is less severe in children than in adults, but can present as MIS-C, even in patients without comorbidities. There is evidence of an exacerbated inflammatory response with potential systemic injury, and it may present with aspects similar to those of Kawasaki disease, toxic shock syndrome, and macrophage activation syndrome. MIS-C can develop weeks after COVID-19 infection, suggesting an immunomediated cause. The most frequent clinical manifestations include fever, gastrointestinal symptoms, rash, mucous membrane changes, and cardiac dysfunction. Elevated inflammatory markers, lymphopenia, and coagulopathy are common laboratory findings. Supportive treatment and early immunomodulation can control the intense inflammatory response and reduce complications and mortality.
ConclusionsMIS-C associated with COVID-19 is serious, rare, and potentially fatal. The emergency department pediatrician must recognize and treat it early using immunomodulatory strategies to reduce systemic injury. Further studies are needed to identify the disease pathogenesis and establish the most appropriate treatment.
In November 2019, some severe pneumonia cases were reported in Wuhan, China. In December 2019, the rapid increase in the number of these cases with an epidemiological association with the local market caught the attention of Chinese health authorities. The cause of these outbreaks was identified in January 2020 as a coronavirus; the World Health Organization (WHO) later called the virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the disease caused by it coronavirus disease (COVID-19). The disease quickly spread to other countries, which led the WHO to declare an international public health emergency on January 30, 2020 and a pandemic on March 1, 2020.1–3
SARS-CoV-2 exerts a pathogenic mechanism by binding the spike protein on its surface to the type 2 angiotensin-converting enzyme (ACE2) receptor, whose physiological action is to act as a regulatory mechanism against the effects of angiotensin.4 ACE2 receptors are significantly expressed in the lung, mainly in type II pneumocytes, the predominant site of the pathogenicity of the virus in the human body, as well as in other organs such as the heart, vascular endothelium, intestinal epithelium, and kidneys, thus providing a favorable mechanism for multiple organ dysfunction.5,6
The literature is consistent in stating that COVID-19, a disease caused by SARS-CoV-2, affects children more rarely and mildly than it affects adults.7,8 Some of the hypotheses that explain this phenomenon include differences in ACE2 receptor expression, differences in the immune system (better innate immune response in children, especially when compared with the elderly, and a lesser pro-inflammatory response with less cytokine production), a lower prevalence of comorbidities, lower exposure to the pathogen in the initial phase of the pandemic, respiratory infections by other coronaviruses that are already circulating in the community, greater colonization by other viruses and bacteria conferring interactions with the microbiota and competition, and a greater risk of children being infected by the second or third generation of SARS-CoV-2, a phenomenon that is generally associated with reduced aggressiveness.9,10
Although COVID-19 is less severe and less frequent in children, as the pandemic progresses, there are reports of a worrying clinical picture in the pediatric age group, with symptoms similar to Kawasaki disease and toxic shock that require assertive conduct by pediatricians, especially those working in emergency services. Similar reports have been reported in several countries under the name “multisystem inflammatory syndrome in children” (MIS-C) temporarily associated with COVID-19.11–13
The pathophysiology of MIS-C is unclear, but it appears to be a consequence of an exacerbated immune system response or maladaptive response of the host. After the virus enters the human cells, the first line of defense against infection should be a quick and well-coordinated immune response; however, when this mechanism is unregulated and excessive, hyperinflammation can occur.14
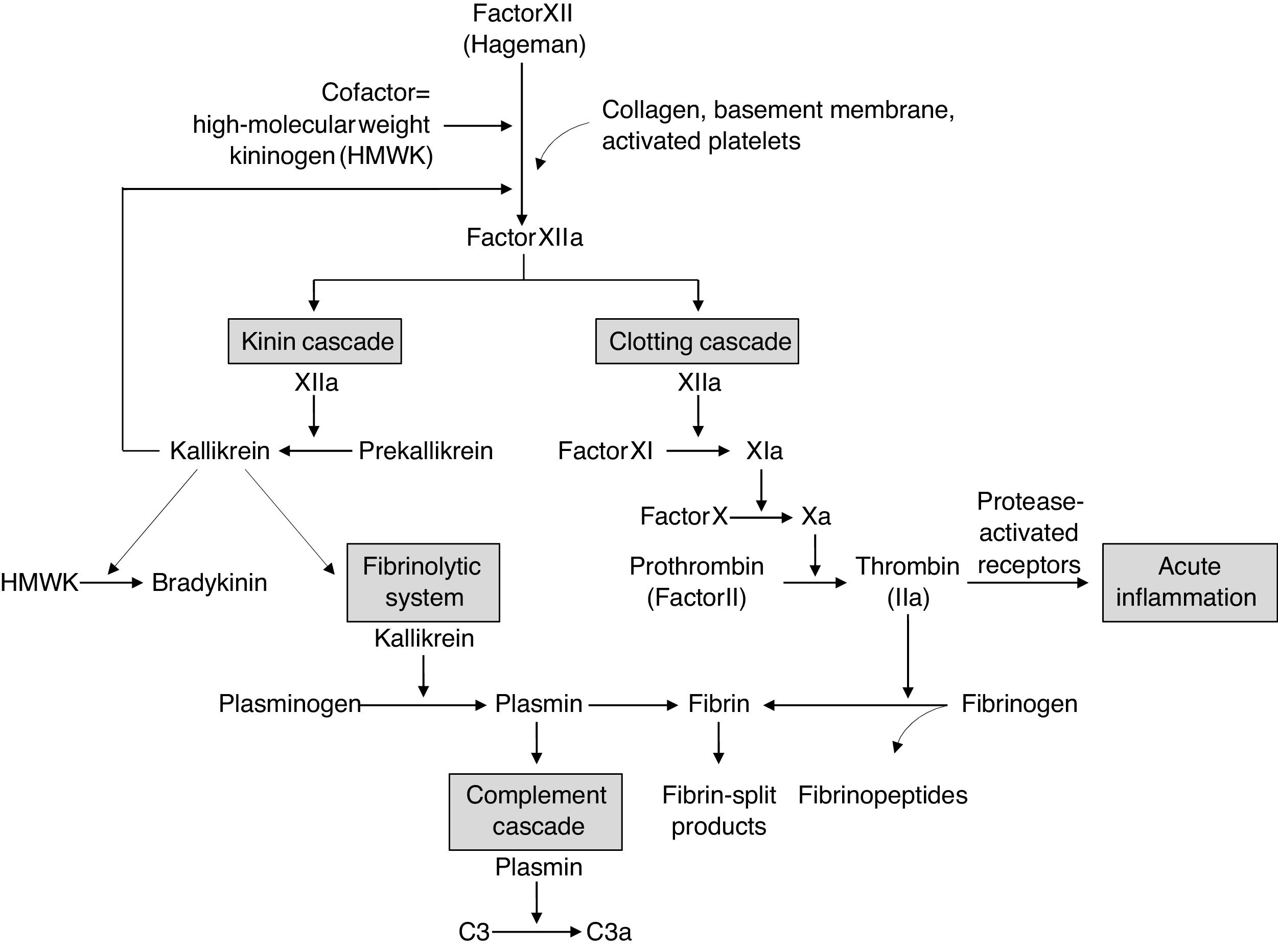
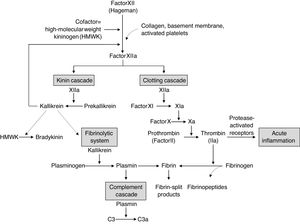
Cytokines that play an important role in inducing immunity and immunopathology during infections in excess can cause the clinical syndrome known as cytokine storm. The inflammatory response caused by SARS-CoV-2 appears to be the major cause of mortality in infected patients.15 The infection of dendritic cells or macrophages by SARS-CoV-2 induces the production of low levels of antiviral cytokines and increases the production of inflammatory cytokines (tumor necrosis factor [TNF], interleukin [IL]-1, IL-6, and interferon-γ).16–19 An apparent action of inflammatory cytokines, especially IL-6, is the expression of tissue factor, which leads to the activation of coagulation and the complement cascade and the release of inflammatory kinins20 (Fig. 1).21 In addition, the excessive production of IL-6, IL-10, and TNF is negatively correlated with the total number of lymphocytes affecting both innate and acquired immunity.22,23
Interrelationship between the four plasma mediator systems triggered by the activation of factor XII (Hageman factor).
