This study aims to describe the epidemiological characteristics and survival rates of children with acute myeloid leukemia treated in hospitals in southern Brazil and compare them with international data.
MethodsA multicenter cohort study was conducted with retrospective data collection of all new patients with acute myeloid leukemia under 18 treated at five referral centers in pediatric hematology-oncology in southern Brazil between January 2005 and December 2015.
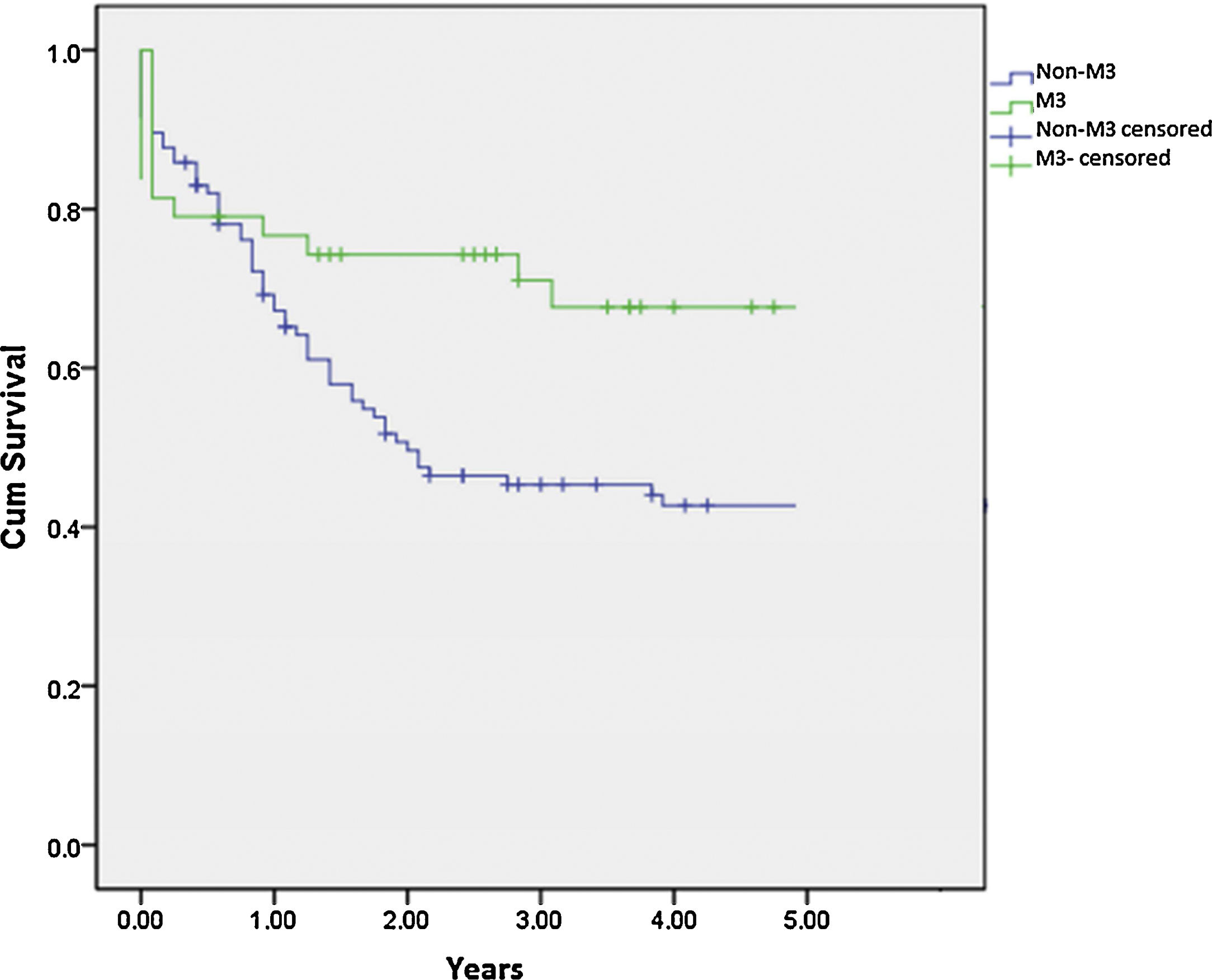
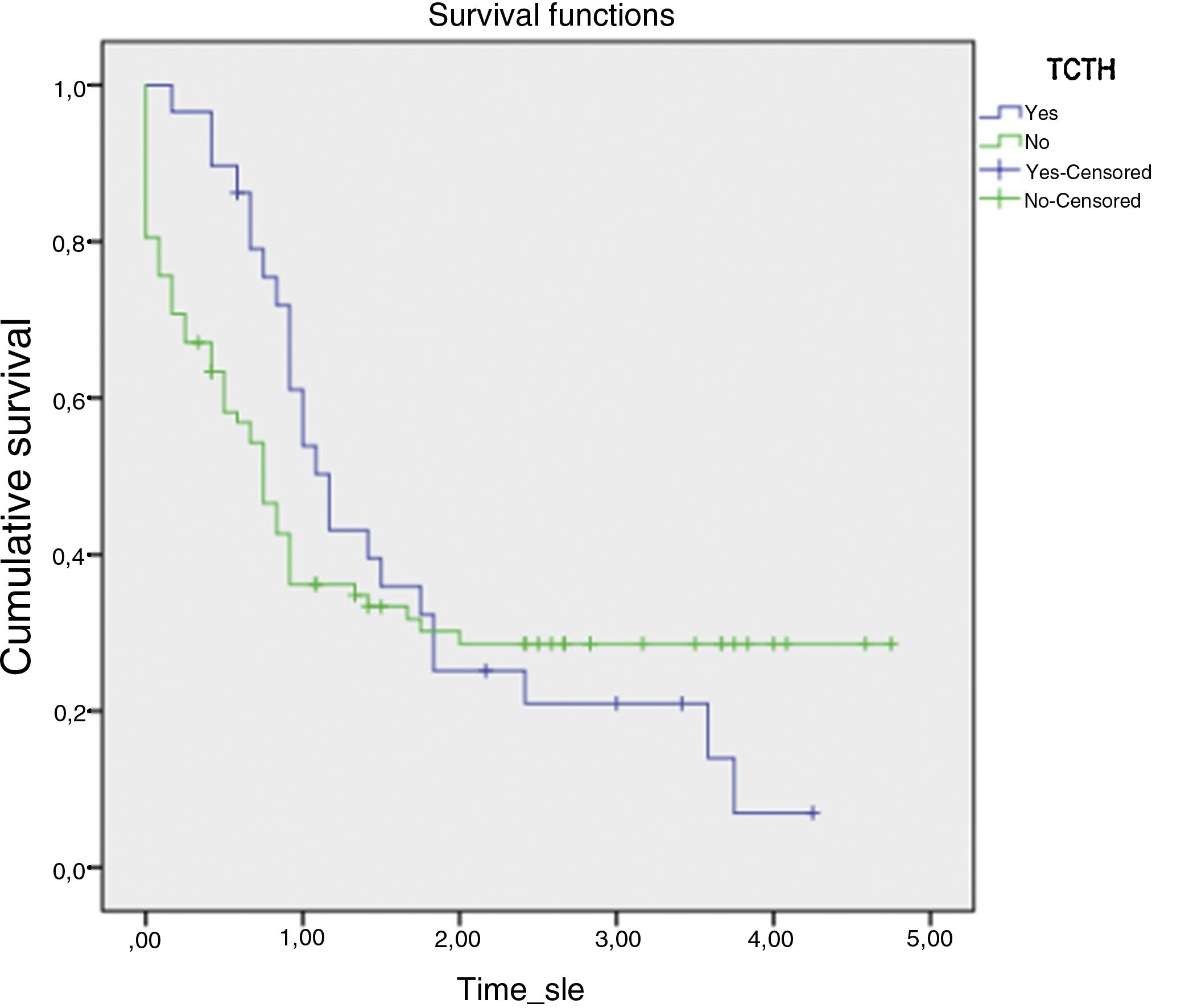
ResultsOf the 149 patients with acute myeloid leukemia, 63.0% (n=94) were male. The median age at diagnosis was 10.5 years (range 0–18 years) and 40.3% (n=60) had a white blood cell count below 50,000/mm2. The most common Franco-American-British (FAB) subtype was M3 (n=43, 28.9%). Nine (6.0%) patients had central nervous system disease. In M3 patients, overall survival (OS) was 69.2% and 3-year event-free survival was 67.7%; in non-M3 patients, these rates were 45.3% and 36.7%, respectively. In non-M3 patients, OS was significantly different between transplanted (61.8%) and non-transplanted (38.2%) patients (p=0.031).
