To evaluate the initial Dornic acidity in raw human milk, after pasteurization and after heating and dilution of a dietary supplement for preterm infants.
MethodsA quantitative, descriptive, and experimental study was carried out with a convenience sample at the human milk bank at a Brazilian public maternity, with specialized care for pregnant women and newborns at risk. The eligibility criteria for the study sample included 93 frozen raw human milk in suitable containers with volumes ≥100mL and initial Dornic acidity ≤8° Dornic (°D). Milk acidity of human milk was measured in four stages: in raw human milk (initial); after pasteurization; after the heating of pasteurized milk and dilution of the supplement; and after thirty minutes of supplementation.
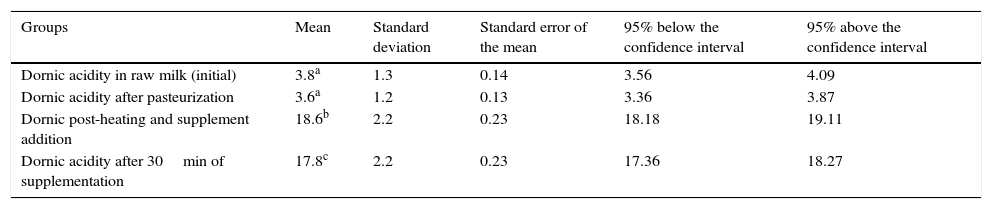
ResultsThe initial acidity was 3.8°D±1.3 (95% CI: 3.56–4.09) with no significant difference in Dornic acidity in pasteurized milk, which was 3.6°D±1.2 (95% CI: 3.36–3.87). The dilution of the supplement in pasteurized milk that was heated significantly increased mean Dornic acidity to 18.6°D±2.2 (95% CI: 18.18–19.11), which remained high after thirty minutes of supplementation at 17.8°D±2.2 (95% CI: 17.36–18.27), considering p<0.05.
ConclusionsThe study observed no significant differences in Dornic acidity of raw human milk and pasteurized human milk; however, the dilution of a human milk supplementation caused a significant increase in acidity. Further investigations are necessary on the influence of this finding on the quality of supplemented milk and its consequences on the health of preterm infants.
Avaliar a acidez Dornic inicial no leite humano cru, após pasteurização e após aquecimento e diluição de um suplemento nutricional para recém-nascidos prematuros.
MétodosEstudo quantitativo, descritivo, experimental com amostragem por conveniência, realizado no Banco de Leite Humano de uma maternidade pública brasileira, com assistência especializada às gestantes e recém-nascidos de risco. Os critérios de elegibilidade das 93 amostras do estudo incluíram leites humanos crus congelados em embalagens apropriadas, com volumes≥100mL e acidez Dornic inicial≤8°Dornic (°D). A acidez Dornic dos leites humanos foi mensurada em quatro momentos: no leite humano cru (inicial); após pasteurização; após aquecimento do leite pasteurizado e diluição do suplemento; e após transcorridos trinta minutos de suplementação.
ResultadosA acidez inicial foi de 3,8°D±1,3 (IC 3,56–4,09) não apresentando diferença significativa em relação à acidez Dornic no leite pasteurizado, que foi 3,6°D±1,2 (IC 3,36–3,87). A diluição do suplemento no leite pasteurizado e aquecido aumentou significativamente a média da acidez Dornic a 18,6°D±2,2 (IC 18,18–19,11), a qual se manteve elevada em 17,8°D±2,2 (IC 17,36–18,27) após 30 minutos da diluição, considerando p<0,05.
ConclusõesO estudo demonstrou que a acidez Dornic do leite humano cru e do leite humano pasteurizado não apresentaram diferenças significativas entre si, porém, a diluição do suplemento de leite humano promoveu elevação significativa da acidez. Maiores investigações da influência desse achado sobre a qualidade do leite suplementado e suas consequências na saúde de prematuros são necessárias.