Source: Kumar et al.21
The massive release of inflammatory mediators with exaggerated activation of the immune system is similar to the syndrome known as cytokine storm, which occurs in a group of conditions that share the same pathogenic mechanism but have different triggering factors.14 The neutrophil, the main protagonist of the cytokine storm, has the capacity to secrete ferritin, which explains the high levels found in several inflammatory syndromes.24 Ferritin, in turn, has an immunosuppressive action and inhibits the differentiation of myeloid cells and T and B lymphocytes,24 worsening the host's responsiveness. High levels of ferritin and hemophagocytosis are related to inflammation severity and are present in several diseases with different etiologies: macrophage activation syndrome, hemophagocytic lymphohistiocytosis (HLH), sepsis, multisystem inflammatory syndrome (MIS), staphylococcal toxic shock,25 and severe cases of COVID-19,26 suggesting that these are different phenotypes of the same inflammatory process.27
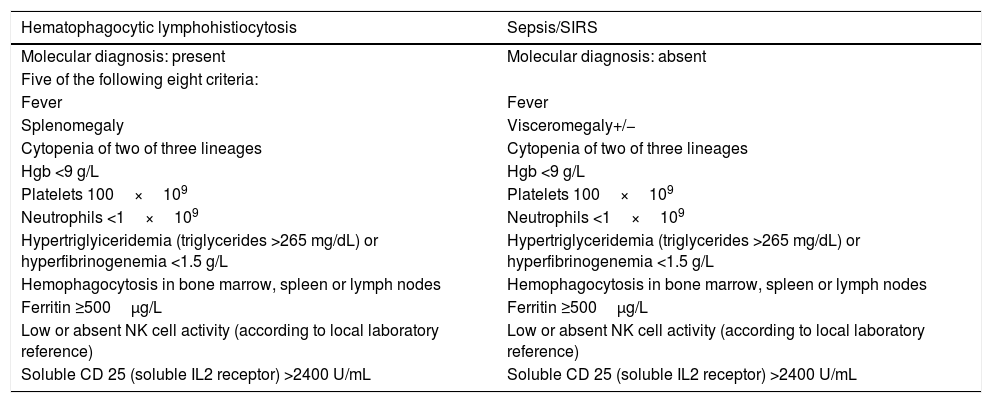
Thus, MIS-C appears to be a clinical syndrome that shares aspects with other inflammatory conditions in which large amounts of cytokines cause the dysfunction of several organs, including Kawasaki disease, sepsis, macrophage activation syndrome, and secondary HLH (Table 1). Its action on the vascular bed is very important, causing hypotension and the leakage of fluids and immune system cells in the lung, heart, and other organs.28 Cardiac involvement, whether myocardial dysfunction, pericarditis, valve dysfunction, or coronary abnormalities, is noteworthy.29
Diagnostic criteria of HLH and clinical and laboratory manifestations of sepsis/SIRS.
| Hematophagocytic lymphohistiocytosis | Sepsis/SIRS |
|---|---|
| Molecular diagnosis: present | Molecular diagnosis: absent |
| Five of the following eight criteria: | |
| Fever | Fever |
| Splenomegaly | Visceromegaly+/− |
| Cytopenia of two of three lineages | Cytopenia of two of three lineages |
| Hgb <9 g/L | Hgb <9 g/L |
| Platelets 100×109 | Platelets 100×109 |
| Neutrophils <1×109 | Neutrophils <1×109 |
| Hypertriglyiceridemia (triglycerides >265 mg/dL) or hyperfibrinogenemia <1.5 g/L | Hypertriglyceridemia (triglycerides >265 mg/dL) or hyperfibrinogenemia <1.5 g/L |
| Hemophagocytosis in bone marrow, spleen or lymph nodes | Hemophagocytosis in bone marrow, spleen or lymph nodes |
| Ferritin ≥500μg/L | Ferritin ≥500μg/L |
| Low or absent NK cell activity (according to local laboratory reference) | Low or absent NK cell activity (according to local laboratory reference) |
| Soluble CD 25 (soluble IL2 receptor) >2400 U/mL | Soluble CD 25 (soluble IL2 receptor) >2400 U/mL |
SIRS, systemic inflammatory response syndrome, HLH, hematophagocytic lymphohistiocytosis.
Source: Castillo and Carcillo, with modifications.5
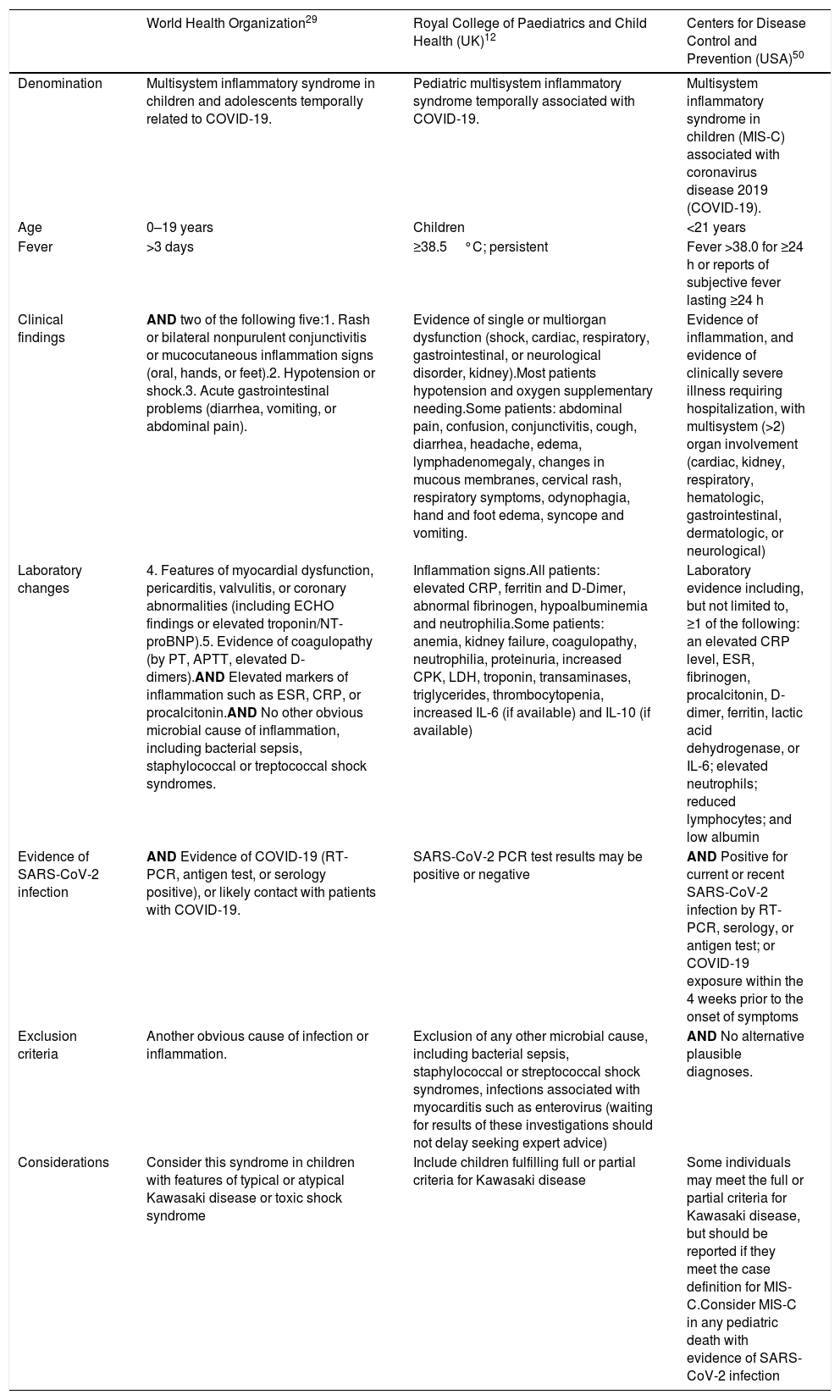
The criteria for the clinical diagnosis of MIS-C, according to the Royal College of Paediatrics and Child Health (RCPCH),12 are shared by other inflammatory diseases (Table 2). Notably, fever, adenomegaly, rash, involvement of the mucous membranes, edema of the hands and feet, and involvement of the coronary arteries are clinical criteria for the diagnosis of Kawasaki disease. The presence of fever and coronary abnormalities are symptoms of Kawasaki-like disease,30 which explains the connection to MIS-C associated with COVID-19.
Case definitions for multisystem inflammatory syndrome during the COVID-19 pandemic.