ConclusionsThese results show a higher prevalence of the Franco-American-British M3 subtype than that reported in the international literature, as well as a decreased OS compared with that of developed countries. Further multicenter Brazilian studies with a larger sample size are encouraged to better understand the characteristics of acute myeloid leukemia, and to improve the treatment and prognosis in this population.
Acute myeloid leukemia (AML) is a rare neoplasm, accounting for 15–20% of all childhood acute leukemias. Approximately 500–600 children and adolescents are diagnosed with AML each year in the United States.1 Despite the development of new drugs and the possibility of allogeneic hematopoietic stem cell transplantation (HSCT), treatment of this disease remains a challenge. Cure rates with current treatment protocols range from 60% to 70% in this age group.2
Recent studies have shown that cytogenetic and molecular abnormalities are involved in the pathogenesis of childhood AML, with clonal chromosome abnormalities in 70–85% of cases.3 Great efforts have been made to improve characterization of the disease and prognostic stratification of patients. Several molecular and cytogenetic events have been identified that allow definition of the distinct subtypes of AML in childhood. Such changes can be used as markers and help better define targets for therapy, thereby reducing the toxicity of current treatment strategies.4
Currently, cytogenetic abnormalities, such as t(8;21) RUNX1/RUNX1T1 and inv(16)/t(16;16) CBFB/MYH11, are considered the core-binding factor mutations of leukemic cells. Pediatric patients with these mutations have a favorable prognosis and do not benefit from matched related-donor HSCT.5 Other genetic abnormalities, such as −5/del(5q), −7/del(7q) or complex karyotype, wild-type NPM1, and FLT3-ITD, are associated with poor prognosis.6 The finding that patients with a normal karyotype may have cryptic mutations, and that these mutations may have prognostic value, provided new insight into these patients, who were previously considered at low risk in most protocols.5
It cannot be denied that, since the 1970s, childhood AML survival rates have improved; however, overall survival (OS) remains suboptimal. Despite improvements in risk stratification, tailoring of treatment to the individual characteristics of each patient and of the disease and better indications for HSCT, the 3-year OS still ranges from 63% to 75%.6
To date, little has been published about childhood AML in Brazil. Considering its relatively low incidence (seven occurrences per 1,000,000 children/year)4 and prognosis, several international collaborative groups have been focused on analyzing epidemiological and prognostic factors in an attempt to improve treatment strategies and increase OS and event-free survival (EFS) rates.4 The present study was therefore designed to describe the epidemiological features and survival rates of patients with childhood AML treated at hospitals of a South Brazil state (Rio Grande do Sul) and compare the findings with international data.