The technological advances of the 20th century in the area of Neonatology allowed for the creation of Neonatal Intensive Care Units and favored the survival of preterm and very low birth weight (VLBW) newborns.1 This reality has imposed several challenges on the care of this population, which requires greater attention in relation to growth, development, and nutrition.2
Regarding the dietary aspects, the World Health Organization recommends the use of the mother's own milk also for preterm infants, as in addition to being better tolerated due to its easy digestibility, it also has high nutritional quality and benefits the mother–child binomial through breastfeeding.3 It also aids the immune protection against infections, sepsis, and necrotizing enterocolitis, favors the preterm infant's mental development,4 and appears to modulate risk factors for cardiovascular diseases in the long term,4 constituting a powerful option among the strategies to reduce child mortality.5
As for the nutritional composition, the human milk (HM) of a preterm infant's mother initially has a higher concentration of protein, lipids, minerals (such as sodium, calcium and phosphorus), electrolytes, and immunological properties, when compared to the milk of a full-term newborn's mother; but at the end of the first month, these differences decrease, making the milk of a preterm infant's mother resemble that of a full-term infant's.6,7 With a decrease in the nutritional reserves of preterm infants, in contrast with their high metabolic demands, inadequate nutritional support can lead to adverse and permanent effects on their growth and development.3
Therefore, HM supplementation has been indicated to meet the nutritional requirements of this population, and to prevent or treat metabolic bone diseases in these individuals,3,8 a well-established nutritional practice in neonatology.9 Among the HM supplements used in Brazil, those based on bovine whey protein hydrolysates, combined with several vitamins and minerals (especially calcium, phosphorus, and potassium), are most commonly used.10
In spite of recommendations for supplementation of the preterm mother's own milk, if the nursing mother cannot meet the baby's requirements, the administration of milk from a HM bank (HMB) is proposed.5,11 In this case, it undergoes strict quality control before distribution, among which is the measurement of Dornic acidity (DA). Variations within the range of 1.0–8.0 Dornic degrees (°D) classify the food for consumption, whereas higher values disqualify it from the microbiological point of view and may also influence the biological availability of nutrients, such as calcium,12,13 which is essential for bone mineralization in preterm infants.14,15
The initial DA of HM is determined by its chemical composition, with special contribution of proteins, phosphates, citrates, and carbon dioxide,16,17 in addition to organic acids17; when a supplement containing these components is added, it can cause changes in that acidity by changing the concentrations of such components in HM.16 However, there are no studies on the assessment of DA in supplemented HM; moreover, aspects about the effectiveness and safety of nutritional supplements for HM for preterm infants are not yet fully understood,9 justifying the performance of this study.
In light of this problem, the present study aimed to evaluate the initial DA in raw HM after pasteurization and after heating and dilution of the supplement used to complement very-low birth weight preterm infants’ nutrition and/or those undergoing treatment for metabolic bone diseases, to verify whether there are fluctuations in the behavior of the DA variable, considering these food handling steps.
MethodsThis was a quantitative, descriptive, experimental study with a convenience sampling, conducted in the state reference HMB of Maternidade Escola Januário Cicco in Natal, state of Rio Grande do Norte, Brazil, which is part of the Brazilian Unified Health System and provides specialized care to at-risk pregnant women, mothers, and newborns. The HM collected and pasteurized on site are intended to benefit preterm newborns in the Neonatal Intensive Care Unit of the hospital and rooming-in wards.
The study was approved by the Research Ethics Committee of the school maternity and Hospital Universitário Onofre Lopes, of the hospital complex of Universidade Federal do Rio Grande do Norte.
Sample collection was preceded by signing an institutional authorization form and took place over 24 days, during the afternoon shift, between the months of August and October 2014.
To estimate the number of samples, the sample size was simulated in relation to the assessed effect size, based on two fixed parameters: alpha=0.05 (5% of statistical probability to reject the null hypothesis) and statistical power=0.8 (test power to detect a genuine, actual effect, if it exists). Also, without predicting a direction, the difference between the means was investigated from a two-tailed analysis. It was observed that for an expected effect of 0.5 (mean effect according to Cohen), it would be necessary to collect a minimum sample size of 34 elements.