| World Health Organization29 | Royal College of Paediatrics and Child Health (UK)12 | Centers for Disease Control and Prevention (USA)50 | |
|---|---|---|---|
| Denomination | Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19. | Pediatric multisystem inflammatory syndrome temporally associated with COVID-19. | Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). |
| Age | 0–19 years | Children | <21 years |
| Fever | >3 days | ≥38.5°C; persistent | Fever >38.0 for ≥24 h or reports of subjective fever lasting ≥24 h |
| Clinical findings | AND two of the following five:1. Rash or bilateral nonpurulent conjunctivitis or mucocutaneous inflammation signs (oral, hands, or feet).2. Hypotension or shock.3. Acute gastrointestinal problems (diarrhea, vomiting, or abdominal pain). | Evidence of single or multiorgan dysfunction (shock, cardiac, respiratory, gastrointestinal, or neurological disorder, kidney).Most patients hypotension and oxygen supplementary needing.Some patients: abdominal pain, confusion, conjunctivitis, cough, diarrhea, headache, edema, lymphadenomegaly, changes in mucous membranes, cervical rash, respiratory symptoms, odynophagia, hand and foot edema, syncope and vomiting. | Evidence of inflammation, and evidence of clinically severe illness requiring hospitalization, with multisystem (>2) organ involvement (cardiac, kidney, respiratory, hematologic, gastrointestinal, dermatologic, or neurological) |
| Laboratory changes | 4. Features of myocardial dysfunction, pericarditis, valvulitis, or coronary abnormalities (including ECHO findings or elevated troponin/NT-proBNP).5. Evidence of coagulopathy (by PT, APTT, elevated D-dimers).AND Elevated markers of inflammation such as ESR, CRP, or procalcitonin.AND No other obvious microbial cause of inflammation, including bacterial sepsis, staphylococcal or treptococcal shock syndromes. | Inflammation signs.All patients: elevated CRP, ferritin and D-Dimer, abnormal fibrinogen, hypoalbuminemia and neutrophilia.Some patients: anemia, kidney failure, coagulopathy, neutrophilia, proteinuria, increased CPK, LDH, troponin, transaminases, triglycerides, thrombocytopenia, increased IL-6 (if available) and IL-10 (if available) | Laboratory evidence including, but not limited to, ≥1 of the following: an elevated CRP level, ESR, fibrinogen, procalcitonin, D-dimer, ferritin, lactic acid dehydrogenase, or IL-6; elevated neutrophils; reduced lymphocytes; and low albumin |
| Evidence of SARS-CoV-2 infection | AND Evidence of COVID-19 (RT-PCR, antigen test, or serology positive), or likely contact with patients with COVID-19. | SARS-CoV-2 PCR test results may be positive or negative | AND Positive for current or recent SARS-CoV-2 infection by RT-PCR, serology, or antigen test; or COVID-19 exposure within the 4 weeks prior to the onset of symptoms |
| Exclusion criteria | Another obvious cause of infection or inflammation. | Exclusion of any other microbial cause, including bacterial sepsis, staphylococcal or streptococcal shock syndromes, infections associated with myocarditis such as enterovirus (waiting for results of these investigations should not delay seeking expert advice) | AND No alternative plausible diagnoses. |
| Considerations | Consider this syndrome in children with features of typical or atypical Kawasaki disease or toxic shock syndrome | Include children fulfilling full or partial criteria for Kawasaki disease | Some individuals may meet the full or partial criteria for Kawasaki disease, but should be reported if they meet the case definition for MIS-C.Consider MIS-C in any pediatric death with evidence of SARS-CoV-2 infection |
ECHO, echocardiography; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PT, prothrombin time; APTT, activated partial thromboplastin time; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; RT-PCR, reverse transcription – polymerase chain reaction; CPK, creatine phosphokinase; LDH, lactate dehydrogenase; IL, interleukin; MIS-C, multisystem inflammatory syndrome in children.
Source: Whittaker et al.41 with modifications.
The laboratory criteria of MIS-C (Table 2) also include symptoms that overlap with HLH, such as elevated triglycerides and ferritin and the involvement of two hematological series.25 HLH, primary or secondary to infection, is a complex clinical syndrome characterized by an excessive but ineffective inflammatory response, intense macrophage activation, tissue invasion by T lymphocytes, and the secretion of large amounts of cytokines, possibly due to the stimulation of Toll-like receptors. The ability of SARS-CoV-2 to bind to these receptors justifies the presence of common laboratory parameters for COVID-19 and HLH.
Studies are needed to better understand MIS-C. The challenges are related to the pathophysiological mechanisms, diagnostic criteria, and treatment strategies.31 The disease is rare, affecting 2 in 100,000 children under 21 years of age, and it has a temporal relationship with COVID-19, appearing two to four weeks after SARS-CoV-2 infection. The initial approach to managing pediatric patients with suspected MIS most often occurs in the emergency department, which requires that professionals working in this area, especially pediatricians, be prepared. This review aims to contribute to this preparation.
Clinical presentationThere is limited knowledge regarding SARS-CoV-2 infection in children. The clinical picture is oligosymptomatic when compared with most adult patients32; most symptoms are those of upper respiratory tract infection and mild pneumonia33 with some reports of gastrointestinal symptoms.34 However, some publications (Table 3) recently reported acute and severe clinical manifestations in children and adolescents, with an evolution to cardiogenic shock and the need for intensive care (Tables 2 and 3).
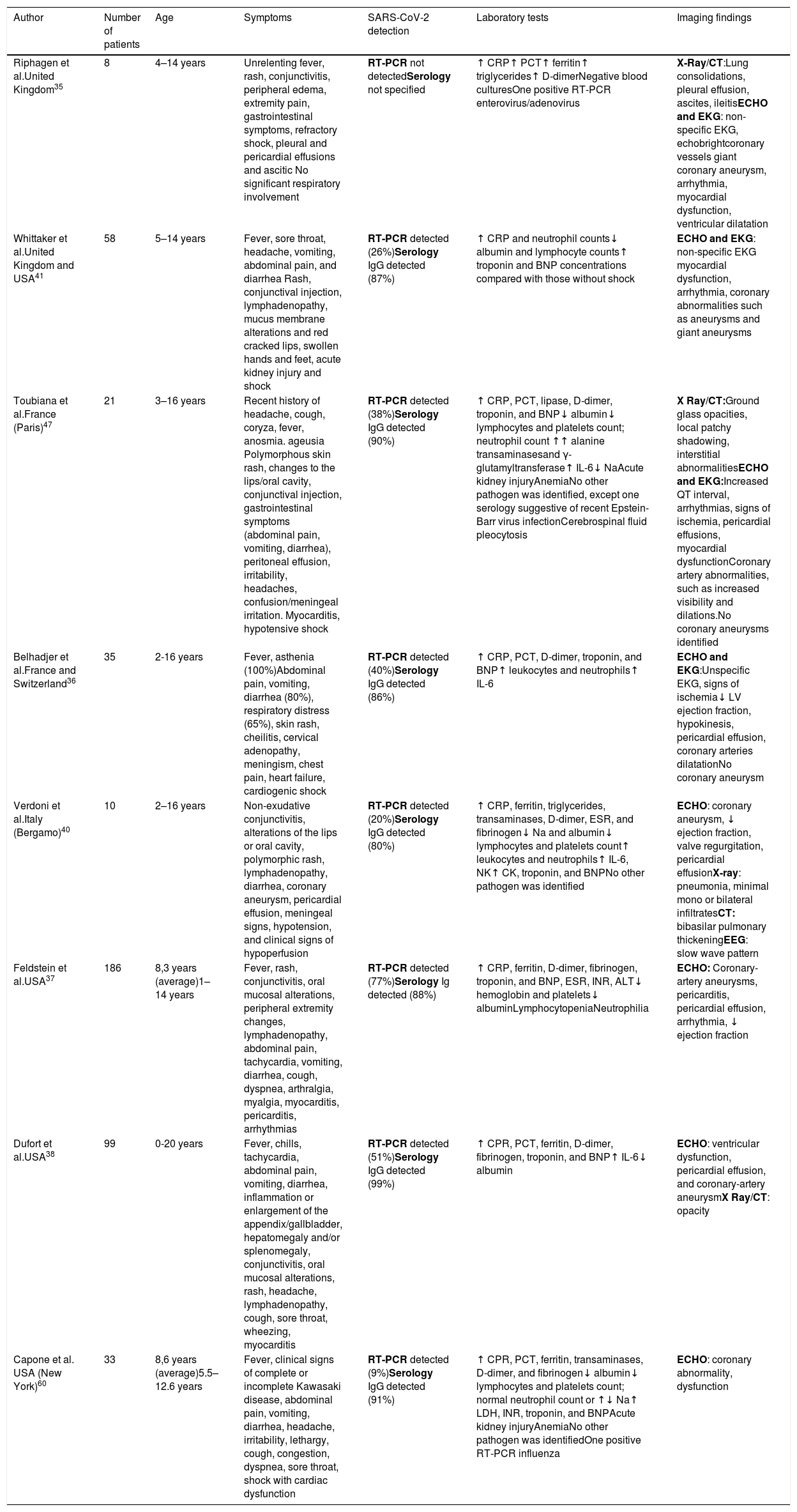
Imaging and laboratory findings of children and adolescents presenting with SARS-CoV-2-related symptoms and signs of MIS-C, according to the authors.