Materials and methodsThe authors conducted a multicenter cohort study with retrospective data collection of all new AML cases in patients younger than 18 years treated at five public referral hospitals in a South Brazil state, references for AML treatment, from January 2005 to December 2015 (Hospital de Clínicas de Porto Alegre, Hospital São Vicente de Paula/Passo Fundo, Hospital da Criança Santo Antônio/Irmandade Santa Casa de Misericórdia de Porto Alegre, Hospital da Criança Conceição/Porto Alegre, Universidade Federal de Pelotas/Pelotas). The diagnosis was confirmed by morphological analysis and flow cytometric immunophenotyping of bone marrow aspirate and by anatomopathological study of bone marrow biopsy. HSCT indication was based on the occurrence of relapsed AML. Patients who reached the age of 18 during the study time period continued to be followed. There was no loss to follow-up and patients with secondary AML were excluded from the analysis and was no lost to follow-up by the patient. The study was approved by the ethics committee of the institution, with secondary approvals from the ethics committees of all participating centers. Informed consent was waived due to the non-interventional design of the study and the retrospective nature of data collection.
The patients’ medical records were reviewed for data on demographics (sex, place of origin, and date of birth), presentation at diagnosis (date of diagnosis, French-American-British [FAB] classification, and karyotype), treatment (systemic chemotherapy regimen, autologous and/or allogeneic BMT), and follow-up after diagnosis (current status, date of recurrence, date of diagnosis of a second neoplasm, date of death, or date of last contact).
Statistical analysisThe number of patients aged <18 years with a diagnosis of AML who received care at the five participating centers during the study period determined the sample size. Continuous variables were expressed as standard deviation (SD) or median and range. Qualitative variables were expressed as absolute and relative frequencies. Survival curves were estimated by the Kaplan–Meier method. Cox regression models were used to evaluate survival assessment considering the following risk factors: more than 10 years of age, risk karyotype and leukometry of the diagnosis above 50,000 leukocytes/mm. The incidence rate was calculated from the number of new cases of a disease divided by the number of people at risk. Data were analyzed using SPSS, v. 20.0 for Windows. The level of significance was set at 5% for all analyses.
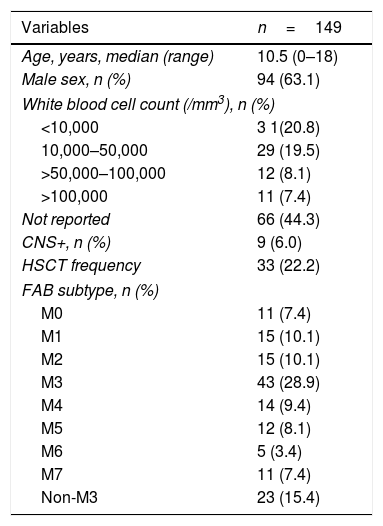
ResultsA total of 149 patients with a diagnosis of childhood AML treated at five pediatric hematology-oncology referral hospitals in southern Brazil over a 10-year period were analyzed. Most patients were male (n=94, 63.0%), and 60 (40.3%) had a white blood cell (WBC) count below 50,000/mm3. The most common FAB subtype was M3 in 28.9% of patients (n=43). Table 1 shows the main characteristics of the patient cohort.
Baseline characteristics of the pediatric AML cohort.
| Variables | n=149 |
|---|---|
| Age, years, median (range) | 10.5 (0–18) |
| Male sex, n (%) | 94 (63.1) |
| White blood cell count (/mm3), n (%) | |
| <10,000 | 3 1(20.8) |
| 10,000–50,000 | 29 (19.5) |
| >50,000–100,000 | 12 (8.1) |
| >100,000 | 11 (7.4) |
| Not reported | 66 (44.3) |
| CNS+, n (%) | 9 (6.0) |
| HSCT frequency | 33 (22.2) |
| FAB subtype, n (%) | |
| M0 | 11 (7.4) |
| M1 | 15 (10.1) |
| M2 | 15 (10.1) |
| M3 | 43 (28.9) |
| M4 | 14 (9.4) |
| M5 | 12 (8.1) |
| M6 | 5 (3.4) |
| M7 | 11 (7.4) |
| Non-M3 | 23 (15.4) |
Baseline characteristics of the AML cohort.