Study eligibility criteria included 104 samples of raw HM from nursing mothers that had expressed their milk at home, in the HM collection room at the school maternity, or at the HMB; were received for pasteurization frozen in glass containers with plastic lids, with volumes >100mL and an initial DA<8°D. The exclusion criteria were the occurrence of repetition samples (from the same donor) and failure to follow the steps established by the study methodology with a given sample. There were 11 sample losses, totaling 93 assessed samples.
N/9 sodium hydroxide (N/9 NaOH–Dornic solution) was used as the titrant solution to determine the DA of the HM. Each 0.01mL used to neutralize 1mL of HM corresponded to 1°D (one Dornic degree). For the detection of the turning point, one drop of phenolphthalein indicator solution in hydroalcoholic solution at 1% was added, which initially appeared as a clear solution and, after the pH turned, became light pink in color, indicating the pH change. Subsequently, the spent titrant volume was read using an acid meter.12,13
The nutritional supplement added to HM was a commercial nutrient powder formula for at-risk newborns, based on bovine whey protein hydrolysate, maltodextrin, vitamins, and minerals; a single type was used for all evaluations carried out in study. According to the protocol of nutrition and dietetics of the maternity service, the HM was heated to 37°C before the multicomponent supplement dilution. The manufacturer's recommendations were followed regarding product dilution (1g/20mL HM), although at the proportional amount of 0.05g for 1mL of HM used in the measurements.
In the case of very-low birth weight preterm infants, whose sucking and swallowing reflexes are delayed due to the neurological system immaturity, it becomes impossible to carry out the continuous and prompt diet administration and the supplemented milk is not fully consumed at once. Short intervals during the diet administration are required to prevent reflux and/or bronchoaspiration, both in the case of offering the food using a dosing cup directly to the mouth of preterm infant, and through enteral feeding for those who cannot be fed orally.
Considering the consequences of the interval between preparation and the completion of the administration, as well as the stages of its previous handling, the DA of the HM included in the study was measured at four different times in order to characterize its possible fluctuations: in the raw HM (initial acidity); after pasteurization; after the pasteurized HM was heated and nutritional supplement was diluted; and 30 (thirty) minutes after HM supplementation.
The first moment (DA measured in raw HM) was based on data collection of samples by consulting the HMB records, as the activity is developed and recorded daily by local professionals. The researchers were responsible for carrying out the measurements at the second, third, and fourth moments of the study.
The data collected from the samples were stored in a specific form and subsequently stored in Microsoft Excel® spreadsheets (Microsoft Excel®, V 2010. Microsoft®, WA, USA). The DA of samples at the respective moments of the study was measured in triplicate, using the arithmetic mean of the values obtained from the individual analysis of each aliquot. The mean DA obtained at the appropriate study moments were used for the statistical tests.
The one-way ANOVA statistical test was applied to determine the variability between the means of each stage of the study; Tukey's post-test was then used for multiple comparisons. The level of significance was set at 5%.
ResultsAt first, the statistical test showed that at least one of the groups had a statistically significant difference compared to the others (p<0.0001).
The post-test indicated no significant difference (p>0.05) between DA in raw HM and after pasteurization. However, the mean DA in raw HM showed a statistically significant difference (p<0.05) in DA means after the pasteurized HM was heated and the nutritional supplement was added when compared with the DA registered 30min after supplementation.
A difference was also observed between the means of DA after HM pasteurization, the DA of pasteurized HM after heating and supplement addition and DA 30min after supplementation, as shown in Table 1.
Mean, standard deviation, standard error of the mean, and variation above and below the 95% confidence intervals of Dornic acidity of human milk at each stage of the study.
| Groups | Mean | Standard deviation | Standard error of the mean | 95% below the confidence interval | 95% above the confidence interval |
|---|---|---|---|---|---|
| Dornic acidity in raw milk (initial) | 3.8a | 1.3 | 0.14 | 3.56 | 4.09 |
| Dornic acidity after pasteurization | 3.6a | 1.2 | 0.13 | 3.36 | 3.87 |
| Dornic post-heating and supplement addition | 18.6b | 2.2 | 0.23 | 18.18 | 19.11 |
| Dornic acidity after 30min of supplementation | 17.8c | 2.2 | 0.23 | 17.36 | 18.27 |
a,b,c Different letters represent statistically significant difference (p<0.05).