| Author | Number of patients | Age | Symptoms | SARS-CoV-2 detection | Laboratory tests | Imaging findings |
|---|---|---|---|---|---|---|
| Riphagen et al.United Kingdom35 | 8 | 4–14 years | Unrelenting fever, rash, conjunctivitis, peripheral edema, extremity pain, gastrointestinal symptoms, refractory shock, pleural and pericardial effusions and ascitic No significant respiratory involvement | RT-PCR not detectedSerology not specified | ↑ CRP↑ PCT↑ ferritin↑ triglycerides↑ D-dimerNegative blood culturesOne positive RT-PCR enterovirus/adenovirus | X-Ray/CT:Lung consolidations, pleural effusion, ascites, ileitisECHO and EKG: non-specific EKG, echobrightcoronary vessels giant coronary aneurysm, arrhythmia, myocardial dysfunction, ventricular dilatation |
| Whittaker et al.United Kingdom and USA41 | 58 | 5–14 years | Fever, sore throat, headache, vomiting, abdominal pain, and diarrhea Rash, conjunctival injection, lymphadenopathy, mucus membrane alterations and red cracked lips, swollen hands and feet, acute kidney injury and shock | RT-PCR detected (26%)Serology IgG detected (87%) | ↑ CRP and neutrophil counts↓ albumin and lymphocyte counts↑ troponin and BNP concentrations compared with those without shock | ECHO and EKG: non-specific EKG myocardial dysfunction, arrhythmia, coronary abnormalities such as aneurysms and giant aneurysms |
| Toubiana et al.France (Paris)47 | 21 | 3–16 years | Recent history of headache, cough, coryza, fever, anosmia. ageusia Polymorphous skin rash, changes to the lips/oral cavity, conjunctival injection, gastrointestinal symptoms (abdominal pain, vomiting, diarrhea), peritoneal effusion, irritability, headaches, confusion/meningeal irritation. Myocarditis, hypotensive shock | RT-PCR detected (38%)Serology IgG detected (90%) | ↑ CRP, PCT, lipase, D-dimer, troponin, and BNP↓ albumin↓ lymphocytes and platelets count; neutrophil count ↑↑ alanine transaminasesand γ-glutamyltransferase↑ IL-6↓ NaAcute kidney injuryAnemiaNo other pathogen was identified, except one serology suggestive of recent Epstein-Barr virus infectionCerebrospinal fluid pleocytosis | X Ray/CT:Ground glass opacities, local patchy shadowing, interstitial abnormalitiesECHO and EKG:Increased QT interval, arrhythmias, signs of ischemia, pericardial effusions, myocardial dysfunctionCoronary artery abnormalities, such as increased visibility and dilations.No coronary aneurysms identified |
| Belhadjer et al.France and Switzerland36 | 35 | 2-16 years | Fever, asthenia (100%)Abdominal pain, vomiting, diarrhea (80%), respiratory distress (65%), skin rash, cheilitis, cervical adenopathy, meningism, chest pain, heart failure, cardiogenic shock | RT-PCR detected (40%)Serology IgG detected (86%) | ↑ CRP, PCT, D-dimer, troponin, and BNP↑ leukocytes and neutrophils↑ IL-6 | ECHO and EKG:Unspecific EKG, signs of ischemia↓ LV ejection fraction, hypokinesis, pericardial effusion, coronary arteries dilatationNo coronary aneurysm |
| Verdoni et al.Italy (Bergamo)40 | 10 | 2–16 years | Non-exudative conjunctivitis, alterations of the lips or oral cavity, polymorphic rash, lymphadenopathy, diarrhea, coronary aneurysm, pericardial effusion, meningeal signs, hypotension, and clinical signs of hypoperfusion | RT-PCR detected (20%)Serology IgG detected (80%) | ↑ CRP, ferritin, triglycerides, transaminases, D-dimer, ESR, and fibrinogen↓ Na and albumin↓ lymphocytes and platelets count↑ leukocytes and neutrophils↑ IL-6, NK↑ CK, troponin, and BNPNo other pathogen was identified | ECHO: coronary aneurysm, ↓ ejection fraction, valve regurgitation, pericardial effusionX-ray: pneumonia, minimal mono or bilateral infiltratesCT: bibasilar pulmonary thickeningEEG: slow wave pattern |
| Feldstein et al.USA37 | 186 | 8,3 years (average)1–14 years | Fever, rash, conjunctivitis, oral mucosal alterations, peripheral extremity changes, lymphadenopathy, abdominal pain, tachycardia, vomiting, diarrhea, cough, dyspnea, arthralgia, myalgia, myocarditis, pericarditis, arrhythmias | RT-PCR detected (77%)Serology Ig detected (88%) | ↑ CRP, ferritin, D-dimer, fibrinogen, troponin, and BNP, ESR, INR, ALT↓ hemoglobin and platelets↓ albuminLymphocytopeniaNeutrophilia | ECHO: Coronary-artery aneurysms, pericarditis, pericardial effusion, arrhythmia, ↓ ejection fraction |
| Dufort et al.USA38 | 99 | 0-20 years | Fever, chills, tachycardia, abdominal pain, vomiting, diarrhea, inflammation or enlargement of the appendix/gallbladder, hepatomegaly and/or splenomegaly, conjunctivitis, oral mucosal alterations, rash, headache, lymphadenopathy, cough, sore throat, wheezing, myocarditis | RT-PCR detected (51%)Serology IgG detected (99%) | ↑ CPR, PCT, ferritin, D-dimer, fibrinogen, troponin, and BNP↑ IL-6↓ albumin | ECHO: ventricular dysfunction, pericardial effusion, and coronary-artery aneurysmX Ray/CT: opacity |
| Capone et al. USA (New York)60 | 33 | 8,6 years (average)5.5–12.6 years | Fever, clinical signs of complete or incomplete Kawasaki disease, abdominal pain, vomiting, diarrhea, headache, irritability, lethargy, cough, congestion, dyspnea, sore throat, shock with cardiac dysfunction | RT-PCR detected (9%)Serology IgG detected (91%) | ↑ CPR, PCT, ferritin, transaminases, D-dimer, and fibrinogen↓ albumin↓ lymphocytes and platelets count; normal neutrophil count or ↑↓ Na↑ LDH, INR, troponin, and BNPAcute kidney injuryAnemiaNo other pathogen was identifiedOne positive RT-PCR influenza | ECHO: coronary abnormality, dysfunction |
RT-PCR, reverse transcription – polymerase chain reaction; PCT, procalcitonin; CRP, C-reactive protein; TG, triglycerides; CT, computed tomography; ECHO, echocardiography; EKG, electrocardiogram; EEG, electroencephalography; IL, interleukin; CK, creatine kinase; LDH, lactate dehydrogenase; IgG, immunoglobulin G; BNP, B-type natriuretic peptide; Na, natrium; NK, natural killer; ESR, erythrocyte sedimentation rate; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; MIS-C, multisystem inflammatory disorder in children and adolescents; INR, international normalized ratio; LV, left ventricular.
At the end of April 2020, the United Kingdom issued an alert regarding the growing number of pediatric patients who had a clinical picture compatible with toxic shock, signs similar to Kawasaki disease, changes in hemodynamic parameters, and gastrointestinal manifestations.28 Evolution with cardiogenic shock, the need for ventilatory support and vasoactive drugs, coronary dilation, and elevated inflammatory test results without evidence of pulmonary involvement were observed in eight patients with the initial picture of high fever, skin rash, non-purulent conjunctivitis, abdominal pain, and diarrhea. One patient died. SARS-CoV-2 positivity was identified through the investigation of viral RNA in two patients and adenovirus in another; in others, there was a history of contact with first-degree relatives with COVID-19.35
A study of 14 pediatric intensive care units in France and Switzerland reported 35 children who developed severe cardiac decompensation with signs of MIS-C associated with COVID-19. The laboratory diagnosis was made by polymerase chain reaction (PCR) detection in a nasopharyngeal swab and/or positive serology in 31 of the 35 patients. In this case series, the clinical manifestations at admission were fever, asthenia, and gastrointestinal symptoms such as abdominal pain, diarrhea, and vomiting in 80% of patients. Some signs suggestive of Kawasaki disease, such as skin rash, cervical adenopathy, cheilitis, and meningism, were also observed, without meeting all classic clinical criteria of the disease. The main laboratory findings revealed changes in inflammatory tests (PCR, erythrocyte sedimentation rate, brain natriuretic peptide [BNP], interleukin-6, D-dimer, troponin I) and on echocardiography, with a relevant reduction in ejection fraction and left ventricular hypokinesia in most patients. All patients required intensive care unit (ICU) admission, 80% used inotropic drugs, 28% used extracorporeal membrane oxygenation (ECMO) and two-thirds required mechanical ventilation.36 The improvement in myocardial dysfunction observed in this series suggests that the mechanism of heart failure may be more closely associated with the inflammatory process, leading to the distention of myocardial fibers and activation of BNP rather than to the direct lesions of the virus. Another noteworthy observation is that the disease occurred in an older age group compared to that usually affected by Kawasaki disease.
The New York State database on MIS-C has similarities with the European series. On July 3, 2020, 230 cases had been reported with a total of two deaths. The most prevalent symptoms are prolonged fever (for more than five days), abdominal pain, diarrhea, vomiting, dyspnea or tachypnea, paleness or cyanosis of extremities, decreased urine output, and lethargy or mental confusion. Positive antigenic or serological tests for SARS-CoV-2 were observed in 94% of patients.11
Two studies from the US37,38 presented the largest case series related to MIS-C. Using the same New York database, Dufort EM et al.38 published a study of 191 patients, among whom 95 had confirmed MIS-C and four had suspected MIS-C. Regarding the definition of confirmed cases, clinical criteria, laboratory criteria (alteration of at least two exams), and serological evidence or molecular testing (RT-PCR) for SARS-CoV-2 were used. Suspected cases were classified according to the clinical condition and epidemiological criteria. All patients had fever, 97% had tachycardia, 80% had gastrointestinal symptoms, 60% had rash, 57% had conjunctivitis, and 27% had mucosal changes. In this study, some symptoms little described by other authors stand out, such as hepatomegaly, splenomegaly, ascites, inflammation, and enlargement of the cecal appendix and gallbladder. In addition, it is evident that skin manifestations are common in children under 5 years of age, and cases of myocarditis or cardiogenic shock are predominant in adolescents. The authors also highlighted the presence of neurological symptoms in 13% of children and 38% of adolescents. Dufort EM38 and other authors emphasized the presence of encephalopathy, headache, signs of cerebellar alteration, muscle weakness, and reduced reflexes.39
A study of 186 patients across the United States reinforced the clinical and laboratory worsening, mainly among patients over 10 years of age, with inflammatory markers and cardiac enzymes with more significant changes.37
A study in Bergamo, Italy, compared a group of children with Kawasaki-like disease diagnosed before the COVID-19 pandemic with a group diagnosed during the pandemic. In the latter, an increase in the number of cases was observed by up to 30 times, with more pronounced cardiac alterations, a higher frequency of macrophage activation syndrome, and, as in reports from Europe,36 a predominance of older children affected.40
A UK publication described 58 children from eight ICU facilities with the clinical and laboratory criteria for MIS-C associated with COVID-19 caused by SARS-CoV-2 in 2020. The mean age was 9 years, and fever occurred in 100% of cases, vomiting in 45%, abdominal pain in 53%, diarrhea in 52%, and conjunctivitis in 45%. Shock due to myocardial dysfunction was observed in 50% of the patients; of those, 79% required mechanical ventilation and the use of inotropic drugs. There was evidence of SARS-CoV-2 infection (by serology and/or reverse transcription-PCR [RT-PCR]) in 78% of the total patient group. The authors compared the findings of these patients with those hospitalized from 2002 to 2019, diagnosed with Kawasaki, shock, and toxic shock syndrome, and observed older age (9 years versus 2.7 years) and inflammatory tests with elevated levels.41 This corroborates the observation that the inflammatory state compatible with MIS-C associated with COVID-19 has a peculiar presentation.