AML, acute myeloid leukemia; FAB, French-American-British classification of AML; CNS, central nervous system.
Nine (6.0%) patients had central nervous system (CNS) disease, and one (0.6%) patient had pulmonary disease. Ten (6.7%) patients had extramedullary leukemia (EML). Of these, four were classified as M4/M5, one as M1, one as M3, one as M6, one as M7, and two as unspecified non-M3 FAB subtypes.
Regarding karyotype, only 102 (68.4%) patients had available information. Of these, 26 (25.5%) had a normal karyotype.
Fifteen patients (10.0%) were <1 year of age at diagnosis, 23 (15.4%) were 1–4 years of age, 22 (14.8%) were 4–9 years of age, 57 (38.3%) were 9–15 years of age, and 32 (21.5%) were >15 years of age. At diagnosis, Cox regression analysis showed no association between WBC count, age, and risk of death or relapse (p>0.1).
Eight patients (5.4%) had associated syndromes: six had Down syndrome, one had trisomy 13 syndrome, and one had Fanconi anemia. Among patients with Down syndrome, four were male; five patients were classified as M7 subtype and one as M0 subtype. None of these patients had EML, and no radiotherapy or transplant was performed. Only one patient with Down syndrome relapsed; this patient died 17 months after the initial diagnosis. Two other patients with Down syndrome died, one due to refractory disease and the other due to infection. The patient with trisomy 13 syndrome underwent HSCT at first remission and is still alive after 75 months of follow-up. The patient with Fanconi anemia had two bone marrow relapses and died 9 months after the diagnosis due to disease progression.
This study found an incidence of 13.2 occurrences of AML per 1,000,000 children aged <18 years in the state of Rio Grande do Sul. In the United States, the incidence is approximately 8.9 occurrences per 1,000,000 children.1 A possible explanation is race based. It is known that race can influence the incidence of childhood AML, with data showing a higher prevalence of the disease in patients of Latin-American origin.7 Also, there was a higher prevalence of M3 over other FAB subtypes of AML. The Brazilian Collaborative Study Group of Infant Acute Leukemia found a prevalence of 11% (5/45) of acute promyelocytic leukemia (APL) in their AML sample.7 This is consistent with the present finding of 28% (43/106) of cases classified as FAB M3. Although the diagnosis in some patients was molecularly confirmed, in most cases the diagnosis was confirmed by immunophenotype or karyotype.
TreatmentMost patients with APL (31/43 patients) were treated with the GIMEMA-AIEOP AIDA protocol, which consists of all-trans retinoic acid (ATRA) and idarubicin as induction, followed by three polychemotherapy consolidation courses.8 The PETHEMA LPA-99 protocol was used in three other patients (also including idarubicin and ATRA as the induction regimen),9 and two patients received treatment similar to that of the North American Leukemia Intergroup Protocol C9710 (ATRA+daunorubicin+cytarabine).10 The other seven patients had no available information on the treatment protocol used.
Information on treatment protocol was accurately recorded in 79 of 106 patients with non-promyelocytic leukemia (74.5%). Most treatment strategies (n=44, 55.7%) were based on the Berlin-Frankfurt-Münster (BFM) group protocols (1983, 1993, 1998, and 2004), in which there is a period of maintenance therapy and etoposide induction.11 Fourteen patients (17.7%) received treatment based on the St. Jude AML02 multicenter trial, 13 (16.4%) were treated with a Brazilian protocol,12 five (6.3%) were treated with the ‘7+3’ regimen, and three (3.8%) were treated with other protocols.