Milk is a buffer solution, with a low concentration of free hydrogen ions (H+). Because of the buffer resulting from its intrinsic composition, small changes detected in pH values are preceded by considerable increases in DA.12,18
About the association between these two chemical parameters, in general it is considered that DA is more sensitive to the total content of solutes in HM and that the lower the pH, the higher the DA of the food. As the pH varies depending on the energy content of HM, it is suggested that DA is also directly influenced by the lipid content of the food. Milk with higher fat concentrations has increased chances of developing high acidity.18
The pasteurization of HM with high acidity does not aim to improve quality.18 Therefore, it is important to know if there was bacterial growth prior to this processing, as that produces fermentation and acidification of the milk, which in turn lead to a reduction in nutritional and immunological components inherent to the food, disqualifying its use.12,13,19
Acidified HM cannot meet the specific nutritional needs of very-low birth weight or immunologically-vulnerable preterm infants. The high acidity and the release of protons originating from the ionization of lactic acid in an aqueous medium cause destabilization of soluble proteins and casein micelles, favoring its coagulation; increase the osmolarity; alter the flavor (taste and smell); and reduce the immunological value of the food. This cationic attack, by destabilizing the casein in suspension in HM, impairs the availability of the calcium and phosphorus content, which are chemically associated to casein, creating stable micelles, thus making the absorption of these minerals dependent on the protein digestion process.19–21
In this study, the DA found in raw HM (3.8°D±1.3) showed no statistical difference when compared with that in pasteurized HM (3.6°D±1.2), suggesting that the analyzed milks were adequate for consumption by newborns, with no further interference of the initial DA values on the nutritional content of the food.
After the addition of the nutritional supplement to HM pasteurized and heated to 37°C, there was a significant increase in the mean DA (18.6°D±2.2, p<0.05). This increase was maintained even after 30min post-dilution (17.8°D±2.2, p<0.05). Both means were higher than the 8°D limit recommended for HM consumption by newborns.
Considering the importance of HM acidity on its nutritional quality, it should be emphasized that acid or alkaline overloads result in metabolic acidosis or alkalosis, and its use can cause necrotizing enterocolitis in very-low birth weight preterm infants.18
It is important to emphasize that the HM (either raw or pasteurized and frozen) can undergo lipolysis, releasing greater amounts of free fatty acids, which contribute to the increase in acidity, as interpreted by Novak and Cordeiro.22 Consequently, higher concentrations of these components in the milk, combined with the high supply of minerals such as calcium through the addition of supplements, would result in the formation of insoluble soaps, another factor contrary to the absorption of the mineral by the body.6 Also, it is reasonable to suggest that, despite being the same event (increase in DA), when promoted by different reasons (such as the occurrence of lipolysis, the increase of acidifying components due to HM supplementation, or the development of acidity through the formation of lactic acid as the result of bacterial activity), it is not possible to affirm that the consequences are the same, especially as to interfering with calcium bioavailability and metabolism of preterm receptors. Therefore, further studies on the subject are required to clarify these issues.
Furthermore, the use of multicomponent HM supplements may increase the risk of infection by contamination secondary to the manipulation of the supplement powder and cause changes in food osmolarity, affecting the absorption of its nutrients.23 In this respect, a limitation of the present study is related to the absence of microbiological tests to rule out the possibility of contamination by the supplement, as well as the previous lack of knowledge on the product acidity, conditions that could influence the interpretation of the results found here.