Studies from Germany, Austria, and Portugal have also reported severe cases with gastrointestinal symptoms and increased inflammatory cytokines with an evolution compatible with Kawasaki-like or toxic shock syndrome associated with the detection of SARS-CoV-2 by RT-PCR or serology.42
No MIS-C case reports from China, Japan, or South Korea were retrieved.33 The multiple cases reported in the literature have led the WHO, the US Centers for Disease Control and Prevention, and the RCPCH to publish similar definitions of possible cases eligible for MIS-C (Table 2).12,29
It is important to note that the period of MIS-C development after SARS-CoV-2 infection remains a matter of debate. The disease can develop between the first and the second week after infection with the virus37; however, in patients with negative RT-PCR and serology test results, the evolution to MIS-C can be considered within six weeks after patient exposure to COVID-19.38
Laboratory and imaging testsComplementary tests, an integral part of the diagnostic criteria, are not always available, which can lead to diagnostic difficulties and treatment delays. It is important for the pediatrician to raise the clinical suspicion and, in the face of scarce resources, arrange for referral to a more equipped emergency service.
Real time RT-PCR43,44 is the most recommended method for identifying SARS-CoV-2, and the ideal period for its collection is between the fifth and sixth symptomatic day (range: third to seventh day). If the sample is collected before the third day of illness and the result is negative, a new collection is recommended on the third day.
Seroconversion is believed to occur after the seventh day of illness in 50% of patients and in 14 days in all patients. It is more relevant for epidemiological studies and can assist in COVID-19 diagnosis.43,45 The possibility of cross-reactions leading to false-positive results in negative serum has not been ruled out.46
In observational studies of patients who developed MIS-C, the probability of identifying SARS-CoV-2 infection by serology was two to three times higher than that by RT-PCR.47 These findings are expected, since MIS-C is a late form of the disease that occurs when the presence of the virus is no longer expected and the antibody rates are on the rise. This makes serology particularly useful in correlating SARS-CoV-2 and MIS-C.45
Inflammatory markers of organic functionConsidering that MIS-C is a late manifestation of COVID-19, laboratory findings observed in the infectious phase of the disease maintain some diagnostic relevance even in the late inflammatory phase and may change, significantly or not, as the condition worsens (Table 3).
Despite the lack of an absolute consensus, most authors accept that disease deterioration changes blood count and inflammatory test results, impairs liver and renal function (hyperbilirubinemia, hypertriglyceridemia, and hypoalbuminemia), changes coagulation, changes myocardial enzymes, and elevates kinins.48
The aspects that alert to MIS-C point to the presence, although not always, of one or more changes in inflammatory test results, such as:
- •
Elevation of C-reactive protein (CRP), erythrocyte sedimentation rate, procalcitonin, D-dimer, ferritin, lactic dehydrogenase, fibrinogen, IL-6, or neutrophils; and
- •
Reduction of lymphocytes and/or albumin.29,49,50
Feldstein et al.37 in a study published at the end of June 2020, noticed that among the multiple tests performed on patients affected by MIS-C, at least four were altered, primarily increased CRP, lymphopenia, neutrophilia, increased ferritin, hypoalbuminemia, and myocardial dysfunction.
Verdoni et al.40 when observing children and young people diagnosed with Kawasaki-like disease who developed coronary artery aneurysm in the pre- and post-SARS-CoV-2 era, noticed a tendency toward inflammation and more pronounced cardiac injury markers in which CRP, troponin, ferritin, and BNP were proven useful as predictors of disease worsening.40 In the same line of investigation, Riphagen et al. highlighted that patients who had elevated cardiac enzymes also had non-specific changes on electrocardiography (ECG) and echocardiography.51 Whittaker et al. warned of the recommendation to investigate these patients using echocardiography.41
Imaging tests, including ultrasound, chest X-ray, and chest tomographyPulmonary involvement in MIS-C is generally mild or non-existent. Imaging tests are indicated for patients who have respiratory symptoms (tachypnea, dyspnea, and/or hypoxemia). In this situation, if available, bedside ultrasound (point of care ultrasound) is preferable over others since it provides information comparable to chest tomography with the advantages of avoiding ionizing exposure and displacement of the patient, allowing simultaneous assessment of the clinical and imaging information, and reducing the risks of contamination of employees.52
On a progressive scale of worsening, the most frequent ultrasound findings are irregular pleura, B lines, small pleural consolidations, involvement of the upper and anterior areas, consolidation, air bronchography, and pleural effusion.53
Echocardiography and ECGEchocardiography and ECG are of fundamental relevance in MIS-C, since myocardial and coronary involvement are critical points of unfavorable outcomes.
The most significant findings of a review54 of cardiac changes in COVID-19 were:
- •
ECG: Various aspects that mimic acute coronary syndrome, non-specific changes in the ST segment, an altered or inverted T wave, or elevation or depression of PR or ST as a result of myocardial inflammation (indicative of severity and worse prognosis).
- •
Echocardiography: Dysfunction, hypocontractility, or even segmental absence of contractility in the myocardial chambers. In this case, alterations indicated severity and worse prognosis.
In a prospective observational study of 21 children and young people with MIS-C, pericardial effusion (48%), pleural effusion (14%), myocarditis (76%) with a moderate to severe drop in left ventricular ejection fraction (from 57% to 10%), and coronary abnormalities (38%), such as more pronounced brilliance or dilation without the identification of an aneurysm, were observed. Analyzing the ECG tracings, the authors identified prolonged QT interval, occasional ventricular arrhythmias, and diffuse ST elevation.47
Myocardial impairment has been found in autopsies of adults.54 In the English literature, no articles correlating anatomopathological findings and disease caused by SARS-CoV-2 in childhood were retrieved.
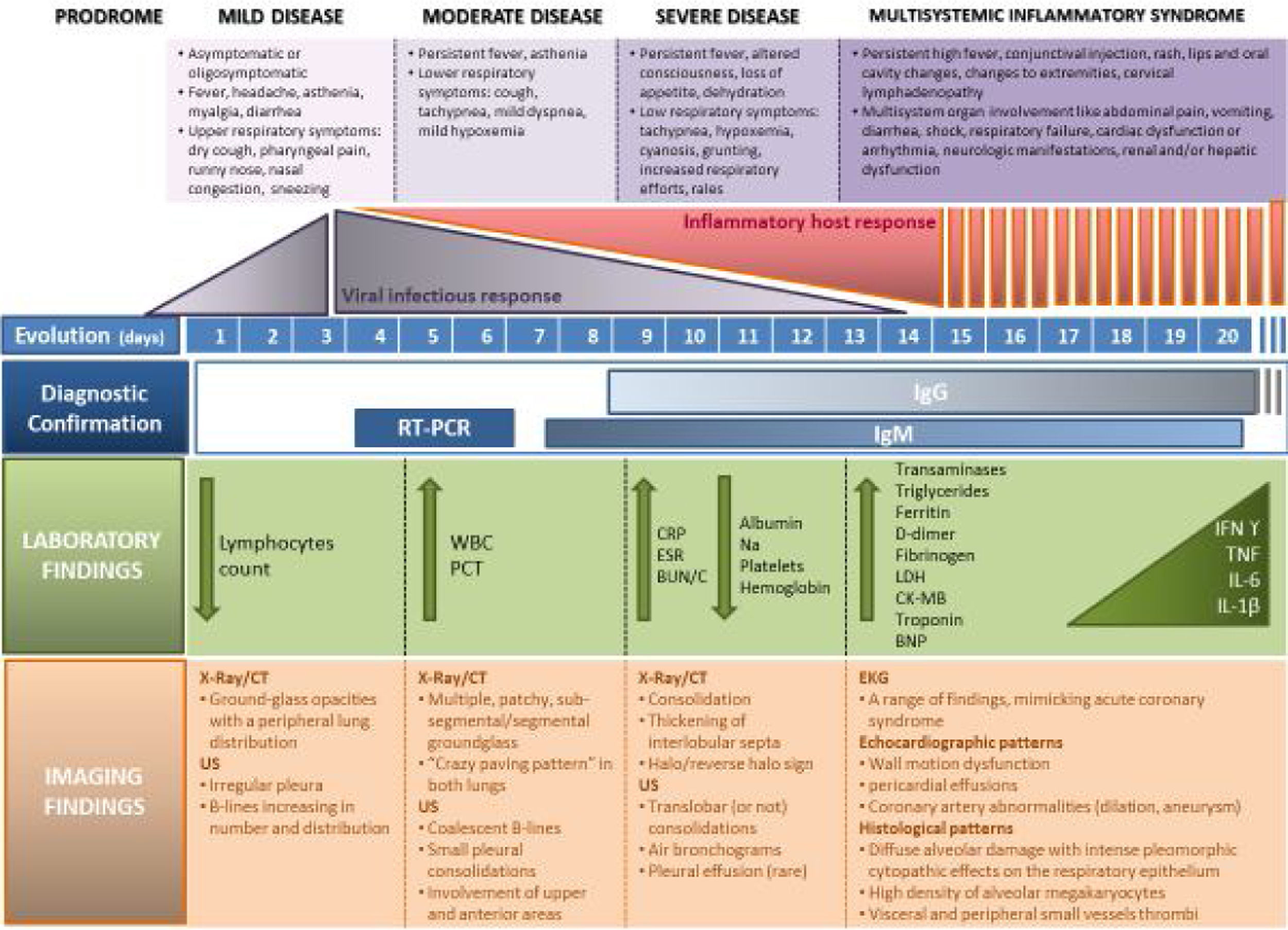
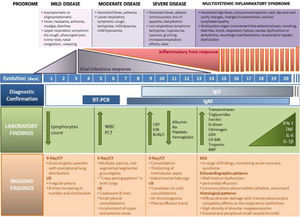
Fig. 2 describes the laboratory and imaging findings according to COVID-19 progression (mild, moderate, or severe and MIS-C).
