To determine eight parameters of oxidative stress markers in erythrocytes from children with sickle cell disease and compare with the same parameters in erythrocytes from healthy children, since oxidative stress plays an important role in the pathophysiology of sickle cell disease and because this disease is a serious public health problem in many countries.
MethodsBlood samples were obtained from 45 children with sickle cell disease (21 males and 24 females with a mean age of 9 years; range: 3–13 years) and 280 blood samples were obtained from children without hemoglobinopathies (137 males and 143 females with a mean age of 10 years; range: 8–11 years), as a control group. All blood samples were analyzed for methemoglobin, reduced glutathione, thiobarbituric acid reactive substances, percentage of hemolysis, reactive oxygen species, and activity of the enzymes glucose 6-phosphate dehydrogenase, superoxide dismutase, and catalase. Data were analyzed using Student's t-test and were expressed as the mean±standard deviation. A p-value of <0.05 was considered significant.
ResultsSignificant differences were observed between children with sickle cell disease and the control group for the parameters methemoglobin, thiobarbituric acid reactive substances, hemolysis, glucose 6-phosphate dehydrogenase activity, and reactive oxygen species, with higher levels in the patients than in the controls.
ConclusionsOxidative stress parameters in children's erythrocytes were determined using simple laboratory methods with small volumes of blood; these biomarkers can be useful to evaluate disease progression and outcomes in patients.
Determinar parâmetros de estresse oxidativo em eritrócitos de crianças com doença falciforme e compará-los com os mesmos parâmetros em eritrócitos de crianças saudáveis, pois o estresse oxidativo desempenha um importante papel na fisiopatologia da doença falciforme, considerada um sério problema de saúde pública em muitos países.
MétodosForam obtidas amostras de sangue de 45 crianças com doença falciforme (21 meninos e 24 meninas com média de 9 anos, variação de 3 a 13 anos) e 280 amostras de sangue de crianças sem hemoglobinopatias (137 meninos e 143 meninas com média de 10 anos, variação de 8 a 11 anos), como grupo controle. Em todas as amostras foram determinados meta-hemoglobina, glutationa reduzida, substâncias reativas ao ácido tiobarbitúrico, porcentagem de hemólise, espécies reativas de oxigênio e atividade das enzimas glucose6-fosfato desidrogenase, superóxido dismutase e catalase. Os dados foram analisados com o teste t de Student e foram expressos como média±desvio padrão. Um valor de p<0,05 foi considerado significativo.
ResultadosForam observadas diferenças significativas entre as crianças com doença falciforme e o grupo controle para os parâmetros meta-hemoglobina, substâncias reativas ao ácido tiobarbitúrico, porcentagem de hemólise, espécies reativas de oxigênio e atividade da enzima glucose6-fosfato desidrogenase, com níveis aumentados nos pacientes.
ConclusõesFoi possível determinar parâmetros de estresse oxidativo em eritrócitos de crianças, com técnicas laboratoriais simples e pequenos volumes de sangue. Esses biomarcadores podem ser úteis na avaliação da progressão e dos resultados de tratamentos da doença.
Sickle cell disease is one of the most common hematologic disorders in the world and is a serious public health problem in many countries, including Brazil.1 There are over 2 million Brazilian carriers of the sickle gene, and this disease is estimated to have an incidence of one in every 1000 live births. In 2001, a decree of the Ministry of Health included screening for hemoglobinopathies in the pre-existing screening programs.2
Sickle cell disease has been characterized as a multi-system disease, associated with episodes of acute illness and progressive organ damage, which begins in infancy and is primarily responsible for a shortened life expectancy in affected patients.3 Rates of morbidity and mortality are still high for patients with sickle cell disease. In Brazil, up to 25% of the children affected died during their first 5 years of life, but early diagnosis and treatment might reduce these rates and improve their quality of life.4
Sickle hemoglobin results from a substitution of glutamic acid to valine at the sixth amino acid position of the β-globin chain.5 This ostensibly minor change is the origin of hemoglobin S, and is responsible for significant changes in the stability and solubility of the molecule.6 The tendency of deoxygenated hemoglobin S to undergo polymerization underlies the innumerable expressions of the sickling syndromes with intravascular hemolysis.7 Free plasma hemoglobin is able to initiate lipid peroxidation, and the heme, which readily dissociates from methemoglobin, may contribute significantly to oxidative stress,8 which might play a significant role in the pathophysiology of sickle cell disease-related microvascular dysfunction, vaso-occlusion, and development of organ damage.9 Biomarkers of oxidative stress can therefore be potentially useful, both to identify patients who are at high risk of oxidative damage and to evaluate the effects of anti-oxidative therapies.10
The purpose of this work was to evaluate the parameters of oxidative stress in erythrocytes from children with sickle cell disease, including percentages of hemolysis, methemoglobin, reduced glutathione, thiobarbituric acid-reactive substances, glucose 6-phosphate dehydrogenase activity, reactive oxygen species, and the anti-oxidant enzymes catalase and superoxide dismutase.