Conversely, studies have emphasized that the increase in osmolarity in HM caused by addition of these supplements would be related to the development of necrotizing enterocolitis in preterm infants by increasing the content of solutes, which is also reinforced by the activity of human amylase. The enzyme, present and active in pasteurized HM, when acting on the carbohydrates (dextrins) that constitute the supplement degrades them into monosaccharides and oligosaccharides in osmolarity to more or less time as the carbohydrate source, modulating the increase in osmolarity.24,25
However, the HM supplementation offered to preterm infants becomes important considering the findings that breast milk from their nursing mothers, even with a different composition in relation to the milk of mothers of full-term newborns, still has insufficient protein, calcium, and phosphorus contents.23,26
In a systematic review carried out by the Cochrane Database of Systematic Reviews, it was concluded that the multicomponent supplementation of HM for preterm infants was associated with a quicker increase in weight, length, and head circumference, although no effects were observed on bone mineralization in the assessed group.27
In a study carried out in 2010 in the intensive care unit of a hospital in Porto Alegre, state of Rio Grande do Sul, Brazil, the authors compared two groups of 19 preterm infants: one group received only HM and the other received the HM supplemented with FM85®, milk fortifier (FM 85®, Nestlé, München, Germany). Both groups were followed regarding the individual anthropometric measurements, whereas bone mineralization was assessed by whole-body bone densitometry with dual energy X-ray absorptiometry (DEXA) and laboratory tests of alkaline phosphatase control, calcium, phosphorus, as well as urinary calcium and phosphorus. The results showed a clear improvement in the supplemented group when compared with the non-supplemented group; a decrease in alkaline phosphatase was also observed in the latter group, demonstrating better mineral homeostasis in this situation.28
Many studies on HM supplementation for preterm infants with bone mineralization deficits have been developed to demonstrate the consequences of this conduct for this specific population.6,8,9,19 However, the results are still conflicting, sometimes showing benefits for mineralization, sometimes showing no significant responses when compared to HM without supplementation and/or specific formulas for preterm infants. However, it must be considered that the methodologies used to assess these outcomes are quite diverse – ranging from the “classic” investigation based on anthropometric data, X-rays, and serum biochemical analysis of calcium, phosphorus, alkaline phosphatase, parathyroid hormone, vitamin D metabolites, calcium, and phosphorus in urine; or more recently, by DEXA – making it difficult to compare the findings. Moreover, the different types of supplementation used in HM and the sample size (usually small) are obstacles to attain consistent conclusions from previously performed studies.28
Thus, the present study is relevant, as it proposed the observation of acid–basic characteristics of supplemented HM in vitro, reducing the influences caused by metabolic activity in vivo, which can become confounding factors when interpreting results about the physicochemical food modifications after supplementation. Nonetheless, the methodology used here did not allow for a direct assessment of the consequences of increased DA in supplemented HM on calcium and phosphorus bioavailability, or the understanding of their effects on the health and metabolism of very-low birth weight preterm infants and/or those undergoing treatment for metabolic bone diseases.
Therefore, considering the results obtained in this study, which showed no significant difference between the initial DA of raw and pasteurized HM, as well as the significant acidification of supplemented HM (used for preterm infants with very-low birth weight and/or in cases of metabolic bone diseases), and given the speculation that this acidification observed in the supplemented HM may hinder the absorption of nutrients such as calcium, it must be emphasized that there is not enough evidence from the scientific literature to sustain such assertion. Therefore, further studies, including methodologies that will allow assessing the occurrence of metabolic bone disease in very-low birth weight preterm infants receiving supplemented and non-supplemented HM, with and without DA increase in the consumed milk, are recommended in order to demonstrate the effectiveness and safety of supplementation, which are not yet fully clarified.
Consequently, such studies are also essential to indicate the optimal composition of HM supplements used to feed these preterm infants. Considering that the consequences of HM supplementation remain questionable, indications/prescriptions always require critical accuracy.
Furthermore, considering the results discussed in the present study, it is worth emphasizing the contrast between the initial disposal of HM samples carried out in HMB due to the presence of DA>8°D, which is interpreted as an increase in the production of lactic acid due to biological contamination of food, and the elevation of this parameter in HM caused by addition of the nutritional supplement, suggesting that samples with high DA discarded by HMBs would not always be justified by high contamination, and could therefore be caused by changes in the chemical composition of the food itself, as proposed by a previous study. Therefore, further discussion is necessary to propose solutions to minimize the disposal of HM supposedly adequate for consumption.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Pereira CI, Dametto JF, Oliveira JC. Evaluation of human milk titratable acidity before and after addition of a nutritional supplement for preterm newborns. J Pediatr (Rio J). 2016;92:499–504.
Study conducted at Universidade Federal do Rio Grande do Norte (UFRN), Natal, RN, Brazil.