Relapse and causes of deathA total of 48 (32.2%) patients relapsed, six of them with APL. Forty-five (93.7%) had a bone marrow relapse and three (6.3%) had a CNS relapse. The most frequently used regimens in second-line therapy were fludarabine, cytarabine, and granulocyte-colony stimulating factor (G-CSF), with or without idarubicin (FLAG/IDA-FLAG). In some patients, idarubicin was replaced by another anthracycline, such as mitoxantrone and daunorubicin, at an equivalent dose. The median time from diagnosis to relapse was 11 months (range, 2–68 months). Twelve (25.0%) patients had a second relapse and one (2.0%) had a third relapse.
Of the six patients with relapsed APL, three underwent an allogeneic bone marrow transplant, two of them died due to progression of disease even after transplant, and one remained alive. Considering the other three patients with non-transplanted APL, two died from disease progression, and one from infection.
A total of 72 (48.3%) patients died. Of the 48 patients who relapsed, 38 (79.1%) died. The leading causes of death were disease progression (n=30, 41.6%) and infection (n=31, 43.0%). Nine (12.5%) patients died of treatment complications: eight with hemorrhagic complications/disseminated intravascular coagulation and one with retinoic acid syndrome. The other two patients died of an error in total parenteral nutrition and head injury. The median time from diagnosis to death was 10 months (range, 4 days to 13.5 years).
HSCTA total of 33 (22.1%) patients underwent HSCT. Of these, three (9.0%) received autologous, 12 (36.3%) related allogeneic, 14 (42.4%) unrelated allogeneic, two (6.0%) haploidentical, and two (6.0%) unrelated umbilical cord blood HSCT types. The median time from diagnosis to transplant was 14 months (range, 6–68 months).
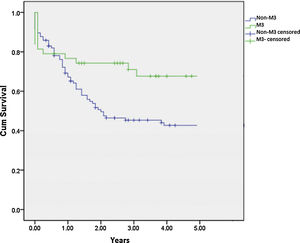
SurvivalTo calculate the survival rate, patients with associated genetic syndromes were excluded. The mean 3-year OS rates in patients with M3 and non-M3 AML were 69.2% (SD, 7.6%) and 45.3% (SD, 5.0%), respectively (p=0.018) (Fig. 1). The mean 3-year EFS was 67.7% (SD, 7.1%) in M3 patients and 36.7% (SD, 4.8%) in non-M3 patients (p=0.005).
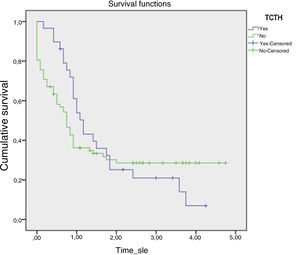
The mean 5-year OS rates in patients with and without bone marrow transplantation were 1.662 (SE, 0.236) and 1.726 (SE, 0.224), respectively (p=0.637) (Fig. 2). In patients with non-M3 AML, OS rates were significantly different between transplanted and non-transplanted patients (61.8% [SD, 8.7%] vs. 38.2% [SD, 5.9%]; (p=0.031).
DiscussionTreatment of childhood cancer has markedly improved over the past decades, and the overall mortality rate has declined by 50% since the 1970s. For childhood, survival for pediatric AML patients was shown to have increased from 17–20% in the 1970s to 57–68% in 2000s.13 Therefore, evaluating epidemiological features and survival probability of patients with childhood AML is essential for continued progress in disease treatment. Despite a few single-center studies and one major multicenter study conducted in Brazil,14 there is still a lack of epidemiological data to support advances in the treatment of childhood AML. To the best of the authors’ knowledge, this is the first multicenter study to document the characteristics and survival rates of patients with childhood AML treated at hematology-oncology referral centers in southern Brazil.