COVID 19 disease and MIS-C related to COVID-19. The figure illustrates 4 escalating progressive phases of the disease, according to evolution, severity, clinical signs and symptoms, infectious vs inflammatory host response, diagnosis tests and lab and imaging findings. RT-PCR, reverse transcription-polymerase chain reaction; PCT, procalcitonin; CRP, C-reactive protein; TG, triglycerides; CT, computed tomography; ECHO, echocardiography; EKG, electrocardiogram; EEG, electroencephalography; IL, interleukin; CK, creatine kinase; LDH, lactate dehydrogenase; IgG, immunoglobulin G; BNP, B-type natriuretic peptide; Na, natrium; NK, natural killer; WBC, white blood count; ESR, erythrocyte sedimentation rate; BUN, blood urea nitrogen; C, creatinine; SARS CoV-2, severe acute respiratory syndrome coronavirus 2; MIS-C, multisystem inflammatory syndrome in children and adolescents; US, ultrasound.
MIS-C temporarily associated with COVID-19 is a rare but serious and potentially fatal presentation; thus, as in any condition where life is at risk, there must be an emphasis on supportive treatment that includes oxygen supplementation; ventilatory, cardiovascular, and renal support; and pharmacotherapy in cases in which it is indicated.55 Supportive treatment does not differ from other situations and will not be outlined here, as there are established pediatric guidelines.12
Antibiotic administration cannot be delayed due to the possibility of toxic shock syndrome. It is also important to highlight the team's mandatory use of personal protective equipment from the start of treatment.
Information regarding the indication and appropriate timing of potential treatments is limited, and several centers consider the use of intravenous immunoglobulin (IVIG), corticosteroids, immunomodulators, anticoagulants, and antiplatelet agents. The selection of two or more immunomodulators to control the inflammatory condition has been described by several authors.37,38,41,60,63 Patients with Kawasaki-like conditions who are classified as high risk for immunoglobulin resistance have been receiving a combination of IVIG with corticosteroids, as this treatment appears to reduce the prevalence of coronary abnormalities and duration of fever.30,36
In addition to IVIG, other potential strategies have been suggested to treat the cytokine storm. The transfer of knowledge from the cytokine storm in the context of autoimmune and infectious diseases to a new viral disease is complex and challenging. Evidence of hyperinflammation reinforces the therapeutic possibility of using immunomodulatory agents in COVID-1926,56; in this scenario, therapy with IVIG, corticosteroids, selective cytokine blockers (anakinra, tocilizumab), anticoagulants, remdesivir, and convalescent plasma can reduce the inflammatory response and mortality.
The use of medications is justified by the severity of the situation and the effectiveness in similar clinical conditions, although the effectiveness in MIS-C has yet to be proven. Evidence regarding each of these therapeutic modalities is provided in Table 4.
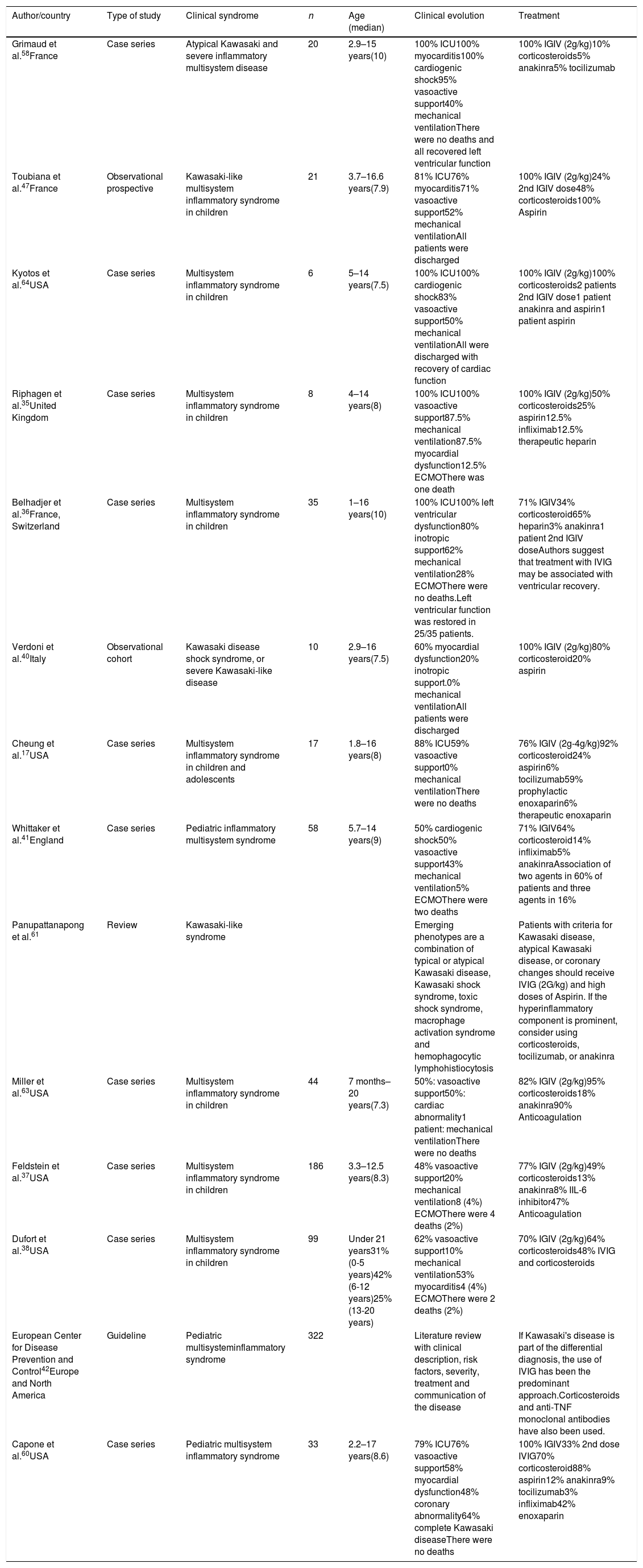
Treatment of multisystem inflammatory syndrome related to COVID-19.