MethodsChemicalsMeta-phosphoric acid, 2-mercaptoethanol, pyrogallol, 2,2-azobis(2-amidinopropane)hydrochloride (AAPH), ethylenediaminetetraacetic acid (EDTA), and 5,5-dithiobis-2-nitrobenzoic acid (DTNB) were obtained from Sigma–Aldrich (St. Louis, MO, USA). Sodium and potassium phosphates, saponin, trichloroacetic acid, and thiobarbituric acid were supplied by Vetec Ltda (Rio de Janeiro, RJ, Brazil). Sodium citrate, tris(hydroxymethyl)aminomethane, and methanol were obtained from Merck (Darmstadt, Germany). G6-PD activity was determined using a PD410 kit by Randox Laboratories (Antrim, United Kingdom). All organic solvents were of high quality and were double-distilled, and all the other chemicals were of analytical grade.
Blood samplesBlood samples were obtained from 45 children diagnosed with sickle cell disease (21 males and 24 females with a mean age of 9 years; range: 3–13) at the hematopediatric department of Hospital de Clínicas, Universidade Federal do Paraná (UFPR). A control group consisted of 280 children without hemoglobinopathies (137 males and 143 females with a mean age of 10 years old; range: 8–11 years) who were participants of the university extension project entitled “Incidence of anemia and parasitic infections in school-aged children in municipal schools of metropolitan region of Curitiba-Parana – Brazil,” from UFPR. The use of human subjects was approved by the Ethical Committee for Research Involving Humans, Hospital de Clínicas, UFPR. Informed consent was obtained from the guardians for all the children. Children with any hematological alteration were excluded from the study.
A venous blood sample of 5mL was collected from each patient in K3-EDTA coated tubes. Aliquots (200μL) of whole blood were separated for determination of G6-PD activity. Then, samples were centrifuged at 3000×g for 10min. The plasma and the buffy coat were removed by aspiration, and the erythrocytes were washed with phosphate buffered saline (PBS) (NaCl, 150mmol/L; NaH2PO4, 1.9mmol/L; and Na2HPO4, 8.1mmol/L) three times. Finally, red blood cells were suspended in PBS solution and water to obtain suspensions with hematocrits of approximately 10% and 40% for PBS solution and of approximately 40% for water solution. Hemoglobin concentration was measured in all suspensions. Not all analyses were performed in each specimen due to the limited volumes available.
Hematologic parametersThe complete blood count was determined using the Pentra 80 electronic cell counter (Horiba Medical, Japan).
Methemoglobin concentrationMethemoglobin concentration was determined according to a method based on Naoum et al.11 adapted to small volumes. Aliquots (100μL) of 10% erythrocyte suspensions were hemolyzed with 100μL of 1% saponin and were stabilized in 1000μL of 60mmol/L phosphate buffer; the absorbance was then determined at 630nm (for methemoglobin) and at 540nm (for oxyhemoglobin). Methemoglobin concentration was expressed as a percentage in relation to hemoglobin concentration.
Reduced glutathione determinationReduced glutathione (GSH) concentration was determined by a method previously described by Beutler,12 by evaluating the reduction of 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) by sulfhydryl compounds from the formation of a yellow colored anionic product whose absorbance was measured at 412nm. Aliquots of 50μL of 40% suspension of red blood cell in PBS were used. The GSH concentration was expressed in μmol/gHb.
Lipid peroxidationLipid peroxidation of red blood cell membranes was assessed based on Cesquini et al.13 Aliquots (600μL) of a 10% suspension of red blood cell were added to 250μL of 25% trichloroacetic acid and 600μL of 1% thiobarbituric acid, boiled for 15min at 100°C, and cooled for 5min at 0°C. The absorbance of the thiobarbituric acid reactive substances (TBARS) formed was then read at 532nm using ¿=156/(mmolecm) and the concentrations are expressed in nmol/gHb.
Measurement of hemolysisHemolysis of red blood cell was carried out as described by Banerjee et al.,14 adapted to microplates by mixing 10% suspension of red blood cell in PBS with varying amounts of AAPH solution (providing final concentrations of 50, 100, and 150mmol/L). This reaction mixture was incubated for 3h at 37°C with shaking. The extent of hemolysis was determined spectrophotometrically by measuring the absorbance of the hemolysate at 540nm in a microplate reader (Thermo Scientific, Thermo Plate, USA). Red blood cells in a solution of 200mmol/L of AAPH were used as the 100% hemolysis control.