In this cohort, AML occurred predominantly in males (63%), which is consistent with the literature reporting a male predominance in the incidence of AML.1 However, AML was diagnosed at a median age of 10.5 years, which is in contrast with the literature that reports a mean age of 5 years at diagnosis.15 Also noteworthy is that the most prevalent age group for AML was 9–15 years (n=57, 38.3%). This prevalence differs from that reported in other Brazilian and international studies, which show a first incidence peak before 1 year of age and a second peak between 15 and 20 years of age, with a mean age of 5 years at diagnosis.15
Another point to consider is the prevalence of EML. Although the prevalence of CNS disease of 6% (n=9) in the present study was similar to that reported in previous studies,16 the overall prevalence of diagnosed EML of 6.7% (n=10) was much lower than that reported in the literature. Most studies report a rate of 20–40% of extramedullary involvement in childhood AML cases; however, some authors consider hepatomegaly and CNS disease in the diagnosis of EML, while others do not.16 The lower prevalence of non-CNS EML observed in the present study may be due to lack of information in the medical records, a higher incidence of the M3 subtype or, perhaps, due to regional peculiarities. Moreover, children with EML are more frequently classified as M4/M5 subtypes, which is consistent with the present findings (four of ten patients with EML were classified as M4/M5). However, prognosis in these cases is still controversial.17 While some authors argue that EML has a worse prognosis,18 others argue that there is no relationship between extramedullary involvement and prognosis.19
A high WBC count (>100×109/L) is generally related to a worse prognosis.20 In the present cohort, only 83 patients had WBC count recorded at diagnosis, with a median WBC count of 20,300/mm3. Of these, only 11 had a WBC count >100,000/mm3, but there was no relationship between WBC count and risk of death or relapse (p=0.46). The small number of patients with high WBC counts at diagnosis might have influenced this analysis.
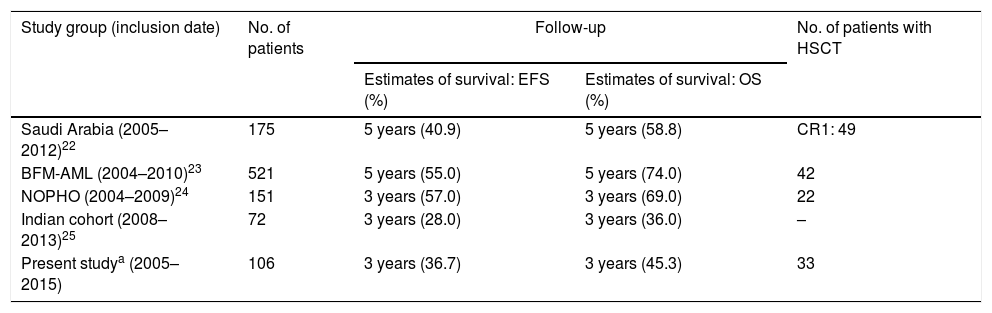
It is known that developing countries have lower OS rates than developed countries.21 In developed countries, the 5-year OS for childhood AML ranges from 60% to 70% (Table 2).22–25 In a study of 193 children with de novo AML conducted in Saudi Arabia, the 5-year OS was 58.8%. In a study of 72 patients in India, the 3-year OS was 36% and EFS was 28%,26 while in Thailand the 5-year OS was 30.3%.26 In a recent Brazilian multicenter study, with the purpose of evaluating the molecular characteristics of 703 patients with de novo childhood AML (2000–2015), the estimated cumulative 5-year OS was 37.7% (SD, 2.8%).26 However, the generalizability of the results of this survival analysis presents some weaknesses. Because the study was based on a convenience sample, the geographic regions of the country were unequally represented and only a small portion of the cases (<10%) were in southern Brazil. In addition, their main objective was the molecular characterization of childhood AML. Given the continental size of Brazil and its genetic, social and environmental heterogeneity, comprehensive regional studies are essential for a realistic characterization of the diseases in this population. In the present study, 3-year OS was 45.3% and 3-year EFS was 36.7%. In a study conducted in Florianópolis, a city also located in southern Brazil, the mortality rate was 43%,20 similar to that of the present study (48.3%). These results differ considerably from those obtained from studies conducted in developed countries.11 The survival differences may be attributed to lack of supportive care, death due to treatment toxicity, difficulties in risk stratification with limited access to molecular examination, and prolonged time to HSCT in developing countries.27
Survival in different studies.