| Author/country | Type of study | Clinical syndrome | n | Age (median) | Clinical evolution | Treatment |
|---|---|---|---|---|---|---|
| Grimaud et al.58France | Case series | Atypical Kawasaki and severe inflammatory multisystem disease | 20 | 2.9–15 years(10) | 100% ICU100% myocarditis100% cardiogenic shock95% vasoactive support40% mechanical ventilationThere were no deaths and all recovered left ventricular function | 100% IGIV (2g/kg)10% corticosteroids5% anakinra5% tocilizumab |
| Toubiana et al.47France | Observational prospective | Kawasaki-like multisystem inflammatory syndrome in children | 21 | 3.7–16.6 years(7.9) | 81% ICU76% myocarditis71% vasoactive support52% mechanical ventilationAll patients were discharged | 100% IGIV (2g/kg)24% 2nd IGIV dose48% corticosteroids100% Aspirin |
| Kyotos et al.64USA | Case series | Multisystem inflammatory syndrome in children | 6 | 5–14 years(7.5) | 100% ICU100% cardiogenic shock83% vasoactive support50% mechanical ventilationAll were discharged with recovery of cardiac function | 100% IGIV (2g/kg)100% corticosteroids2 patients 2nd IGIV dose1 patient anakinra and aspirin1 patient aspirin |
| Riphagen et al.35United Kingdom | Case series | Multisystem inflammatory syndrome in children | 8 | 4–14 years(8) | 100% ICU100% vasoactive support87.5% mechanical ventilation87.5% myocardial dysfunction12.5% ECMOThere was one death | 100% IGIV (2g/kg)50% corticosteroids25% aspirin12.5% infliximab12.5% therapeutic heparin |
| Belhadjer et al.36France, Switzerland | Case series | Multisystem inflammatory syndrome in children | 35 | 1–16 years(10) | 100% ICU100% left ventricular dysfunction80% inotropic support62% mechanical ventilation28% ECMOThere were no deaths.Left ventricular function was restored in 25/35 patients. | 71% IGIV34% corticosteroid65% heparin3% anakinra1 patient 2nd IGIV doseAuthors suggest that treatment with IVIG may be associated with ventricular recovery. |
| Verdoni et al.40Italy | Observational cohort | Kawasaki disease shock syndrome, or severe Kawasaki-like disease | 10 | 2.9–16 years(7.5) | 60% myocardial dysfunction20% inotropic support.0% mechanical ventilationAll patients were discharged | 100% IGIV (2g/kg)80% corticosteroid20% aspirin |
| Cheung et al.17USA | Case series | Multisystem inflammatory syndrome in children and adolescents | 17 | 1.8–16 years(8) | 88% ICU59% vasoactive support0% mechanical ventilationThere were no deaths | 76% IGIV (2g-4g/kg)92% corticosteroid24% aspirin6% tocilizumab59% prophylactic enoxaparin6% therapeutic enoxaparin |
| Whittaker et al.41England | Case series | Pediatric inflammatory multisystem syndrome | 58 | 5.7–14 years(9) | 50% cardiogenic shock50% vasoactive support43% mechanical ventilation5% ECMOThere were two deaths | 71% IGIV64% corticosteroid14% infliximab5% anakinraAssociation of two agents in 60% of patients and three agents in 16% |
| Panupattanapong et al.61 | Review | Kawasaki-like syndrome | Emerging phenotypes are a combination of typical or atypical Kawasaki disease, Kawasaki shock syndrome, toxic shock syndrome, macrophage activation syndrome and hemophagocytic lymphohistiocytosis | Patients with criteria for Kawasaki disease, atypical Kawasaki disease, or coronary changes should receive IVIG (2G/kg) and high doses of Aspirin. If the hyperinflammatory component is prominent, consider using corticosteroids, tocilizumab, or anakinra | ||
| Miller et al.63USA | Case series | Multisystem inflammatory syndrome in children | 44 | 7 months–20 years(7.3) | 50%: vasoactive support50%: cardiac abnormality1 patient: mechanical ventilationThere were no deaths | 82% IGIV (2g/kg)95% corticosteroids18% anakinra90% Anticoagulation |
| Feldstein et al.37USA | Case series | Multisystem inflammatory syndrome in children | 186 | 3.3–12.5 years(8.3) | 48% vasoactive support20% mechanical ventilation8 (4%) ECMOThere were 4 deaths (2%) | 77% IGIV (2g/kg)49% corticosteroids13% anakinra8% IIL-6 inhibitor47% Anticoagulation |
| Dufort et al.38USA | Case series | Multisystem inflammatory syndrome in children | 99 | Under 21 years31% (0-5 years)42% (6-12 years)25% (13-20 years) | 62% vasoactive support10% mechanical ventilation53% myocarditis4 (4%) ECMOThere were 2 deaths (2%) | 70% IGIV (2g/kg)64% corticosteroids48% IVIG and corticosteroids |
| European Center for Disease Prevention and Control42Europe and North America | Guideline | Pediatric multisysteminflammatory syndrome | 322 | Literature review with clinical description, risk factors, severity, treatment and communication of the disease | If Kawasaki's disease is part of the differential diagnosis, the use of IVIG has been the predominant approach.Corticosteroids and anti-TNF monoclonal antibodies have also been used. | |
| Capone et al.60USA | Case series | Pediatric multisystem inflammatory syndrome | 33 | 2.2–17 years(8.6) | 79% ICU76% vasoactive support58% myocardial dysfunction48% coronary abnormality64% complete Kawasaki diseaseThere were no deaths | 100% IGIV33% 2nd dose IVIG70% corticosteroid88% aspirin12% anakinra9% tocilizumab3% infliximab42% enoxaparin |
ICU, intensive care unit; ECMO, extracorporeal membrane oxygenation.
In addition to life support treatment, IVIG is the predominant therapeutic option for MIS-C in different centers when Kawasaki disease is part of the differential diagnosis.42 The RCPCH proposes that mild or moderate cases of MIS-C associated with COVID-19 be treated with supportive therapy, that severe cases be admitted to the ICU, and that the prescription of immunomodulators should be discussed with physicians who have experience using this type of medication.12 Thus, specialists such as infectologists, rheumatologists, immunologists, or hematologists must be involved in the emergency treatment of MIS-C.
The US National Institutes of Health reinforces a similar concept, recognizing that there is limited information regarding the treatment of MIS-C associated with COVID-19, and stating that supportive therapy is essential; however, many centers consider using IVIG, corticosteroids, and other immunomodulators, although definitive data are not available.57
Most patients included in the MIS-C case series with a differential diagnosis of atypical Kawasaki or Kawasaki-like disease received IVIG (2g/kg), with frequent admission to the ICU and evolution with shock and myocardial dysfunction requiring vasoactive support.40,47,58–60 Case reports of COVID-19 and Kawasaki-like disease also suggested IVIG as therapy.61
Siddiqi et al.62 proposed the use of immunomodulatory agents as therapeutic options in adults in the COVID-19 stage characterized by a state of hyperinflammation before multisystem organic damage occurs. At this stage, corticosteroids may be justified with or without the use of cytokine inhibitors or IVIG. In general, the prognosis and recovery of this critical stage of the disease are poor; thus, its rapid recognition and treatment are essential for a better evolution.
Belhadjer et al.36 described a case series of 35 children admitted to the ICU diagnosed with MIS-C, all of whom had myocardial dysfunction and 80% of whom required hemodynamic support with inotropic agents. Twenty-five patients received IVIG therapy and recovered myocardial function, thus showing an association between IVIG use and good prognosis.
Miller et al.63 reported on 44 patients with MIS-C and gastrointestinal symptoms; among them, none needed hemodynamic support and 81% received IVIG. The severity of the clinical presentation is common in the reports of MIS-C case series and deserves attention due to the high rate of patients requiring ICU admission and the high proportion of children with myocardial dysfunction, cardiogenic shock, and the need for vasoactive support. Most of these patients received IVIG treatment and recovered myocardial function; the evolution to death was an exception.17,41,51,60,64
Dufort et al.38 reported on 99 children in New York State under 20 years of age who were diagnosed with MIS-C; of them, 70% received IVIG. It is noteworthy that the two children among them who died did not receive immunomodulatory treatment.38 Another study of 26 states in the United States described 186 children and adolescents with fever for at least 24hours, multisystem involvement, laboratory evidence of inflammation, and SARS-CoV-2 infection. IVIG treatment was used in 77% of these patients; 21% received a second dose. The authors propose that the choice of treatment should be based on the mechanisms of aggression, i.e., active viral replication in the affected tissues, the host's exuberant inflammatory response, or both. Antiviral agents are beneficial in the first situation, whereas immunomodulatory agents are preferred in cases of immune dysregulation.37
The mechanism of action of IVIG is unknown in Kawasaki disease or in MIS-C associated with COVID-19. It is likely associated with the global anti-inflammatory effect, with modulation in the production of cytokines, neutralization of toxins and other pathogens, and increased regulatory activity of T cells, among others.30,65
CorticosteroidsThe use of corticosteroids in patients with SARS and Middle East respiratory syndrome (recent coronavirus epidemics) has not shown any influence on mortality in addition to increasing viral load and delaying viral clearing.66,67 Furthermore, the current WHO guideline does not recommend the universal use of corticosteroids in the treatment of severe COVID-19, unless there was some other clinical condition in which the medication had a proven therapeutic effect.29 Immunosuppression can be beneficial in cases of hyperinflammatory states.26 Although a randomized study using dexamethasone in adults reported an association between its use and a significant reduction in mortality, these results cannot yet be applied to the pediatric age group.57
A meta-analysis of 2,746 patients with Kawasaki disease demonstrated that the association of corticosteroids and immunoglobulin is related to a reduced risk of coronary aneurysm compared to isolated IVIG therapy.68 The European consensus of Kawasaki disease recommends the use of corticosteroids in some conditions, such as resistance to IVIG, presentation as HLH, presence of shock, evidence of coronary and or peripheral aneurysms, age less than 1 year, and Kobayashi score ≥4.69 The suggested corticosteroid regimen is methylprednisolone 1.6–2mg/kg/day for 5–7 days or methylprednisolone 30mg/day for 3 days.30
Corticosteroids (methylprednisolone and hydrocortisone) correspond to a treatment modality most used in MIS-C according to publications of pediatric case series as shown in Table 4.17,34,35,40,41,47,57,58,61 In most studies, the dose of methylprednisolone was 2mg/kg and the duration of therapy was not well defined.
There is much doubt regarding the efficacy and safety of using corticosteroids in COVID-19, and clinical studies are needed in all age groups.
IL-6 inhibitorsTocilizumab is a recombinant monoclonal antibody that binds directly to the IL-6 receptor, which, in addition to crossing the blood–brain barrier and releasing prostaglandin E2, triggers the increase in temperature.55 The inhibition of IL-6 receptors promotes a reduction in cytokine production, thus configuring some indication of possible treatment for hyperinflammation, although there are no studies in pediatric populations.