Activity of glucose6-phosphate dehydrogenase (G6-PD)Aliquots (200μL) of whole blood before erythrocyte isolation were washed with 2mL PBS three times. G6-PD activity was determined using the Cobas Mira automated analyzer (Roche, Mannheim, Germany) with the PD410 commercial kit (Randox, Antrim, United Kingdom) as described in the manufacturer's manual.
Superoxide dismutase activityThe enzyme activity was based on a method adapted from Beutler12 of the auto-oxidation of pyrogallol. Aliquots of 200μL of packed red blood cell were hemolyzed with 300μL of cold deionized water, and a chloroform-ethanol extract was prepared. The mixture was centrifuged at 2300×g for 10min. Varying amounts of the clear supernatant extract (0, 20, 40, 60, 80, 100 and 300μL) were added to a solution of tris–HCl and water. After 10min, 20μL of a 1mmol/L pyrogallol solution was added to each tube and the absorbance was measured at 412nm in a microplate. The amount of extract required to inhibit pyrogallol auto-oxidation by 50% was used to determine the level of enzyme activity.
Catalase activityThe enzyme activity was determined by a method adapted from Beutler12 that measures the rate of decomposition of hydrogen peroxide by catalase spectrophotometrically at 240nm. Aliquots of 50μL of 40% suspension of red blood cell were added to 450μL of a hemolyzing solution of β-mercaptoethanol (0.7mmol/L) and EDTA (0.27mol/L). This solution was diluted 1:100 in PBS and 10μL of the final solution was added to 990μL of hydrogen peroxide solution. The decrease in absorbance of the system was measured for 10min.
Intracellular reactive oxygen speciesReactive oxygen species were determined according to a method based on López-Revuelta et al.15 adapted to small volumes of blood samples in a microplate. Erythrocytes (995μL of 10%, v/v suspension in PBS) were incubated with 5μL of dichlorodihydrofluorescein-diacetate (DCFDA, 10mol/L) at 37°C for 30min. This suspension was diluted in 9.0mL of PBS and 37.5μL of this was then added to 112.5μL of PBS in 96-well plates. Determination of reactive oxygen species was performed using a GloMax®-Multi Microplate Multimode Reader fluorimeter (Promega Corporation, USA). Under these conditions, DCFDA was hydrolyzed to 2′,7′-dichlorodihydrofluorescein (DCFH2), which then became available for oxidation by reactive oxygen species to produce fluorescent 2,7-dichlorofluorescein (DCF). Fluorescence was determined at 530nm after excitation at 495nm. Reactive oxygen species formation was expressed as fluorescence units (UF)/gHb.
Statistical analysesStatistical analysis was performed using Statistica 8.0 software (StatSoft, USA). No outliers were identified. The Kolmogorov–Smirnov test was used to assess the normality and all parameters were distributed normally. Data were expressed as mean±standard deviation and compared between groups using Student's t-test; a p-value <0.05 was considered significant.
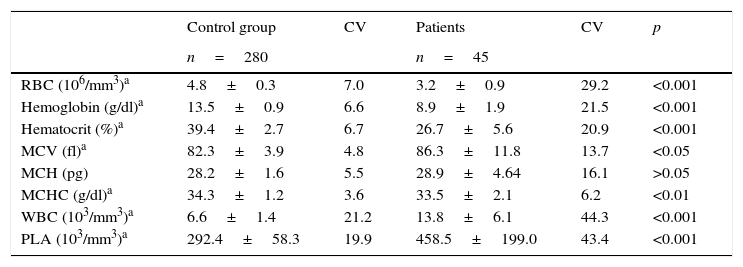
ResultsData from blood counts of healthy children and patients with sickle cell disease are illustrated in Table 1. Statistically significant differences were observed for all parameters, except for medium corpuscular hemoglobin (MCH; p<0.05).