| Study group (inclusion date) | No. of patients | Follow-up | No. of patients with HSCT | |
|---|---|---|---|---|
| Estimates of survival: EFS (%) | Estimates of survival: OS (%) | |||
| Saudi Arabia (2005–2012)22 | 175 | 5 years (40.9) | 5 years (58.8) | CR1: 49 |
| BFM-AML (2004–2010)23 | 521 | 5 years (55.0) | 5 years (74.0) | 42 |
| NOPHO (2004–2009)24 | 151 | 3 years (57.0) | 3 years (69.0) | 22 |
| Indian cohort (2008–2013)25 | 72 | 3 years (28.0) | 3 years (36.0) | – |
| Present studya (2005–2015) | 106 | 3 years (36.7) | 3 years (45.3) | 33 |
BFM-AML, Berlin-Frankfurt-Münster protocol for acute myeloid leukemia; NOPHO, Nordic Society of Pediatric Hematology and Oncology; HSCT, hematopoietic stem cell transplantation; OS, overall survival; EFS, event-free survival.
The role of HSCT in childhood AML remains controversial.28 However, despite the controversy surrounding this issue, most indications for HSCT – as well as the cytogenetic markers of high risk and standard risk – are well known and have already been incorporated in the World Health Organization (WHO) classifications.11 In the present study, there was a significant difference in 3-year OS between transplanted and non-transplanted patients (61.8% vs. 38.2%). However, lack of information on the pre-transplant status of the recipients precluded a more detailed analysis.
Treatment complications remain a major cause of morbidity and mortality in childhood AML, even in developed countries, with infection as the leading cause.28 In the present study, infection (n=31, 43%) and disease progression (n=30, 42%) were the major causes of death. In the AML-BFM 93 and AML-BFM 98 clinical trials, patients died more frequently in the first 14 days of treatment (n=104, 11.5%) due to disease complications (leukostasis and bleeding). After this period, the major cause of death was infection, mainly bacterial and fungal.29 Among 492 children with AML enrolled in a multicenter study in the United States and Canada, 58 died of infection, with a cumulative incidence of infection-related mortality of 11% (SD, 2%).29 The infection-related mortality is expected to be higher in developing countries due to the lack of supportive care. In a study conducted in Central America, of 279 patients with AML, 65 (23%) died of treatment-related complications: 29 of infection and 13 of bleeding.21
This study has limitations inherent due to its retrospective design. It relied on medical record review alone, and some data were missing or incompletely recorded, resulting in a reduced sample size for some of the analyses. In addition, limiting factors include known excess APL, limited disease characterization, high non-disease-related mortality, in addition to limited characterization of HSCT, possible uncontrolled biases in the HSCT, and the absence of a national protocol for AML in Brazil. Despite this shortcoming, it is still possible to draw significant information on the epidemiological aspects of childhood AML treated at southern Brazilian hospitals.
In conclusion, the results show a higher prevalence of the FAB M3 subtype of AML in this population than that reported in the international literature. Although similar to rates reported in other Brazilian studies, OS was decreased compared with that of developed countries. Perhaps increased access to cytogenetic and molecular tests and, more importantly, proper hospital support could improve the survival of children with AML treated in Brazil. Further prospective, multicenter Brazilian studies with a larger sample size are encouraged to better understand the characteristics of AML and improve treatment and prognosis in this population.
Ethics approval and consent to participateEthical approval to conduct this study has been granted by the Ethics Committee of Hospital de Clínicas de Porto Alegre (HCPA). Participating adults as well as those responsible for minors signed informed consents in duplicate, keeping a copy for themselves.
Availability of data and materialsThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
FundingThe research was supported by FIPE/HCPA (Fundo de Incentivo à Pesquisa e Eventos do Hospital de Clínicas de Porto Alegre).
Conflicts of interestThe authors declare no conflicts of interest.