Otocilizumab is indicated in adult patients with autoimmune diseases and is approved by the US Food and Drug Administration (FDA) for use in cytokine release syndrome in critical cases in adults and children. Due to the similarities between the cytokine release syndrome and the cytokine storm in COVID-19, studies are underway to evaluate the safety and efficacy of this therapeutic modality.55
Siltuximab, another monoclonal antibody, binds directly to IL-6 and can be considered a second-line treatment for COVID-19 cases resistant to tocilizumab, although there is no evidence of its effectiveness to date.
Data are lacking in the pediatric age group to recommend or contraindicate the use of IL-6 inhibitors.57
IL-1 inhibitorsThe use of anakinra, a recombinant IL-1 receptor antagonist, has been suggested for modulating the effects of cytokines. A randomized study in adult sepsis patients reported increased survival in patients with hyperinflammation who received anakinra without increasing adverse effects.70 Although there is no evidence of a correlation between serum IL-1 levels and COVID-19 severity, ongoing multicenter studies have evaluated the role of anakinra associated with emapalumab in reducing hyperinflammation in patients with severe SARS-CoV-2 infection.
Anakinra was used as additional therapy in MIS-C in 18.2% of cases in the American series63 and in 5% of cases in the United Kingdom,41 and can be considered in the treatment of MIS-C that is refractory to IVIG and corticosteroids.71 The research field remains open, as its role in this situation cannot be properly assessed.
TNF inhibitors (infliximab)TNF is one among the factors that trigger the cytokine storm; therefore, its role in the treatment of SARS-CoV-2 infection deserves investigation. A meta-analysis using anti-TNF showed improved survival in patients with sepsis, a situation in which the role of cytokines is also relevant,72 thus opening a therapeutic possibility in severe cases of COVID-19.
Medications with antiviral activityRemdesivir shows in vitro and in vivo activity against SARS-CoV-2 and other beta-coronaviruses.73 A multicenter randomized placebo-controlled study with adult patients hospitalized with COVID-19 pulmonary infection observed that the use of remdesivir decreased the length of hospital stay.74 The FDA has issued emergency use authorization for remdesivir for the treatment of severe COVID-19 in adults and children. In Brazil, remdesivir was not available at the time of this publication.
Other antivirals have been tested in adults with severe COVID-19. The combination of lopinavir and ritonavir was used in a cohort of adult patients hospitalized with severe SARS-CoV-2 pneumonia with no evidence of superior results to those of standard treatment.75 No studies with these medications in COVID-19 in children were retrieved.
Acetylsalicylic acidThere are reports of use of acetylsalicylic acid (ASA) in cases of MIS-C in situations that meet Kawasaki-like criteria. Since exacerbated inflammation is associated with organ damage and coronary aneurysm, the anti-inflammatory action of ASA at a dose of 30–50mg/kg/day can be useful, although there is no reliable evidence that a reduction in the rate of coronary abnormalities occurs in Kawasaki disease. The antiplatelet action of ASA at a dose of 3–5mg/kg/day is indicated 48h after fever defervescence and should be maintained for 6-8 weeks in cases where there are no coronary changes. Some authors have suggested the use of continuous ASA in patients with coronary abnormalities to prevent thrombosis. It is important to highlight that bleeding is a contraindication to ASA use and that care should be taken in the presence of thrombocytopenia and renal dysfunction.30,69
AnticoagulationAnticoagulant therapy in pediatrics was mainly derived from studies in adults. Cytokine storm causes activation of the coagulation cascade, resulting in thrombosis,20,26 in addition to evidence of severe vascular injury in severe COVID-19.76,77 Autopsy findings confirm the state of hypercoagulability and extensive endothelial injury through the observation of a high frequency of pulmonary microthrombi and other organs.77
There is evidence of laboratory abnormalities of hypercoagulability and a high prevalence of thromboembolic events in adults with COVID-19.78 Clinical studies demonstrated that 20–55% of hospitalized patients show laboratory evidence of coagulopathy (elevated D-dimer, prolonged prothrombin time, mild thrombocytopenia, and/or decreased fibrinogen).79 There is also evidence that a high D-dimer level is associated with a worse prognosis and an increased mortality rate.80 A retrospective study in adults with high D-dimer levels showed that those who received enoxaparin had a lower mortality rate than those without thromboprophylaxis.80 Such observations have led to the indication of anticoagulant therapy in adults. In this population, prophylactic anticoagulation with low molecular weight heparin has been a standard recommendation for all cases that require hospitalization for COVID-19 and have no contraindications.81
In a series of MIS-C cases, the use of anticoagulation was quite variable, ranging from 12.5% to 90.1% of cases.17,36,51,63 The impact of anticoagulation on the clinical outcome cannot be assessed due to the numerous factors involved, thus requiring the individualization of this therapy in the pediatric age group.
Microthrombosis, and even thrombus in large vessels, has been a worrying manifestation in COVID-19; thus, the hematologist should be involved in the initial care of a child with suspected MIS-C that has some evidence of hypercoagulability or critical status. It is important that the pediatric emergency physician is aware of possible early interventions with the support of specialists to obtain the best outcomes.
Enoxaparin, the most commonly used and studied anticoagulant in pediatric patients, can be administered subcutaneously without requiring exclusive vascular access, and has a half-life of 3–6h via renal elimination. Its effect is monitored by measuring anti-Xa, and its action can be partially reversed with the administration of protamine. Laboratory control prior to heparinization includes complete blood count to assess the presence of thrombocytopenia, coagulogram, and renal function assessment, which are already part of the control of every critical patient.82Table 5 shows the dose of enoxaparin used in children.
The spectrum of involvement of COVID-19 in adults and children differs; therefore, the recommendations for adults do not apply directly to the pediatric population. Anticoagulation should be performed in the context of clinical trials or in consultation with specialists. Pediatric clinical trials using anticoagulants for thromboprophylaxis are ongoing.83
Convalescent plasmaIn a Cochrane review evaluating the use of convalescent plasma in patients with COVID-19, eight studies of 32 patients were identified. The authors found a very low level of evidence considering the efficacy and safety of using this therapeutic modality. Randomized controlled studies using convalescent plasma are in progress; however, pediatric patients have not been included.84
Extracorporeal membrane oxygenationECMO can be considered a therapeutic modality in referral services in patients with hypoxemia and refractory hypercapnia, septic and/or cardiogenic shock refractory to vasoactive drugs, and failure of a single organ with minor comorbidity. Contraindications for ECMO must follow established guidelines, which broadly include severe and multiple comorbidities, immunocompromised status, chronic lung disease, conditions that are contraindications for anticoagulation, and severe neurological conditions.85
ECMO was approved by the FDA as a therapy during the COVID-19 pandemic, and the WHO suggests its use for COVID-19 patients with refractory hypoxemia despite the use of protective ventilation, emphasizing that it is a very limited resource that must be rationed in a pandemic situation.
In a pediatric series, ECMO was used in a minority of MIS-C cases, ranging from 5% to 12.5%.36,41,51 The assessment of the true impact of this therapy is not yet possible.
The role of ECMO and its indication during the pandemic must be considered, taking into account the scarcity of resources and specialized professionals, since it is a highly complex procedure and requires an expert team.
The great challenge for pediatricians is recognizing cases of children in the emergency department who present clinically with MIS-C associated with COVID-19 and defining the indication and the ideal time for the immunomodulatory therapeutic approach. For the proper management of patients in critical condition due to a little-known disease, specialist involvement is necessary in the emergency room.
Algorithm: Proposal approach to MIS-C related to COVID-19 at the Emergency DepartmentThe proposal approach is present in the algorithm in Fig. 3.
Final considerationsIn a short period of time, much has been learned about MIS-C temporally associated with COVID-19; however, numerous uncertainties persist. Although it is a rare disease, it comprises a variable spectrum of symptoms and severity and may have a turbulent evolution consisting of multiple organ failure and the need for intensive care.
The pediatric emergency department is the main gateway for children and adolescents with suspected MIS. This review aimed to contribute to the dissemination of current knowledge and instruct pediatricians in early diagnosis and treatment. Pediatric specialists should be involved in decision-making, either in person or remotely, and immunomodulatory therapy should not be delayed in severe cases.
Patients with clinical instability should be referred to the ICU, but in the absence of available spaces, supportive therapy, immunomodulation, and even anticoagulation, when properly indicated, should be initiated in the emergency department. The emergency room pediatrician should not hesitate to seek help and guidance.
Many challenges must still be overcome in understanding this disease. It is important to clarify whether MIS-C is a new condition or shares a common mechanism with other pathologies. Evidence points to an immune-mediated disease with SARS-CoV-2 as a trigger. Causality with SARS-CoV-2 cannot be proven, but the temporality associated with COVID-19 is remarkable, highlighting the importance of conducting serology and viral research.
COVID-19 in children appears to present differently from that in elderly adults or those with comorbidities, but it bears some resemblance to the involvement of young adults, since the cytokine storm syndrome seems more evident, affecting the myocardium. Aggression to the heart seems to be constant in MIS-C, with myocarditis being observed in older children and adolescents and Kawasaki-like disease observed in preschoolers. Similarities with Kawasaki-like disease may increase our understanding of Kawasaki disease, whose etiology is currently unknown.
Finally, it is worth mentioning that the emergency department pediatrician must also be aware of differential diagnoses, especially sepsis and toxic shock syndrome, that require aggressive supportive therapy and antibiotic therapy.
Conflicts of interestThe authors declare no conflicts of interest.