Hematological values in healthy children (control group) and patients with sickle cell disease.
| Control group | CV | Patients | CV | p | |
|---|---|---|---|---|---|
| n=280 | n=45 | ||||
| RBC (106/mm3)a | 4.8±0.3 | 7.0 | 3.2±0.9 | 29.2 | <0.001 |
| Hemoglobin (g/dl)a | 13.5±0.9 | 6.6 | 8.9±1.9 | 21.5 | <0.001 |
| Hematocrit (%)a | 39.4±2.7 | 6.7 | 26.7±5.6 | 20.9 | <0.001 |
| MCV (fl)a | 82.3±3.9 | 4.8 | 86.3±11.8 | 13.7 | <0.05 |
| MCH (pg) | 28.2±1.6 | 5.5 | 28.9±4.64 | 16.1 | >0.05 |
| MCHC (g/dl)a | 34.3±1.2 | 3.6 | 33.5±2.1 | 6.2 | <0.01 |
| WBC (103/mm3)a | 6.6±1.4 | 21.2 | 13.8±6.1 | 44.3 | <0.001 |
| PLA (103/mm3)a | 292.4±58.3 | 19.9 | 458.5±199.0 | 43.4 | <0.001 |
RBC, red blood cells; MCV, medium corpuscular volume; MCH, medium corpuscular hemoglobin; MCHC, medium corpuscular hemoglobin concentration; WBC, white blood cells; PLA, platelets; CV, Pearson's coefficient of variation (%). Data are presented as mean±standard deviation.
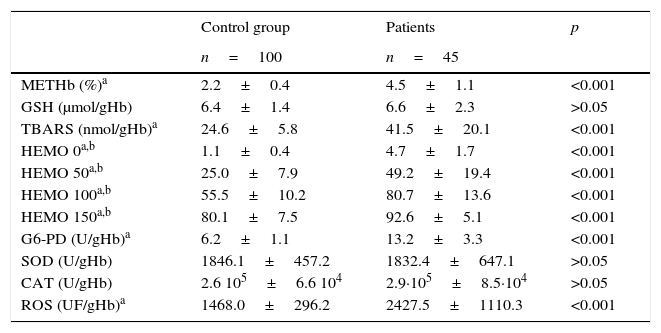
Data from oxidative stress parameters are illustrated in Table 2, comparing patients with sickle cell disease with healthy children. Statistically significant differences were observed for methemoglobin, TBARS, percentage of hemolysis, G6-PD activity, and reactive oxygen species (p<0.05).
Oxidative stress parameters in normal children (control group) and patients with sickle cell disease.
| Control group | Patients | p | |
|---|---|---|---|
| n=100 | n=45 | ||
| METHb (%)a | 2.2±0.4 | 4.5±1.1 | <0.001 |
| GSH (μmol/gHb) | 6.4±1.4 | 6.6±2.3 | >0.05 |
| TBARS (nmol/gHb)a | 24.6±5.8 | 41.5±20.1 | <0.001 |
| HEMO 0a,b | 1.1±0.4 | 4.7±1.7 | <0.001 |
| HEMO 50a,b | 25.0±7.9 | 49.2±19.4 | <0.001 |
| HEMO 100a,b | 55.5±10.2 | 80.7±13.6 | <0.001 |
| HEMO 150a,b | 80.1±7.5 | 92.6±5.1 | <0.001 |
| G6-PD (U/gHb)a | 6.2±1.1 | 13.2±3.3 | <0.001 |
| SOD (U/gHb) | 1846.1±457.2 | 1832.4±647.1 | >0.05 |
| CAT (U/gHb) | 2.6 105±6.6 104 | 2.9·105±8.5·104 | >0.05 |
| ROS (UF/gHb)a | 1468.0±296.2 | 2427.5±1110.3 | <0.001 |
METHb, methemoglobin; GSH, reduced glutathione; TBARS, thiobarbituric acid reactive substances; HEMO, hemolysis; G6-PD, glucose 6-phosphate dehydrogenase; SOD, superoxide dismutase; CAT, catalase; ROS, reactive oxygen species. Data presented as mean±standard deviation.
Normal erythrocytes suffer oxidative stress due to the production of reactive oxygen species that results from oxygen metabolism. However, this is efficiently repaired by the highly powerful antioxidant systems of the cell without any problematic effect. Oxidative stress occurs as a result of an imbalance between reactive oxygen species production and antioxidant defenses.16
In sickle cell disease, oxidative stress may result from high levels of meta hemoglobin S, which is less stable than meta hemoglobin A, leading to intravascular hemolysis,17 ischemia-reperfusion injury, chronic inflammation, and higher auto-oxidation of sickle hemoglobin.18,19 Many potential antioxidants are of interest in relation to sickle cell disease,20 and several studies have demonstrated significant increases in stress markers and differing behavior in antioxidant defense systems in patients with sickle cell disease when compared to those in healthy subjects.21
The present results for blood counts confirm several features of sickle cell disease that are already known, such as the hemolytic anemia,21 evidenced by low levels of hemoglobin7 and increased levels of white blood cells and platelets.6
As previously demonstrated,8 methemoglobin levels are increased in individuals with sickle cell disease. There is an electron transfer in the bonding interaction between the heme and the oxygen (O2) in oxygenated hemoglobin. When hemoglobin deoxygenates, the heme iron normally remains in the ferrous state.20 In this exchange, alterations wherein hemoglobin autoxidizes result in methemoglobin, with the heme iron in ferric state.8 Alterations in erythrocyte function or structure can lead to an enhanced flow of methemoglobin that can lead to oxidative stress.15
The increased intra- and extra-erythrocytic oxidative stress induces lipid peroxidation and membrane instability.14 TBARS is one of the existing biomarkers, and this evaluation is an indirect quantification of lipid peroxidation processes, which makes it a good indicator of pro-oxidant stimuli. In accordance with results reported previously,19,20,22 the present study observed significantly higher levels of TBARS in patients with sickle cell disease than in the controls.
Rigid and deformed sickle erythrocytes have a shortened lifespan and undergo both intravascular and extravascular hemolysis.23 Higher percentages of hemolysis in erythrocyte from children with sickle cell disease than in the control group were observed, both in basal suspensions of erythrocytes and in suspensions incubated with an oxidizing agent.
G6-PD is an important enzyme related to the antioxidant defense in erythrocytes.20 Higher activity of this enzyme in patients with sickle cell disease was found than in the control group. It was previously reported that erythrocytes from patients with sickle cell disease have an increased percentage of reticulocytes, while the activity of G6-PD in reticulocytes is normal, but declines exponentially as the red cells age.24
Sickle cells spontaneously generate approximately two times more reactive oxygen species than normal red blood cells.25 In accordance with the findings of George et al.,26 elevated levels of reactive oxygen species in sickle erythrocytes were also demonstrated.
Reduced glutathione (GSH) is present at high concentrations in erythrocytes and acts by itself or via glutathione peroxidase as a major reducing source to maintain cell integrity.17 The measurements of GSH and its oxidized form glutathione disulfide (GSSG) have been considered useful indicators of in vivo oxidative stress.27 The majority of studies of adults with sickle cell disease reported some deficits of endogenous synthesis of GSH, probably due to its consumption by increased oxidant production.26,28 Although Rusanova et al.22 showed high levels of GSH in pediatric patients with sickle cell disease, the present study found no difference in GSH levels between children with sickle cell disease and the control group.
Superoxide dismutase can convert superoxide to hydrogen peroxide, and catalase can remove excess hydrogen peroxide.16 According to Silva et al.,20 the increased pro-oxidant generation in sickle cell disease results in an antioxidant deficiency. However, there are some discrepancies between studies on superoxide dismutase and catalase levels in this disease, with some studies observing increased activity and others observing decreased levels.29 An increase in these enzymes activity potentially constitutes a defense mechanism in response to increased oxidative stress,19 or might be a consequence of increased reticulocyte content in blood samples from patients with sickle cell disease. However, a decrease in enzyme levels was related to disease severity in patients.20,22 These seemingly contradictory findings could be due to differences in the extent of oxidative stress, disease severity, enzyme polymorphism, and the enzyme co-factor.29 The present results showed no difference between the activities of these enzymes in children with sickle cell disease and those in healthy children, according with Cho et al.30 with regard to catalase. These results may be due to large individual variability found among patients.
In light of evidence suggesting that an excess of oxidative stress has implications in sickle cell disease pathophysiology, the assessment of oxidative stress parameters in these patients may provide useful information regarding the use of current medications and may lead to the development of new therapeutic strategies.10,19,20 Monitoring the oxidative stress involves the observation of different parameters associated with pro-oxidant and antioxidant biomarkers.27 However, the use of an isolated biomarker and the measurement of individual antioxidants are not likely to be useful indexes of oxidative status. The oxidant–antioxidant balance involves biochemical reactions that require the evaluation of many endpoints.26
The present study evaluated eight oxidative stress markers, including pro-oxidant and antioxidant parameters. The results indicate the presence of a hyperoxidative status in children with sickle cell disease, which can be observed by their high levels of methemoglobin, TBARS, hemolysis, reactive oxygen species, and G6-PD activity. Simple techniques were used to determine these parameters using small volumes of blood. These parameters that appeared altered in children with sickle cell disease can be useful in the evaluation of disease progression and treatment.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Hermann PB, Pianovski MA, Henneberg R, Nascimento AJ, Leonart MS. Erythrocyte oxidative stress markers in children with sickle cell disease. J Pediatr (Rio J). 2016;92:394–9.